Abstract
The purpose of this study was to investigate the expression of Yes-associated protein (YAP) in different metastatic sites in metastatic breast cancer and to determine the clinical implications of these patterns. Immunohistochemical staining was used to investigate the expression of YAP and phospho-YAP in tissue microarrays from 122 cases of metastatic breast cancer (bone metastasis = 29, brain metastasis = 38, liver metastasis = 12, and lung metastasis = 43). The expression levels of YAP and phospho-YAP differed according to the metastatic site in metastatic breast cancer. Specifically, nuclear expression of phospho-YAP was high in brain metastasis but low in lung metastasis (P = 0.010). The effects of YAP and phospho-YAP expression on clinical outcomes were investigated by univariate analysis. This analysis showed that nuclear YAP positivity (P = 0.008) and nuclear phospho-YAP positivity (P = 0.003) were both associated with shorter overall survival. In conclusion, the level of YAP expression varies according to the metastatic site in metastatic breast cancer. Moreover, high YAP expression was correlated with poor prognosis.
Keywords: Breast cancer, metastasis, YAP
Introduction
Yes-associated protein (YAP) has been reported to play a role in controlling organ size by regulating cell proliferation and survival [1]. YAP is a transcriptional coactivator that regulates cellular responses in the nucleus by interacting with transcription factors. Kinase-mediated phosphorylation of YAP results in its sequestration from the nucleus to the cytoplasm, thereby downregulating the transcription of its target genes [2]. Recent studies have shown that YAP is associated with tumorigenesis; in breast cancer, YAP has been proposed to act as both a tumor suppressor and an oncogene. YAP has been proposed to act as a tumor suppressor because human breast cancer tissue exhibits decreased YAP expression compared with normal breast tissue [3]. Furthermore, knockdown of YAP in a breast cancer cell line suppressed anoikis, thereby resulting in increased cell migration and invasiveness. Tumor growth was also enhanced in a YAP-knockout mouse model [4]. In contrast, other studies suggested that YAP has an oncogenic role, since overexpression of YAP in a breast cancer cell line increased proliferation [5]. Moreover, YAP overexpression increased tumor formation and tumor growth in a study of mouse tumor xenografts [6].
Breast cancer is a cancer with high rates of morbidity and mortality, since it is prone to distant metastasis. The major metastatic sites of breast cancer are the lungs, brain, liver, and bone [7,8]; most studies have focused on brain and bone metastasis [9-14]. The most common mechanism of tumor metastasis is reciprocal interaction between tumor cells and the host tissue. This interaction consists of a cycle of adhesion, proteolysis, invasion, and angiogenesis [8,15]. However, since tumors show different metastatic patterns, the seed and soil hypothesis has been proposed. This hypothesis states that a specific tumor (seed) can survive only in a specific visceral organ (soil) [16]. Metastatic breast cancers are known to exhibit characteristic findings according to the metastatic site. Brain metastasis has been shown to be associated with young age, ER negativity, prior lung metastasis, HER-2 overexpression, EGFR overexpression, and basal subtype [11-13], whereas bone metastasis has been shown to be associated with lower histologic grade, ER positivity, ER positivity/PR negativity, the strand growth pattern, and the presence of fibrotic foci in the invasive ductal carcinoma [10,17,18]. As a result, specific metastatic sites are associated with distinct characteristics in metastatic breast cancer. Therefore, the purpose of this study was to investigate the expression of YAP at different metastatic sites in metastatic breast cancer and to determine the clinical implications of these patterns.
Materials and methods
Patient selection
Cases with invasive primary breast cancer with metastasis to distant organs (liver, lungs, brain, and bone) were selected from the data files of the Department of Pathology of Severance Hospital, South Korea. Only patients with diagnosis of invasive ductal carcinoma were included. This study was approved by the Institutional Review Board of our institution. A total of 122 cases were included; 32 of these had samples of both the primary breast cancer tissue and the paired distant metastatic cancer tissue. All slides were reviewed again before analysis and their pathologic diagnoses were approved by 2 different pathologists (JSK and WHJ). The histological grade of each sample was assessed using the Nottingham grading system [19].
