Abstract
Accumulating evidence have emerged important roles for microRNAs (miRNAs) participating in epithelial-mesenchymal transition (EMT) process and are associated with metastasis in cervical cancer. We hypothesized that miR-223 played an important role in cell metastasis of cervical cancer. Here, we found miR-223 was downregulated in human cervical cancer cell lines and clinical tumor tissues. Result of wound healing and cell migration assays revealed that miR-223 inhibited cell migration, whereas miR-223-in showed the opposite effect. In terms of mechanism, miR-223 influenced the expression of the EMT-associated proteins by upregulating the epithelial markers E-cadherin and α-cadherin and downregulating the mesenchymal marker vimentin. In conclusion, miR-223 inhibited cell metastasis of human cervical cancer by modulating epithelial-mesenchymal transition.
Keywords: miR-223, human cervical cancer, cell metastasis
Introduction
Cervical cancer is a gynecological malignancy with the second most common malignancy in female worldwide [1]. Despite advanced in treatment with surgery, radiotherapy, and chemotherapy, the prognosis for cervical cancer remains poor. Therefore, finding novel diagnostic and prognostic markers and new therapeutic targets for treatment of cervical cancer are of great significant.
Recent studies have identified that MicroRNAs (miRNAs) are a class of small noncoding RNAs that regulate the targeted gene expression by targeting the 3’-untranslated regions (3’-UTRs) of target mRNAs, resulting in mRNA degradation or translational suppression [2-4]. Increasing evidence indicated that miRNAs regulate a variety of biological processes, including cell proliferation, differentiation, migration and apoptosis [5-9]. However, the function of miR-223 in cervical cancer remains poorly understood. In the present study, we found that miR-223 was significantly decreased in cervical cancer clinical tissues. Our results revealed that miR-223 functioned as a tumor suppressor by inhibiting cell metastasis in vitro. Together, these results suggest that miR-223 could be a therapeutic target for the treatment of cervical cancer.
Materials and methods
Clinical specimens
Eight pairs of cervical cancer tissues and adjacent noncancerous tissues were obtained from Department of Obstetrics and Gynecology, Zhujiang Hospital, Southern Medical University (Guangzhou, People’s Republic of China). The study was approved by the ethics committee of Zhujiang Hospital, Southern Medical University (Guangzhou, People’s Republic of China). Writ ten informed consent was obtained from each participant before the study. All tissues confirmed by pathology and immunohistochemistry were collected and frozen in liquid nitrogen and stored at -80°C.
Cell culture
Human cervical cancer cell lines Siha, Hela and C-33A were purchased from the American Type Culture Collection (Manassas, VA, USA) and were cultured in RPMI 1640 (HyClone, USA) containing 10% (v/v) fetal bovine serum (FBS, HyClone, USA), 100 mg/mL of streptomycin, and 100 IU/mL of penicillin. All cells were incubated at 37°C in a humidified chamber containing 5% CO2.
Plasmids and transfection
The miR-223 mimics, miR-223-in and the corresponding negative controls (NC) were purchased from GeneCopoeia Co. (Guangzhou, China). Transfection of plasmids and siRNAs were performed using lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol.
RNA extraction and real-time quantitative PCR
Total RNA from cultured cells and fresh frozen tissues was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Reverse transcription and quantitative PCR were performed using the miRNA-specific TaqMan MiRNA Assay Kit to detect the expression level of miR-223 by using the Applied Biosystems 7,900 Sequence Detection system. The primer of miR-223 (HmiRQP0341) was synthesized by GeneCopoeia Co. (Guangzhou, China). The relative miR-223 expression levels after normalization to U6 small nuclear RNA were calculated using 2-ΔΔCt method.
Wound healing assay
Hela cells after transfection were seeded in 6-well plates at 70% confluence and cultured until they formed a monolayer that occupied 100% of the surface area. Next, a linear wound was made by scratching the monolayer with a 200 μl pipette tip and washed three times with serum-free RPMI 1640 to remove the cell debris. Cells were incubated for 24 h. Images were captured and the gap size was measured. The experiment was repeated three times.
Migration assays
Migration assays were performed using Transwell chambers with a pore size of 8 mm. Cells were transfected with miR-223, miR-223-in or the relative control mimics and incubated for 48 h. 5×104 transfected cells were placed in the upper chamber. Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum was added to the lower chamber as a chemo-attractant. Chambers were incubated in 5% CO2 at 37°C for 24 h, and then cells on the upper surface were removed. Cells that had migrated to the bottom surface were washed twice with cold phosphate-buffered saline, fixed in methanol and stained with 0.1% crystal violet. Stained cells were counted under a microscope.
