Abstract
It is extremely difficult to discriminate between follicular thyroid carcinoma (FTC) and follicular thyroid adenoma (FTA) before surgery, because the morphologies of carcinoma cells and adenoma cells obtained by fine needle aspiration biopsy (FNAB) are similar. Molecular markers may be helpful on this issue. The purpose of this study was to assess the role of GPER1, EGFR and CXCR1 in differential diagnosis between FTC and FTA. GPER1, EGFR and CXCR1 mRNA expression levels were examined in 15 FTCs and 10 FTAs using real-time RT-PCR. FTC showed to have significantly increased mRNA levels of the three molecules compared to FTA (P < 0.001 for all the three molecules). GPER1, EGFR and CXCR1 protein expression in 106 FTCs and 128 FTAs were analyzed using immunohistochemistry. The rates of GPER1, EGFR and CXCR1 high expression were 73.6%, 72.6% and 70.8% in FTC and 30.5%, 28.1% and 27.3% in FTA, respectively. Statistical analysis showed that GPER1, EGFR and CXCR1 protein expression were correlated with one another in FTC and concomitant high expression of the three molecules had stronger correlation with the occurrence of FTC than did each alone. The positive predictive values (PPV) for concomitant high expression of the three molecules for discriminating between FTC and FTA were 91.0% for GPER1/EGFR, 93.8% for GPER1/CXCR1, 92.3% for EGFR/CXCR1 and 98.2% for GPER1/EGFR/CXCR1, respectively. These results indicated that the evaluation of GPER1, EGFR and CXCR1 concomitant high expression may be helpful in differential diagnosis between FTC and FTA.
Keywords: GPER1, EGFR, CXCR1, follicular thyroid carcinoma (FTC), follicular thyroid adenoma (FTA)
Introduction
Follicular thyroid carcinoma (FTC) is the second most common thyroid cancer after papillary thyroid carcinoma (PTC). It accounts for 10-20% of all thyroid malignancies, and similar to PTC, it is more prevalent in women than in men [1]. FTC is more aggressive than its papillary counterpart with hematogenous spread as its metastatic signature [2]. It is classified into two categories based on the degree of invasion: widely invasive FTC (WI-FTC) and minimally invasive FTC (MI-FTC). The former has widespread infiltration of adjacent thyroid tissue and/or blood vessels whereas the latter has limited capsular and/or vascular invasion. It is extremely difficult to differentiate between malignant FTC and benign follicular thyroid adenoma (FTA) before surgery, because the morphologies of carcinoma cells and adenoma cells obtained by fine needle aspiration biopsy (FNAB) are similar [3]. The final diagnosis should be determined by postoperative pathological examination by the evaluation of the specific characteristics of FTC such as capsular infiltration and/or vascular invasion [4]. If we could preoperatively diagnose FTC, it would facilitate determination of surgical indication for follicular tumor. To date, studies have been intensively performed to find molecular markers able to discriminate between FTC and FTA, which can be applied to preoperatively obtained specimens, such as immunostaining of FNAB samples.
G protein-coupled estrogen receptor 1 (GPER1), formerly known as GPR30, is a novel seven-transmembrane receptor belonging to the G protein-coupled receptor family, binds estrogen with high affinity and functions alongside the traditional nuclear estrogen receptors (ERα and ERβ) to regulate cellular and physiological responsiveness to estrogen. Activation of GPER1 leads to multiple intracellular responses related to proliferation, invasion and migration. GPER1 is widely expressed in numerous tissues throughout the body and is often highly expressed in cancer cell lines, particularly those from aggressive tumors [5], and has been shown to be an important prognostic factor in breast, endometrial, ovarian cancers [6-8]. High expression of GPER1 in these estrogen-related tumors has been associated with metastases and poor survival.
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase important in transducing extracellular signals from the cell surface to the cell interior. Studies have shown that EGFR is transactivated through stimulation of GPER1 to generate a survival response, facilitating proliferation, invasion and migration in several types of cancer [9-11]. Over expression of EGFR is frequently found in epithelial cancers such as breast cancer [12], esophageal squamous cell carcinomas [13] and papillary thyroid cancer [14], and high expression of EGFR occurs at an advanced stage of malignancy characterized by metastatic competence and poor prognosis.
CXCR1 is one of two high-affinity receptors for the CXC chemokine interleukin-8 (IL-8), a major mediator of immune and inflammatory responses implicated in many disorders, including tumor growth [15]. CXCR1 is mainly expressed in neutrophils and is originally characterized by its ability to induce chemotaxis of leukocytes. However, CXCR1 has been shown to act on multiple cell types, as our recent study showed that CXCR1 is involved in invasion and migration of ER-negative breast cancer cells through cross-talk with GPER1 via EGFR-ERK/PI3K pathway [16]. Moreover, it was found that over expression of CXCR1 is correlated with drug-resistance, invasion, and metastasis in several solid tumors [17].
Recently, our group examined the expression of GPER1, EGFR and CXCR1 in PTC, and found that high expression of GPER1, EGFR and CXCR1 was significantly correlated with lymph node metastasis in PTC. Concomitant high expression of the three molecules had stronger correlation with lymph node metastasis than did each alone, demonstrating that the evaluation of GPER1, EGFR and CXCR1 expression in PTC may be useful in predicting the risk of lymph node metastasis [18]. In the present study, we will examine GPER1, EGFR and CXCR1 expression in FTC and FTA, and evaluate potential usefulness of the three molecules in discriminating between FTC and FTA.