Tissue microarray
A representative area was selected on each H&E-stained tumor slide and a corresponding spot was marked on the surface of the paraffin block. Using a biopsy needle, the selected area was punched out and a 3-mm tissue core was placed into a 6 × 5 recipient block. Tissue was extracted from each invasive tumor. More than 2 tissue cores were extracted from each tumor to minimize extraction bias. Each tissue core was assigned to a unique tissue microarray location number that was linked to a database containing other clinicopathologic data.
Immunohistochemistry (IHC)
The antibodies used for IHC in this study are listed in Table 1. IHC staining was conducted using formalin-fixed, paraffin-embedded 3-μm thick tissue sections from all tissue microarrays. Slides were deparaffinized and rehydrated using xylene and alcohol. A Ventana Discovery XT automated system (Ventana Medical System, Tucson, AZ, USA) was used for all staining. Appropriate positive and negative controls were included in each replicate. Antigen retrieval was performed using CC1 buffer (Cell Conditioning 1; citrate buffer pH 6.0, Ventana Medical System).
Table 1.
Source, clone, and dilution of each antibody used in this study
| Antibody | Company | Clone | Dilution |
|---|---|---|---|
| YAP-related | |||
| YAP | Santa Cruz Biotechnology, Inc., California, USA | 9A1 | 1:100 |
| Phospho-YAP (Ser127) | Abcam, Cambridge, UK | EP1675Y | 1:100 |
| Molecular subtype-related | |||
| ER | Thermo Scientific, San Diego, CA, USA | SP1 | 1:100 |
| PR | DAKO, Glostrup, Denmark | PgR | 1:50 |
| HER-2 | DAKO, Glostrup, Denmark | Polyclonal | 1:1500 |
| Ki-67 | Abcam, Cambridge, UK | MIB | 1:1000 |
Interpretation of IHC results
A cut-off value of 1% or more positively stained nuclei was used to define ER and AR positivity [20]. HER-2 staining was used to classify each sample into one of the following categories, set by the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP): 0 = no immuntaining; 1+ = weak incomplete membranous staining, less than 10% of all tumor cells; 2+ = complete membranous staining, either uniform or weak in at least 10% of all tumor cells; and 3+ = uniform intense membranous staining in at least 30% of all tumor cells [21]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed. Cases with 0 to 1+ staining were regarded as HER-2-negative.
The final IHC staining results are expressed as the product of the proportion of stained cells and the immunostaining intensity. Stained cell proportions were scored as follows: 0, negative; 1, positive (less than 30% of all cells stained); and 2, positive (greater than or equal to 30% of all cells stained). The immunostaining intensity was scored as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. A product between 0 and 1 was regarded as negative, between 2 and 4 as low positive, and between 5 and 6 as high positive [22]. The Ki-67 labeling index (LI) was defined as the percentage of cells with nuclear expression out of all of the cancer cells.
Tumor phenotype classification
Breast cancer phenotypes were classified according to the ER, PR, HER-2 and Ki-67 IHC, and HER-2 FISH results as follows [23]: luminal A type: ER and/or PR positive, HER-2 negative, and Ki-67 LI < 14%; luminal B type: HER-2 negative-ER and/or PR positive, HER-2 negative, and Ki-67 LI ≥ 14%; HER-2 positive-ER and/or PR positive and HER-2 overexpressed and/or amplified; HER-2 type: ER and PR negative and HER-2 overexpressed and/or amplified; TNBC type: ER, PR, and HER-2 negative.
Statistical analysis
Data were analyzed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). Correlations of immunostaining results between primary breast cancer and metastatic breast cancer were analyzed by McNemar’s test. Student’s t-test and Fisher’s exact test were used to evaluate the significance of differences in continuous and categorical variables, respectively. Corrected p-values and the Bonferroni method were used for multiple comparisons. Statistical significance was assumed when P < 0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate time to tumor metastasis and time to survival.
Results
Basal characteristics of patients with metastatic breast cancer
Among the 122 patients in this study, 43 (35.2%) had lung metastases, 38 (31.1%) had brain metastases, 29 (23.8%) had bone metastases, and 12 (9.8%) had liver metastases. The proportions of ER positivity and PR positivity were higher in bone metastasis and liver metastasis (P < 0.001). The proportions of luminal A type were higher in bone and liver metastasis, whereas the proportions of TNBC were higher in brain and lung metastasis (P < 0.001) (Table 2).
Table 2.