Western blotting
Total proteins from cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, China). Equal quantities (40 μg) of protein samples were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Bedford, MA, USA), and blocking was performed with 5% non-fat milk room temperature for 1.5 h, and incubated with the primary antibodies, anti-E-cadherin, anti-Vimentin, anti-α-carenin (1:500; Abcam). Following the initial western blot assay, the membranes were stripped and re-probed with anti-β-actin (1:500; Sigma, St Louis, MO, USA) as a protein loading control.
Statistical analysis
All the statistics were expressed as mean ± standard deviation (SD) and processed using SPSS 17.0 statistical software (Chicago, IL, USA). Values of P < 0.05 were considered significant.
Result
MiR-223 expression was downregulated in cervical cancer cell lines and clinical tissues
To investigate the expression of miR-223 in human cervical cancer, we first evaluated the expression levels of miR-223 in cervical cancer cell lines and cancer tissues. As showed in Figure 1A, real time PCR analysis indicated that expression of miR-223 was differentially decreased in eight human cervical cancer tissues compared with adjacent non-cancerous tissues (ANT), suggesting miR-223 may act as an anti-oncogene in cervical cancer.
Figure 1.
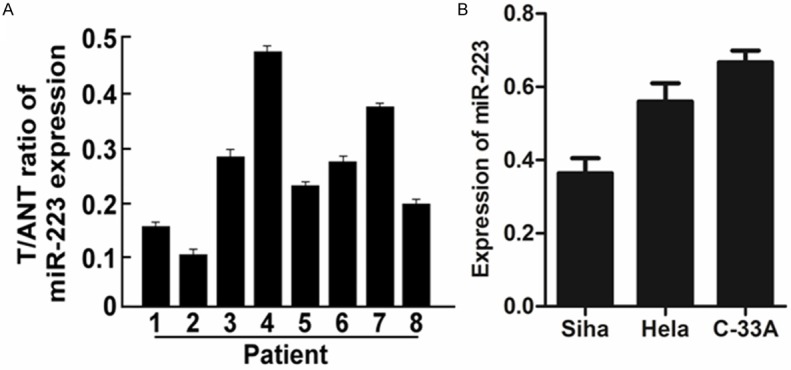
Expression of miR-223 in human cervical cancer clinical specimens and cell lines. A. Relative miR-223 mRNA expression levels in 8 paired human cervical cancer tissues (T) and the adjacent non-cancerous tissues (ANT) from the same patient were detected by PCR analysis. B. Real-time PCR analysis of miR-223 expression in human cervical cancer cell lines, including Siha, Hela and C-33A. Each bar represents the mean of three independent experiments. *P < 0.05.
In addition to cervical cancer tissues, endogenous expression of miR-373 was detected in a panel of cervical cancer cell lines (SiHa, HeLa, C-33A). It was observed that the expression of miR-223 in HeLa cells was higher than that in SiHa cells, lower than that in C-33A cells (Figure 1B). Given the above results, it was decided to use the HeLa cells for the below experiments.
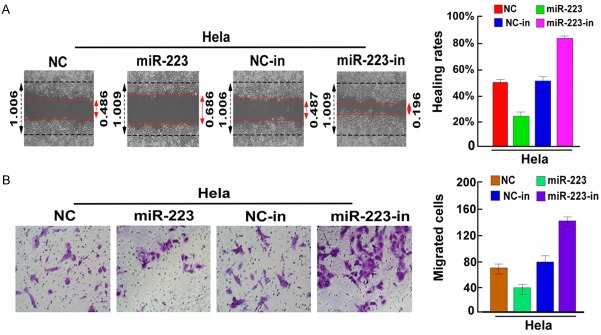
MiR-223 inhibited cell metastasis of cervical cancer cells
To further explore the functional role of miR-223 in cervical cancer, the cervical cancer Hela cells were transfected with the miR-223 and miR-223-in, which were used to increase or reduce expression of miR-223, respectively. Results of wounding healing assays indicated that cells in the miR-223 group had stronger wound healing ability than those in the relative control group. In contrast, cells in the miR-223 inhibitor group grew slower and their wound healing ability was weaker than those in the relative control group (Figure 2A). Furthermore, result of migration assay revealed that cell migration of Hela cells was dramatically enhanced by miR-223 overexpression. However, the miR-223-in showed the opposite effect (Figure 2B). Collectively, these results showed that miR-223 overexpression inhibited cervical cancer cell metastasis in vitro.