Materials and methods
Case selection and tissue sample preparation
Tumor specimens for real-time RT-PCR were obtained from 25 patients who underwent initial thyroidectomy in the Department of Surgery, the First Affiliated Hospital, Chongqing Medical University, between March 2013 and March 2014, including 10 FTAs, 10 MI-FTCs and 5 WI-FTCs. The benign thyroid hyperplasia specimens were obtained from 10 patients with adenomatous nodule. For controls, 10 normal thyroid tissue specimens were taken from the contralateral lobe of FTC specimens. All specimens were immediately snap-frozen in liquid nitrogen and stored at -80°C up to subsequent real-time RT-PCR.
Tumor specimens for immunohistochemical analysis were obtained from 106 pathologically proven FTCs and 128 FTAs between March 2008 and March 2014. In FTAs, there were 33 men and 95 women, 62 patients with the age of < 45 years and 66 with the age of ≥ 45 years, 42 with tumor size of ≤ 2 cm, 52 with tumor size of > 2 and ≤ 4 cm, 34 with tumor size of > 4 cm. All these tumors were encapsulated, exhibited a follicular architecture and lacked either vascular or capsular invasion. Relatively, in FTCs there were 27 men and 79 women, 51 patients with the age of < 45 years and 55 with the age of ≥ 45 years, 35 with tumor size of ≤ 2 cm, 42 with tumor size of > 2 and ≤ 4 cm, 29 with tumor size of > 4 cm. Moreover, in FTCs, 35 were WI-FTCs and 71 were MI-FTCs, and 34 had vascular invasion only, 16 had capsular infiltration only and 56 had both. Besides, benign thyroid hyperplasia specimens were obtained from 115 patients with adenomatous nodule, 90 normal thyroid tissues were taken from the contralateral lobe of FTC specimens, which exhibit apparently normal morphology as a control. The study protocol was approved by the Research Ethics Committee of Chongqing Medical University and informed consent was obtained from all patients.
RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from frozen thyroid tissues using TriZol reagent (Invitrogen, Camarillo, CA, USA), and residual genomic DNA was eliminated by DNase I digestion (Ambion, USA). RNA purity was confirmed by spectrophotometry. Total RNA was reverse transcribed to cDNA by using Super Script III Reverse Transcriptase (Invitrogen, USA) according to the manufacturer’s protocol. The final cDNA product was amounted to 25 μL and stored at -80°C.
Real-time PCR was performed by using SYBR-Green real-time PCR method on the ABI-Prism 7000 sequence detector (Applied Biosystems, USA). The primers are shown in Table 1. The predicated product size of the primers for GPER1, EGFR and CXCR1 was 240 bp, 106 bp, and 214 bp, respectively. Quantities of gene specific mRNA expression were determined by the CT method. Samples were analyzed in triplicate. Average threshold cycle (CT) values for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as an internal calibrator. The 2-ΔΔCT method was used for relative quantitation [19]. Results are presented as the mean ± standard deviation of three independent experiments. The real-time PCR mix was made on the basis of the prescription from the supplier: 6 μL sterile water, 1 μL sense and 1 μL antisense primers, 10 μL Platinum SYBR Green QPCR Super Mix-UDG w/ROX (Invitrogen, USA) and 2 μL target cDNA in a total volume of 20 μL. Run conditions were: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min.
Table 1.
Primers used for real-time RT-PCR
| Gene | Primers | Product size |
|---|---|---|
| GPER1 | Forward: 5-AGTCGGATGTGAGGTTCAG-3 | 240 bp |
| Reverse: 5-TCTGTGTGAGGAGTGCAAG-3 | ||
| EGFR | Forward: 5-AGCTTCTTGCAGCGATACAGCTCAGAC-3 | 106 bp |
| Reverse: 5-TGGGAACGGACTGGTTTATGTATTCAGG-3 | ||
| CXCR1 | Forward: 5-GCAGCTCCTACTGTTGGACA-3 | 214 bp |
| Reverse: 5-GGGCATAGGCGATGATCACA-3 | ||
| GAPDH | Forward: 5-GGAGTCCACTGGCGTCTTCA-3 | 191 bp |
| Reverse: 5-GGGGTGCTAAGCAGTTGGTG-3 |
Tissue microarray
Formalin-fixed, paraffin-embedded blocks were routinely prepared from surgical specimens of FTC, FTA, adenomatous nodule and normal thyroid tissue. Representative areas containing FTC, FTA, adenomatous nodule and normal thyroid tissue were identified by a pathologist. Duplicate tissue cores with a diameter of 0.6 mm were taken from each specimen (Beecher Instruments, Silver Springs, USA) and arrayed on a recipient paraffin block, using standard procedures [20]. Serial 5-μm-thick sections were cut with a Leica microtome (Leica Microsystems, Wetzlar, Germany) and mounted onto polylysine-coated slides.
Immunohistochemical staining
Sections from tissue microarray blocks were dewaxed and hydrated. Antigen retrieval was achieved by microwaving in 0.01 M citrate buffer (pH 6.0) for 10 min. After microwave treatment, the slides were treated with 3% hydrogen peroxide for 30 min to block the endogenous peroxidase and followed by blocking with 10% normal goat serum (50062Z, Invitrogen, USA) in PBS at room temperature for 1 h. The slides were then incubated overnight at 4°C in the primary rabbit polyclonal anti-GPER1 antibody (1:100 dilution, ab39742; Abcam, USA), anti-EGFR antibody (1:100 dilution, BS1533; Bioworld Technology, USA), or anti-CXCR1 antibody (1:100 dilution, bs-1009R; Bioss, China). For negative isotype controls, the sections were incubated in rabbit immunoglobulin G (1:1000, NI01-100UG; Merck Millipore, Germany). After defrosting at 37°C for 30 min, the slides were washed with PBS and incubated with a secondary biotinylated goat-anti-rabbit antibody (ZB-2010; Zhongshan Golden Bridge Biotechnology, China) for 30 min, peroxidase-labeled streptavidin (ZB-2404; Zhongshan Golden Bridge Biotechnology, China) for 20 min and diaminobenzidine chromogen substrate (Sigma, USA) for 5 min. Slides were counterstained with hematoxylin, dehydrated, and mounted.