Basal characteristics of patients with metastatic breast cancer
| Parameter | Total n = 122 (%) | Bone metastasis n = 29 (%) | Brain metastasis n = 38 (%) | Liver metastasis n = 12 (%) | Lung metastasis n = 43 (%) | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 0.045 | |||||
| ≤50 | 61 (50.0) | 18 (62.1) | 17 (44.7) | 2 (16.7) | 24 (55.8) | |
| >50 | 61 (50.0) | 11 (37.9) | 21 (55.3) | 10 (83.3) | 19 (44.2) | |
| ER | <0.001 | |||||
| Negative | 59 (48.4) | 7 (24.1) | 25 (65.8) | 2 (16.7) | 25 (58.1) | |
| Positive | 63 (51.6) | 22 (75.9) | 13 (34.2) | 10 (83.3) | 18 (41.9) | |
| PR | <0.001 | |||||
| Negative | 83 (68.0) | 13 (44.8) | 37 (97.4) | 3 (25.0) | 30 (69.8) | |
| Positive | 39 (32.0) | 16 (55.2) | 1 (2.6) | 9 (75.0) | 13 (30.2) | |
| HER-2 | 0.057 | |||||
| Negative | 81 (66.4) | 22 (75.9) | 19 (50.0) | 10 (83.3) | 30 (69.8) | |
| Positive | 41 (33.6) | 7 (24.1) | 19 (50.0) | 2 (16.7) | 13 (30.2) | |
| Molecular subtype | <0.001 | |||||
| Luminal A | 44 (36.1) | 19 (65.5) | 4 (10.5) | 8 (66.7) | 13 (30.2) | |
| Luminal B | 20 (16.4) | 4 (13.8) | 9 (23.7) | 2 (16.7) | 5 (11.6) | |
| HER-2 | 27 (22.1) | 4 (13.8) | 12 (31.6) | 1 (8.3) | 10 (23.3) | |
| TNBC | 31 (25.4) | 2 (6.9) | 13 (34.2) | 1 (8.3) | 15 (34.9) | |
| Patient death | 41 (33.6) | 17 (58.6) | 11 (28.9) | 5 (41.7) | 8 (18.6) | 0.004 |
ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor-2; TNBC, triple negative breast cancer.
Expression of YAP and phospho-YAP according to the metastatic site of metastatic breast cancer
We next analyzed the patterns of YAP and phospho-YAP expression observed at different metastatic sites. YAP and phospho-YAP both exhibited nuclear and/or cytoplasmic expression in tumor cells, whereas they were exclusively cytoplasmic in stromal tissue (Figure 1). Moreover, the pattern of nuclear phospho-YAP expression varied according to the metastatic site. Specifically, nuclear phospho-YAP exhibited higher expression in brain metastasis and lower expression in lung metastasis (P = 0.010). The level of YAP expression also showed different tendencies according to the subcellular localization of YAP; for instance, cytoplasmic YAP expression was higher in lung metastasis and lower in bone metastasis (P = 0.075). Nuclear YAP expression was higher in bone metastasis, whereas nuclear YAP was not observed in lung or liver metastasis (P = 0.067). The level of stromal YAP expression also varied according to the metastatic site; stromal YAP expression was higher in bone metastasis, whereas stromal YAP was not expressed in brain or liver metastasis (P = 0.017) (Figure 1 and Table 3).
Figure 1.
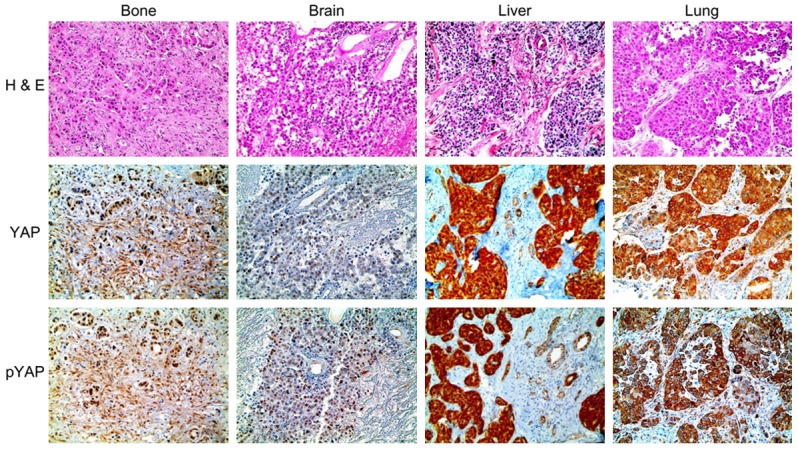
Expression of YAP and phospho-YAP in metastatic breast cancer according to the metastatic site. The expression of nuclear phospho-YAP was higher in brain metastasis and lower in lung metastasis. Cytoplasmic YAP expression was higher in lung metastasis and lower in bone metastasis. Nuclear YAP expression was highest in bone metastasis. Stromal YAP and phospho-YAP expression were highest in bone metastasis.