Figure 2.
MiR-223 upregulation inhibited cell metastasis of cervical cancer. A. Cell migration activity was measured by conducting the wound healing assay 48 h after transfection using miR-223 or miR-223-in or the relative control mimics. B. For cellular migration, transwell assays were performed. Each bar represents the mean of three independent experiments. *P < 0.05.
MiR-223 upregulated E-cadherin and α-carenin and downregulated vimentin expression
Epithelial-mesenchymal transition (EMT) is an essential step for cancer metastasis. The EMT process is characterized by loss of epithelial markers like E-cadherin and α-carenin, attended by increased mesenchymal markers such as vimentin. Result of western blot revealed that the overexpression of miR-223 caused an increase in the expression of the epithelial marker E-cadherin and α-carenin and a corresponding decrease in the expression of the mesenchymal marker vimentin, whereas the miR-223-in had the opposite effect (Figure 3). These results indicated that miR-223 inhibited cell metastasis of human cervical cancer cells by modulating epithelial-mesenchymal transition.
Figure 3.

MiR-223 upregulated E-cadherin and α-carenin and downregulated vimentin expression. A. Levels of E-cadherin, α-carenin and vimentin in Hela cells were quantified using Image J software. β-actin was used as an internal control. B. Western blotting analysis of protein expression of E-cadherin, α-carenin and vimentin in Hela cells. β-actin was used to serve as the loading control. Each bar represents the mean of three independent experiments. *P < 0.05.
Discussion
An increasing number of studies had demonstrated that microRNAs (miRNAs), a class of small non-coding RNAs, are important regulators of a variety of biological processes in cancer, such as cell proliferation, cycle, apoptosis, invasion and migration [10-13]. Aberrant expression of miRNAs is related to initiation and development of cancer [14-16]. Finding of Wu L et al indicated that overexpression of miR-223 inhibited cell proliferation of HCT116 colorectal cancer cells, HeLa cervical cancer cells and HuH-7 hepatoma cells by regulating FOXO1expression [17].
However, the role of miR-223 in cell metastasis of human cervical cancer have until now remained unclear. In this present study, our results indicated that miR-223 expression was markedly downregulated in cervical cancer clinical tissue, suggesting miR-223 as a candidate tumor suppressor in the pathogenesis of cervical cancer.
As demonstrated by our clinical data, miR-223 expression is significantly reduced in cervical cancer clinical tissue, and further experiment (wound healing and migration assasys) revealed that miR-223 overexpression inhibited cervical cancer cell metastasis in vitro. Taken together, we hypothesized that miR-223 inhibits cervical cancer progression by suppressing the epithelial-mesenchymal transition (EMT) process. It was reported that EMT was considered as one of critical steps in cancer cell metastasis [18-21]. Cancer cells lost their epithelial phenotype and cell-cell adhesion and then gained invasive mesenchymal properties [22-24]. Result of western blot showed that overexpression of miR-223 caused an increase in the expression of the epithelial marker E-cadherin and α-carenin and a corresponding decrease in the expression of the mesenchymal marker vimentin, whereas the miR-223-in had the opposite effect.
In summary, the present study has provided further evidence that miR-223 suppressed cell metastasis of cervical cancer and inhibited the EMT progress, and this implied miR-223 might potentially become a novel strategy for targeting EMT.