Immunohistochemical scoring
A semi quantitative assessment of immunohistochemical (IHC) scoring was performed by two observers blinded to the diagnosis. The IHC score was assigned based on staining intensity and percentage of positive cells. The intensity score was assigned as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The proportion score was assigned as 0 (< 5% positive cells), 1 (6-25% positive cells), 2 (26-50% positive cells), 3 (51-75% positive cells), and 4 (> 75% positive cells). Multiplication of the intensity and proportion scores gave rise to the final staining score: 0 (negative), + (1-4), ++ (5-8), and +++ (9-12). For statistical analysis, a final staining score of negative or + was combined into the low expression group, and a final staining score of ++ or +++ was combined into the high expression group.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 statistical software. Data are presented as percentages and mean and standard deviation, according to the distribution. Significance was assessed using Chi-square, Spearman rank and Mann-Whitney U test as appropriate, to compare the groups. P value < 0.05 was considered statistically significant.
Results
GPER1, EGFR and CXCR1 mRNA expression in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens
To compare gene expression of GPER1, EGFR and CXCR1 in FTC and FTA, 10 FTA, 10 MI-FTC and 5 WI-FTC tissue specimens were collected to analyze GPER1, EGFR and CXCR1 mRNA levels using real-time RT-PCR. 10 adenomatous nodule and 10 normal thyroid tissue specimens were used for comparison and as a control. As shown in Table 2, GPER1, EGFR and CXCR1 mRNA levels were significantly higher in FTA compared to adenomatous nodule (P < 0.001 for all the three molecules), while there were not statistically significant differences in GPER1, EGFR and CXCR1 mRNA levels between adenomatous nodule and normal thyroid tissues (P = 0.123, P = 0.082 and P = 0.121, respectively). Obviously, FTC showed to have increased mRNA levels of GPER1, EGFR and CXCR1 compared to FTA. The differences in GPER1, EGFR and CXCR1 mRNA levels between FTC and FTA were statistically significant (P < 0.001 for all the three molecules). However, there were not statistically significant differences in GPER1, EGFR and CXCR1 mRNA levels between WI-FTC and MI-FTC (P = 0.179, P = 0.077 and P = 0.174, respectively), while WI-FTC showing a little higher mRNA expression levels of the three molecules than that of MI-FTC.
Table 2.
mRNA expression of GPER1, EGFR and CXCR1 in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens
| Groups | n | GPER1 | EGFR | CXCR1 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| ΔCT, mean ± SD | P value | ΔCT, mean ± SD | P value | ΔCT, mean ± SD | P value | ||
| Normal thyroid tissue | 10 | 3.91 ± 1.28 | - | 3.70 ± 0.70 | - | 3.03 ± 1.29 | - |
| Adenomatous nodule | 10 | 5.19 ± 2.14 | 0.123a | 4.45 ± 1.08 | 0.082a | 3.92 ± 1.16 | 0.121a |
| FTA | 10 | 18.31 ± 3.40 | < 0.001b | 16.36 ± 1.36 | < 0.001b | 14.48 ± 2.17 | < 0.001b |
| FTC | 15 | 35.22 ± 8.28 | < 0.001c | 33.18 ± 7.96 | < 0.001c | 29.94 ± 7.32 | < 0.001c |
| MI-FTC | 10 | 34.41 ± 9.25 | 0.179d | 32.33 ± 8.07 | 0.077d | 29.30 ± 6.17 | 0.174d |
| WI-FTC | 5 | 40.69 ± 4.34 | 40.43 ± 6.80 | 36.72 ± 14.25 | |||
Mean ± SD of GPER1, EGFR and CXCR1 mRNA expression levels in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens after normalized to GAPDH; Mann-Whitney U test;
adenomatous nodule vs. normal thyroid tissue;
FTA vs. adenomatous nodule;
FTC vs. FTA;
WI-FTC vs. MI-FTC.
Immunohistochemical expression of GPER1, EGFR and CXCR1 in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens
GPER1, EGFR and CXCR1 protein expression were examined by immunohistochemical staining and illustrated in Figure 1. The immuno reactivities of GPER1, EGFR and CXCR1 were detected in the cytoplasm and cell membrane. In adenomatous nodules, there were only a few follicular cells with weak staining for GPER1 (Figure 1A), EGFR (Figure 1B) and CXCR1 (Figure 1C). However, in FTA, there were a number of follicular cells with moderate staining for the three molecules (Figure 1D-F), and in FTC, there were a lot of follicular cells with strong staining for the three molecules (Figure 1G-I). As shown in Table 3, like normal thyroid tissues, the majority of adenomatous nodules have negative or 1 IHC score, no cases showed high expression (≥ 5) of the three molecules. In FTA, more than half of cases have 0~4 IHC score, only a few cases (far less than half of cases) have ≥ 5 IHC score, high expression was present in 39 (30.5%), 36 (28.1%) and 35 (27.3%) of 128 cases for GPER1, EGFR and CXCR1, respectively. However, in FTC, more than half of cases have ≥ 5 IHC score, high expression was present in 78 (73.6%), 77 (72.6%) and 75 (70.8%) of 106 cases for GPER1, EGFR and CXCR1, respectively. Clearly, the rates of GPER1, EGFR and CXCR1 high expression were significantly higher in FTC than in FTA. Statistical analysis for GPER1, EGFR and CXCR1 immunostaining for discriminating between FTC and FTA was shown in Table 4. The positive predictive values (PPV) were 66.7%, 68.1% and 68.2%, respectively.
Figure 1.
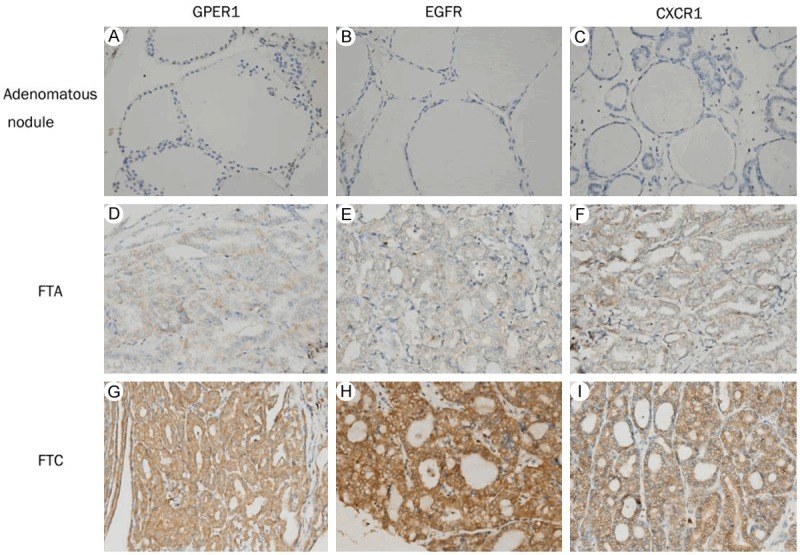
Immunohistochemical staining for GPER1, EGFR and CXCR1. Columns correspond to immunostaining for GPER1, EGFR and CXCR1, respectively. The first row exhibits weak staining of adenomatous nodules with the indicated antibodies (A-C); the second row shows moderate staining of FTA (D-F); and the third row displays strong staining of FTC (G-I). All the pictures are in high-power fields (×400).
Table 3.
Immunohistochemical analysis of GPER1, EGFR and CXCR1 expression in 106 FTC, 128 FTA, 115 adenomatous nodule and 90 normal thyroid tissue specimens according to the scoring system
| Score | GPER1 | EGFR | CXCR1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Normal thyroid tissue (n) | Adenom-atous nodule (n) | FTA (n) | FTC (n) | Normal thyroid tissue (n) | Adenom-atous nodule (n) | FTA (n) | FTC (n) | Normal thyroid tissue (n) | Adenom-atous nodule (n) | FTA (n) | FTC (n) | |
| 0 | ||||||||||||
| Negative | 48 | 66 | 24 | 3 | 50 | 63 | 22 | 2 | 53 | 61 | 25 | 5 |
| + | ||||||||||||
| 1 | 39 | 43 | 17 | 6 | 38 | 48 | 20 | 4 | 35 | 49 | 21 | 5 |
| 2 | 3 | 5 | 20 | 5 | 2 | 4 | 19 | 7 | 2 | 4 | 19 | 7 |
| 3 | 0 | 1 | 15 | 6 | 0 | 0 | 16 | 8 | 0 | 1 | 15 | 8 |
| 4 | 0 | 0 | 13 | 8 | 0 | 0 | 15 | 8 | 0 | 0 | 13 | 6 |
| ++ | ||||||||||||
| 6 | 0 | 0 | 12 | 25 | 0 | 0 | 14 | 25 | 0 | 0 | 12 | 27 |
| 8 | 0 | 0 | 12 | 23 | 0 | 0 | 10 | 28 | 0 | 0 | 10 | 23 |
| +++ | ||||||||||||
| 9 | 0 | 0 | 10 | 19 | 0 | 0 | 7 | 16 | 0 | 0 | 9 | 14 |
| 12 | 0 | 0 | 5 | 11 | 0 | 0 | 5 | 8 | 0 | 0 | 4 | 11 |
| Total | ||||||||||||
| Low | 90 | 115 | 89 | 28 | 90 | 115 | 92 | 29 | 90 | 115 | 93 | 31 |
| High | 39 | 78 | 36 | 77 | 35 | 75 | ||||||
The immunohistochemical scores in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens were determined as the multiplication of proportion score and intensity score.
Table 4.
Discrimination between FTA and FTC by high expression of GPER1, EGFR, and CXCR1 alone
| Variables | High expression rate | ||
|---|---|---|---|
|
| |||
| GPER1 | EGFR | CXCR1 | |
| Sensitivity | 73.6% | 72.6% | 70.8% |
| Specificity | 69.5% | 71.9% | 72.7% |
| Positive predictive value (PPV) | 66.7% | 68.1% | 68.2% |
| Negative predictive value (NPV) | 76.1% | 76.0% | 75.0% |
| Diagnostic accuracy | 71.4% | 72.2% | 71.8% |
Correlation of GPER1, EGFR and CXCR1 protein expression with clinicopathological features in FTA and FTC
The correlation of GPER1, EGFR and CXCR1 protein expression with clinic pathological data was assessed by Chi-square test and summarized in Table 5. No significant correlation was detected between protein expression of the three molecules and age, gender and tumor size in FTA and FTC cases. However, GPER1, EGFR and CXCR1 protein expression were associated with degree of thyroid follicular lesions. Obviously, the rates of GPER1, EGFR and CXCR1 high expression were significantly higher in FTA compared to adenomatous nodule as well normal thyroid tissue (P < 0.001 for all the three molecules). Likewise, the rates of high expression of the three molecules were significantly higher in FTC than in FTA (P < 0.001 for all the three molecules). However, similar to mRNA expression, there were not statistically significant differences in protein expression of the three molecules between WI-FTC and MI-FTC (P > 0.05), while WI-FTC showing to have a little higher rates of GPER1, EGFR and CXCR1 high expression than that of MI-FTC (82.9% vs. 69.0% for GPER1, 82.9% vs. 67.6% for EGFR and 80.0% vs. 66.2% for CXCR1, respectively).
Table 5.