Acknowledgements
All authors designed the study together, performed the experiment together, analyzed the data and wrote the paper; all authors approved the final manuscript.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Xiao L, Zhou X, Liu F, Hu C, Zhu X, Luo Y, Wang M, Xu X, Yang S, Kanwar YS, Sun L. MicroRNA-129-5p modulates epithelial-to-mesenchymal transition by targeting SIP1 and SOX4 during peritoneal dialysis. Lab Invest. 2015;95:817–32. doi: 10.1038/labinvest.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen F, Cai WS, Feng Z, Li JL, Chen JW, Cao J, Xu B. MiR-492 contributes to cell proliferation and cell cycle of human breast cancer cells by suppressing SOX7 expression. Tumour Biol. 2015;36:1913–1921. doi: 10.1007/s13277-014-2794-z. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Gou J, Jia J, Zhao X. MicroRNA-429 functions as a regulator of epithelial-mesenchymal transition by targeting Pcdh8 during murine embryo implantation. Hum Reprod. 2015;30:507–518. doi: 10.1093/humrep/dev001. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, Chang RM, Yu L, Lei X, Xiao S, Yang H, Yang LY. MicroRNA-188-5p Suppresses Tumor Cell Proliferation and Metastasis by Directly Targeting FGF5 in Hepatocellular Carcinoma. J Hepatol. 2015;63:874–85. doi: 10.1016/j.jhep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Zhong C, Ding S, Huang H, Shen Z. MicroRNA-221 promotes human non-small cell lung cancer cell H460 growth. Int J Clin Exp Med. 2015;8:2024–2030. [PMC free article] [PubMed] [Google Scholar]
- 10.Li E, Zhang J, Yuan T, Ma B. MiR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumour Biol. 2014;35:7645–7650. doi: 10.1007/s13277-014-2031-9. [DOI] [PubMed] [Google Scholar]
- 11.Gururajan M, Josson S, Chu GC, Lu CL, Lu YT, Haga CL, Zhau HE, Liu C, Lichterman J, Duan P, Posadas EM, Chung LW. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin Cancer Res. 2014;20:6559–6569. doi: 10.1158/1078-0432.CCR-14-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Ma X, Cai Q, Wang X, Yu B, Cai Q, liu B, Zhu Z, Li C. MiR-199a-3p promotes gastric cancer progression by targeting ZHX1. FEBS Lett. 2014;588:4504–4512. doi: 10.1016/j.febslet.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 13.Dong N, Tang X, Xu B. miRNA-181a inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:993–1001. doi: 10.1167/iovs.14-15860. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Hou L, Huang Y. EZH2-specific microRNA-98 inhibits human ovarian cancer stem cell proliferation via regulating the pRb-E2F pathway. Tumour Biol. 2014;35:7239–7247. doi: 10.1007/s13277-014-1950-9. [DOI] [PubMed] [Google Scholar]
- 15.Qiu F, Sun R, Deng N, Guo T, Cao Y, Yu Y, Wang X, Zou B, Zhang S, Jing T, Ling T, Xie J, Zhang Q. miR-29a/b Enhances Cell Migration and Invasion in Nasopharyngeal Carcinoma Progression by Regulating SPARC and COL3A1 Gene Expression. PLoS One. 2015;10:e0120969. doi: 10.1371/journal.pone.0120969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alizadeh S, Kaviani S, Soleimani M, Abroun S, Kashani-Khatib Z, Asgharzadeh A, Dargahi H, Mousavi R. Mir-55 inhibition can reduce cell proliferation and induce apoptosis in Jurkat (Acute T cell Leukemia) cell line. Iran J Ped Hematol Oncol. 2014;4:141–150. [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M, Zhu Y, Zhao Q, Dong YW, Shao K, Wu A, Wu XZ. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBS Lett. 2012;586:1038–1043. doi: 10.1016/j.febslet.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Zhao L, Chen W, Liu T, Li Z, Li X. miR-210, a modulator of hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cell. Int J Clin Exp Med. 2015;8:2299–2307. [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Deng Z, Wang Z, Wang D, Zhang L, Su Q, Lai Y, Li B, Luo Z, Chen X, Chen Y, Huang X, Ma J, Wang W, Bi J, Guan X. Zipper-interacting protein kinase promotes epithelial-mesenchymal transition, invasion and metastasis through AKT and NF-kB signaling and is associated with metastasis and poor prognosis in gastric cancer patients. Oncotarget. 2015;6:8323–8338. doi: 10.18632/oncotarget.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Zhai L, Zhao C, Lv S. MiR-153 inhibits epithelial-mesenchymal transition by targeting metadherin in human breast cancer. Breast Cancer Res Treat. 2015;150:501–509. doi: 10.1007/s10549-015-3346-y. [DOI] [PubMed] [Google Scholar]
- 21.Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, Hostiuc S. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology. 2015;15:217–225. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhao JL, Zhang L, Guo X, Wang JH, Zhou W, Liu M, Li X, Tang H. miR-212/132 downregulates SMAD2 expression to suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells. IUBMB Life. 2015;67:380–394. doi: 10.1002/iub.1381. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–92. doi: 10.1093/annonc/mdq292. [DOI] [PMC free article] [PubMed] [Google Scholar]