Correlation of GPER1, EGFR and CXCR1 protein expression with clinicopathological parameters in 106 FTCs and 128 FTAs
| Characteristics | Case (n) | GPER1 | EGFR | CXCR1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Low | High | P-value | Low | High | P-value | Low | High | P-value | ||
| Normal thyroid tissue | 90 | 90 | 0 | 90 | 0 | 90 | 0 | |||
| Adenomatous nodule | 115 | 115 | 0 | - | 115 | 0 | - | 115 | 0 | - |
| FTA | 128 | 89 | 39 | < 0.001a | 92 | 36 | < 0.001a | 93 | 35 | < 0.001a |
| < 0.001b | < 0.001b | < 0.001b | ||||||||
| Age (years) | ||||||||||
| < 45 | 62 | 43 | 19 | 0.966 | 45 | 17 | 0.863 | 44 | 18 | 0.678 |
| ≥ 45 | 66 | 46 | 20 | 47 | 19 | 49 | 17 | |||
| Gender | ||||||||||
| Male | 33 | 21 | 12 | 0.393 | 22 | 11 | 0.440 | 22 | 11 | 0.370 |
| Female | 95 | 68 | 27 | 70 | 25 | 71 | 24 | |||
| Tumor size (cm) | ||||||||||
| T1 ≤ 2 | 42 | 33 | 9 | 0.177 | 34 | 8 | 0.179 | 34 | 8 | 0.175 |
| 2 < T2 ≤ 4 | 52 | 36 | 16 | 37 | 15 | 38 | 14 | |||
| > 4 | 34 | 20 | 14 | 21 | 13 | 21 | 13 | |||
| FTC | 106 | 28 | 78 | < 0.001c | 29 | 77 | < 0.001c | 31 | 75 | < 0.001c |
| Pathological type | ||||||||||
| MI-FTC | 71 | 22 | 49 | 0.128 | 23 | 48 | 0.098 | 24 | 47 | 0.142 |
| WI-FTC | 35 | 6 | 29 | 6 | 29 | 7 | 28 | |||
| Age (years) | ||||||||||
| < 45 | 51 | 16 | 35 | 0.265 | 16 | 35 | 0.372 | 17 | 34 | 0.373 |
| ≥ 45 | 55 | 12 | 43 | 13 | 42 | 14 | 41 | |||
| Gender | ||||||||||
| Male | 27 | 9 | 18 | 0.345 | 9 | 18 | 0.420 | 10 | 17 | 0.303 |
| Female | 79 | 19 | 60 | 20 | 59 | 21 | 58 | |||
| Tumor size (cm) | ||||||||||
| T1 ≤ 2 | 35 | 14 | 21 | 0.077 | 14 | 21 | 0.063 | 15 | 20 | 0.069 |
| 2 < T2 ≤ 4 | 42 | 9 | 33 | 11 | 31 | 11 | 31 | |||
| > 4 | 29 | 5 | 24 | 4 | 25 | 5 | 24 | |||
P-values derived using Chi-square test to compare the expression of GPER1, EGFR and CXCR1 between subgroups defined by each clinicopathological parameter.
Stands for significant difference between FTA and normal thyroid tissue;
Stands for significant difference between FTA and adenomatous nodule;
Stands for significant difference between FTC and FTA.
P < 0.05 was considered statistically significant.
Correlation of GPER1, EGFR and CXCR1 protein expression with one another in FTA and FTC
The correlation of GPER1, EGFR and CXCR1 protein expression with one another was assessed by Spearman rank test. As shown in Table 6, only 6/128, 4/128 and 5/128 FTAs showed high expression for both GPER1 and EGFR, both GPER1 and CXCR1, both EGFR and CXCR1, respectively. There was not statistically significant positive correlation between GPER1, EGFR and CXCR1 expression in FTA. In contrast, 61/106 FTCs showed high expression and 12/106 displayed low expression for both GPER1 and EGFR. The correlation between GPER1 and EGFR expression in FTC was statistically significant (rs = 0.208, P = 0.032). Similarly, there was a statistically significant correlation between expression of GPER1 and CXCR1 (rs = 0.226, P = 0.020) in FTC. For both GPER1 and CXCR1, 60/106 FTCs showed high expression. In addition, high expression for both EGFR and CXCR1 was present in 60/106 FTCs. A significantly positive correlation (rs = 0.257, P = 0.008) was also present between expression of EGFR and CXCR1 in FTC.
Table 6.
Correlation of GPER1, EGFR and CXCR1 protein expression with one another in 106 FTCs and 128 FTAs
| Proteins | GPER1 | CXCR1 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Low | High | rs | P value | Low | High | rs | P value | |
| FTAs | ||||||||
| EGFR | ||||||||
| Low | 59 | 33 | -0.188 | 0.034 | 62 | 30 | -0.189 | 0.033 |
| High | 30 | 6 | 31 | 5 | ||||
| CXCR1 | ||||||||
| Low | 58 | 35 | -0.254 | 0.004 | ||||
| High | 31 | 4 | ||||||
| FTCs | ||||||||
| EGFR | ||||||||
| Low | 12 | 17 | 0.208 | 0.032 | 14 | 15 | 0.257 | 0.008 |
| High | 16 | 61 | 17 | 60 | ||||
| CXCR1 | ||||||||
| Low | 13 | 18 | 0.226 | 0.020 | ||||
| High | 15 | 60 | ||||||
P values for Spearman rank test. GPER1, EGFR and CXCR1 were tested pair wise. P < 0.05 was considered statistically significant.
Correlation of GPER1, EGFR and CXCR1 concomitant high expression with the occurrence of FTC
Given that GPER1, EGFR and CXCR1 protein expression were correlated with one another in FTC, we further evaluated the correlation of GPER1, EGFR and CXCR1 concomitant high expression with the occurrence of FTC. As shown in Table 7, the occurrence rate of FTC is significantly higher in cases (91.0%) with concomitant high expression of GPER1/EGFR than in those cases (34.4%) with high expression of only one of the two molecules, or in those cases (16.9%) without high expression for either of the two molecules. Similar results were observed in cases with concomitant high expression of GPER1/CXCR1 and EGFR/CXCR1. There were statistically significant differences in the occurrence rate of FTC between cases with high expression of only one and any two of the three molecules (P < 0.001 for GPER1/EGFR, GPER1/CXCR1 and EGFR/CXCR1). In addition, statistical analysis showed that concomitant high expression of all the three molecules is significantly associated with the occurrence of FTC as compared with cases not showing such expression (P < 0.001). As demonstrated in Figure 2A-C is a representative of FTA showing low expression of GPER1, EGFR and CXCR1; Figure 2D-F is a representative of FTC showing high expression of all the three molecules. Statistical analysis for GPER1, EGFR and CXCR1 concomitant high expression for discriminating between FTC and FTA was shown in Table 8. The PPVs were 91.0% for GPER1/EGFR, 93.8% for GPER1/CXCR1, 92.3% for EGFR/CXCR1 and 98.2% for GPER1/EGFR/CXCR1, respectively.
Table 7.
Correlation of concomitant high expression of GPER1, EGFR and CXCR1 with the occurrence of FTC
| FTA n (%) | FTC n (%) | P value | |
|---|---|---|---|
| GPER1/EGFR | < 0.012a | ||
| Both GPER1/EGFR were low expression | 59 (83.1) | 12 (16.9) | |
| One of GPER1/EGFR was high expression | 63 (65.6) | 33 (34.4) | < 0.001b |
| Both GPER1/EGFR were high expression | 6 (9.0) | 61 (91.0) | |
| GPER1/CXCR1 | < 0.030a | ||
| Both GPER1/CXCR1 were low expression | 58 (81.7) | 13 (18.3) | |
| One of GPER1/CXCR1 was high expression | 66 (66.7) | 33 (33.3) | < 0.001b |
| Both GPER1/CXCR1 were high expression | 4 (6.2) | 60 (93.8) | |
| EGFR/CXCR1 | < 0.020a | ||
| Both EGFR/CXCR1 were low expression | 62 (81.6) | 14 (18.4) | |
| One of EGFR/CXCR1 was high expression | 61 (65.6) | 32 (34.4) | < 0.001b |
| Both EGFR/CXCR1 were high expression | 5 (7.7) | 60 (92.3) | |
| GPER1/EGFR/CXCR1 | |||
| Not all of GPER1/EGFR/CXCR1 was or were high expression | 127 (70.9) | 52 (29.1) | < 0.001c |
| All of GPER1/EGFR/CXCR1 were high expression | 1 (1.8) | 54 (98.2) |
Correlation of concomitant high expression of GPER1, EGFR and CXCR1 with the occurrence of FTC was measured by Chi-square test.
Stands for significant difference among the three groups;
Stands for significant difference between group (2) and group (3);
Stands for significant difference between groups with and without concomitant high expression of all three molecules.
P < 0.05 was considered statistically significant.
Figure 2.
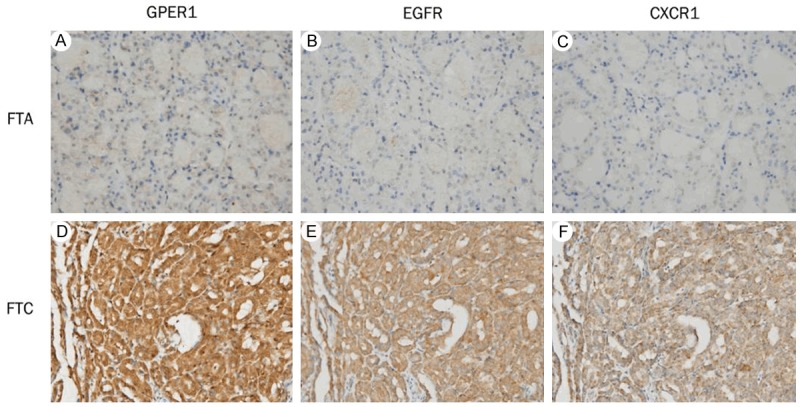
Association of concomitant high expression of GPER1, EGFR and CXCR1 with the occurrence of FTC. Columns correspond to immunostaining for GPER1, EGFR and CXCR1, respectively. The first row is the immunostaining of a representative of FTA showing low expression of all three molecules (A-C); the second row is the immunostaining of a representative of FTC showing high expression of all three molecules (D-F). All the pictures are in high-power fields (×400).
Table 8.
Discrimination between FTA and FTC by concomitant high expression of GPER1, EGFR and CXCR1
| Variables | Concomitant high expression | |||
|---|---|---|---|---|
|
| ||||
| GPER1/EGFR | GPER1/CXCR1 | EGFR/CXCR1 | GPER1/EGFR/CXCR1 | |
| Sensitivity | 83.6% | 82.2% | 81.1% | 93.1% |
| Specificity | 90.8% | 93.5% | 92.5% | 97.0% |
| Positive predictive value (PPV) | 91.0% | 93.8% | 92.3% | 98.2% |
| Negative predictive value (NPV) | 83.1% | 81.7% | 81.6% | 88.9% |
| Diagnostic accuracy | 87.0% | 87.4% | 86.5% | 94.5% |
Discussion
The differential diagnosis between FTC and FTA is difficult and it is often based on the presence of definitive capsular infiltration and/or vascular invasion [3]. Application of these histomorphologic criteria requires extensive sampling of the specimen, frequently with the need for additional deeper tissue sections. Such practice can be expensive and time-consuming, but often necessary [4]. Therefore, molecular markers to facilitate accurate discrimination between FTC and FTA are needed.
In the past decade, GPER1 has been identified as a new member of the estrogen receptor family which binds estrogen with high affinity and mediates response to estrogen through transactivation of EGFR [5,10,11,21]. The activation of EGFR leads to downstream signaling events such as production of CAMP, intracellular calcium mobilization, activation of ERK1/2 and PI3K, which is strongly associated with the proliferation, invasion, metastasis, and drug resistance of various cancer cell lines. CXCR1, one of two high-affinity receptors for IL-8, has been found to be associated with drug resistance, invasion, and metastasis in several types of solid tumor [17], especially, our previous study showed that the cross-talk between GPER1 and CXCR1 through transactivation of EGFR can promote the invasion and migration of nuclear estrogen receptor-negative breast cancer cells [16]. To date, studies have shown that GPER1 is over expressed in several estrogen-related tumors such as breast, endometrial and ovarian carcinoma, which, independent of ERα and ERβ, is associated with invasion, metastasis, drug resistance and poor prognosis of these tumors [6-8]. EGFR and CXCR1 have been observed to be up-regulated in human tumors [12-15,22]. Recently, our group examined the expression of GPER1, EGFR and CXCR1 in PTC, and found that high expression of GPER1, EGFR and CXCR1 was significantly correlated with lymph node metastasis in PTC. Concomitant high expression of the three molecules had stronger correlation with lymph node metastasis than did each alone, demonstrating that the evaluation of GPER1, EGFR and CXCR1 expression may be useful in predicting the risk of lymph node metastasis in PTC [18]. However, almost no studies examined GPER1 expression in follicular thyroid tumor, moreover, no study investigated simultaneously the expression of GPER1, EGFR and CXCR1 to evaluate potential usefulness of the three molecules in discriminating between FTC and FTA. In our present study, we first analyzed GPER1, EGFR and CXCR1 mRNA expression levels in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens using real-time RT-PCR. The results showed that GPER1, EGFR and CXCR1 mRNA levels were significantly higher in FTA than in adenomatous nodule as well normal thyroid tissues. Moreover, there were significantly higher mRNA levels of the three molecules in FTC than in FTA, while there were not statistically significant differences in GPER1, EGFR and CXCR1 mRNA levels between MI-FTCs and MI-FTCs. These results suggested that GPER1, EGFR and CXCR1 may play important roles in proliferation and invasion of thyroid follicular cells, and in the occurrence of capsular infiltration and vascular invasion of FTC.
Then, we examined GPER1, EGFR and CXCR1 protein expression in FTC, FTA, adenomatous nodule and normal thyroid tissue specimens using immunohistochemical staining and assessed the correlation of protein expression of the three molecules with clinic pathological indicators. The results showed that GPER1, EGFR and CXCR1 protein expression were not associated with gender, age and tumor size in FTC as well FTA, and no cases of adenomatous nodule and normal thyroid tissue showed to have high protein expression of GPER1, EGFR and CXCR1. However, high protein expression was present in 30.5%, 28.1% and 27.3% of FTA cases, and 73.6%, 72.6% and 70.8% of FTC cases for GPER1, EGFR and CXCR1, respectively. The rates of GPER1, EGFR and CXCR1 high expression were significantly increased in FTC when compared to FTA (P < 0.001). When we calculated PPV for GPER1, EGFR and CXCR1 immunostaining for discriminating between FTC and FTA, using surgical specimens in our series, the PPV was 66.7%, 68.1% and 68.2%, respectively. However, these values are likely to further decrease when GPER1, EGFR and CXCR1 staining is applied to preoperative diagnosis of FNAB specimens, because the incidence of FTA is much higher than that of FTC at that stage of thyroid nodule screening. Therefore, GPER1, EGFR and CXCR1 should not be alone suggested as a diagnostic marker for FTC in preoperative screening using FNAB specimens.
Subsequently, we assessed the correlation of GPER1, EGFR and CXCR1 protein expression with one another in FTA and FTC. As shown in Table 6, similar to our previous study in PTC [18], GPER1 expression is positively correlated with EGFR expression (rs = 0.208, P = 0.032) and CXCR1 expression (rs = 0.226, P = 0.020) in FTC. In addition, a significantly positive correlation (rs = 0.257, P = 0.008) was also present between expression of EGFR and CXCR1 in FTC. These positive correlations could be supported by the following data. Firstly, previous studies have shown that EGF and EGFR are highly expressed in FTC, which is associated with capsular infiltration and vascular invasion of FTC [23,24]. Subsequently, Vivacqua A at al [25] and Albanito L et al [26] respectively reported that EGF is able to activate the GPER1 promoter and accordingly up-regulate mRNA and protein level of GPER1 via transactivation of EGFR in cancer cells. Furthermore, it is reported that stimulation of GPER1 by estrogen transactivates EGFR which, albeit indirectly, is able to activate NF-κB via ERK and PI3K signaling [16,27]. Then, NF-κB can serve as a transcriptional activator to up-regulate CXCR1 expression [28,29]. Doubtlessly, it is necessary to further explore and elucidate the mechanisms underlying these correlations in FTC. In contrast, there were not statistically significant positive correlations between GPER1, EGFR and CXCR1 expression in FTA. It is suggested that the positive correlation between GPER1, EGFR and CXCR1 expression is associated with malignant transformation of follicular thyroid tumor.
Given that GPER1, EGFR and CXCR1 protein expression in FTC were positively correlated with one another, we farther evaluated the association of concomitant high expression of GPER1, EGFR and CXCR1 with the occurrence of FTC. The results showed that concomitant high expression of any two of the three molecules had stronger correlation with the occurrence of FTC than did each alone. Concomitant high expression of all the three molecules strongly correlates with the occurrence of FTC. When we performed statistical analysis for GPER1, EGFR and CXCR1 concomitant high expression for discriminating between FTC and FTA, the PPVs were 91.0% for GPER1/EGFR, 93.8% for GPER1/CXCR1, 92.3% for EGFR/CXCR1 and 98.2% for GPER1/EGFR/CXCR1, respectively. This result indicated that concomitant high expression of GPER1, EGFR and CXCR1 should be suggested as a valuable armamentarium in the preoperative differential diagnosis between FTC and FTA, such as FNAB.
In summary, our results demonstrated that GPER1, EGFR and CXCR1 may play important roles in proliferation and invasion of thyroid follicular cells. The rates of GPER1, EGFR and CXCR1 high expression were significantly higher in FTC than in FTA. However, GPER1, EGFR and CXCR1 should not be alone suggested as a diagnostic marker for discriminating between FTC and FTA in preoperative diagnosis. Concomitant high expression of any two or all of the three molecules had stronger correlation with the occurrence of FTC than did each alone. Consequently, concomitant high expression of GPER1, EGFR and CXCR1 should be suggested as a valuable armamentarium in the preoperative differential diagnosis between FTC and FTA. Our results provided a possible basis for discriminating between FTC and FTA. Due to limited cases, future studies in larger cohort of cases will be necessary to determine the utility of these molecules as molecular markers for differential diagnosis between FTC and FTA.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81272937), and by Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Personnel Ministry (2011).
Disclosure of conflict of interest
None.
References
- 1.Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6:1771–1779. doi: 10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzaferri EL. Papillary and follicular thyroid cancer: a selective approach to diagnosis and treatment. Annu Rev Med. 1981;32:73–91. doi: 10.1146/annurev.me.32.020181.000445. [DOI] [PubMed] [Google Scholar]
- 3.Cáp J, Ryska A, Rehorková P, Hovorková E, Kerekes Z, Pohnetalová D. Sensitivity and specificity of the fine needle aspiration biopsy of the thyroid: clinical point of view. Clin Endocrinol (Oxf) 1999;51:509–515. doi: 10.1046/j.1365-2265.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 4.Kahn N, Perzin KH. Follicular carcinoma of the thyroid: an evaluation of the histologic criteria used for diagnosis. Pathol Annu. 1983;18:221–253. [PubMed] [Google Scholar]
- 5.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;30:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 6.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, Delellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 7.Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196:386, e1–9. doi: 10.1016/j.ajog.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Smith HO, Arias PH, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, Prossnitz ER. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol. 2009;114:465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 10.Lappano R, Marco PD, De Francesco EM, Chimento A, Pezzi V, Maggiolini M. Cross-talk between GPER and growth factor signaling. J Steroid Biochem Mol Biol. 2013;137:50–56. doi: 10.1016/j.jsbmb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 12.Viale G, Rotmensz N, Maisonneuve P, Bottiqlieri L, Montaqna E, Luini A, Veronesi P, Intra M, Torrisi R, Cardillo A, Campaqnoli E, Goldhirsch A, Colleoni M. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 13.Ozawa S, Ueda M, Ando N, Shimizu N, Abe O. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer. 1989;63:2169–2173. doi: 10.1002/1097-0142(19890601)63:11<2169::aid-cncr2820631117>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Fisher KE, Jani JC, Fisher SB, Foulks C, Hill CE, Weber CJ, Cohen C, Sharma J. Epidermal growth factor receptor overexpression is a marker for adverse pathologic features in papillary thyroid carcinoma. J Surg Res. 2013;185:217–224. doi: 10.1016/j.jss.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 16.Jiang QF, Wu TT, Yang JY, Dong CR, Wang N, Liu XH, Liu ZM. 17β-estradiol promotes the invasion and migration of nuclear estrogen receptor-negative breast cancer cells through cross-talk between GPER1 and CXCR1. J Steroid Biochem Mol Biol. 2013;138:314–324. doi: 10.1016/j.jsbmb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 18.Tang C, Yang L, Wang N, Li L, Xu M, Chen GG, Liu ZM. High expression of GPER1, EGFR and CXCR1 is associated with lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2014;7:3213–3223. [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 21.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang W, Ye L, Shen L, Huang F, Wei Q, Fei X, Chen X, Guan H, Wang W, Li X, Ning G. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35:1780–1787. doi: 10.1093/carcin/bgu060. [DOI] [PubMed] [Google Scholar]
- 23.Gorgoulis V, Aninos D, Priftis C, Evaqelopoulou C, Karameris A, Kanavaros P, Spandidos DA. Expression of epidermal growth factor, transforming growth factor-alpha and epidermal growth factor receptor in thyroid tumors. In Vivo. 1992;6:291–296. [PubMed] [Google Scholar]
- 24.Westermark K, Lundqvist M, Wallin G, Dahlman T, Hackr GW, Heldin NE, Grimellius L. EGF-receptors in human normal and pathological thyroid tissue. Histopathology. 1996;28:221–227. doi: 10.1046/j.1365-2559.1996.d01-427.x. [DOI] [PubMed] [Google Scholar]
- 25.Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, Fuqua SA, Ando S, Maggiolini M. G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells. Mol Endocrinol. 2009;23:1815–1826. doi: 10.1210/me.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albanito L, Sisci D, Aquila S, Brunelli E, Vivacqua A, Madeo A, Lappano R, Pandey DP, Picard D, Mauro L, Ando S, Maggiolini M. Epidermal growth factor induces G protein-coupled receptor 30 expression in estrogen receptor-negative breast cancer cells. Endocrinology. 2008;149:3799–3808. doi: 10.1210/en.2008-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Raychaudhuri B, Vogelbaum MA. IL-8 is a mediator of NF-κB induced invasion by gliomas. J Neurooncol. 2011;101:227–235. doi: 10.1007/s11060-010-0261-2. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG, Waugh DJ. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]