Table 3.
Expression of YAP and phospho-YAP according to the metastatic site in metastatic breast cancer
| Parameter | Total n = 122 (%) | Bone metastasis n = 29 (%) | Brain metastasis n = 38 (%) | Liver metastasis n = 12 (%) | Lung metastasis n = 43 (%) | P-value |
|---|---|---|---|---|---|---|
| YAP (cytoplasmic) | 0.075 | |||||
| Negative | 78 (63.9) | 22 (75.9) | 27 (71.1) | 8 (66.7) | 21 (48.8) | |
| Positive | 44 (36.1) | 7 (24.1) | 11 (28.9) | 4 (33.3) | 22 (51.2) | |
| YAP (nuclear) | 0.067 | |||||
| Negative | 115 (94.3) | 25 (86.2) | 35 (92.1) | 12 (100.0) | 43 (100.0) | |
| Positive | 7 (5.7) | 4 (13.8) | 3 (7.9) | 0 (0.0) | 0 (0.0) | |
| Stromal YAP | 0.017 | |||||
| Negative | 115 (94.3) | 24 (82.8) | 38 (100.0) | 12 (100.0) | 41 (95.3) | |
| Positive | 7 (5.7) | 5 (17.2) | 0 (0.0) | 0 (0.0) | 2 (4.7) | |
| pYAP (cytoplasmic) | 0.280 | |||||
| Negative | 97 (79.5) | 26 (89.7) | 31 (81.6) | 8 (66.7) | 32 (74.4) | |
| Positive | 25 (20.5) | 3 (10.3) | 7 (18.4) | 4 (33.3) | 11 (25.6) | |
| pYAP (nuclear) | 0.010 | |||||
| Negative | 108 (88.5) | 25 (86.2) | 29 (76.3) | 11 (91.7) | 43 (88.5) | |
| Positive | 14 (11.5) | 4 (13.8) | 9 (23.7) | 1 (8.3) | 0 (0.0) | |
| Stromal pYAP | 0.089 | |||||
| Negative | 114 (93.4) | 25 (86.2) | 38 (100.0) | 12 (100.0) | 39 (90.7) | |
| Positive | 8 (6.6) | 4 (13.8) | 0 (0.0) | 0 (0.0) | 4 (9.3) |
Correlations of YAP and phospho-YAP expression levels with primary and metastatic breast cancer according to metastatic site
We analyzed the expression of YAP and phospho-YAP in primary and metastatic cancers in 49 paired cases with both primary and distant metastatic cancer tissue. No significant differences were observed regarding the expression of YAP or phospho-YAP (Table 4).
Table 4.
Correlation analysis of YAP and phospho-YAP expression according to tumor cell compartment with primary and metastatic breast cancer according to metastatic site
| Parameter | Total | Bone metastasis | Brain metastasis | Liver metastasis | Lung metastasis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N = 32 (%) | P-value | N = 7 (%) | P-value | n= 7 (%) | P-value | N = 2 (%) | P-value | N = 16 (%) | P-value | |
| YAP (cytoplasmic) | 1.000 | 0.500 | 1.000 | n/a | 1.000 | |||||
| (+) → (+) | 4 (12.5) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 3 (18.8) | |||||
| (+) → (-) | 5 (15.6) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 3 (18.8) | |||||
| (-) → (+) | 6 (18.8) | 2 (28.6) | 1 (14.3) | 0 (0.0) | 3 (18.8) | |||||
| (-) → (-) | 17 (53.1) | 5 (71.4) | 3 (42.9) | 2 (100.0) | 7 (43.8) | |||||
| YAP (nuclear) | 1.000 | n/a | 1.000 | n/a | n/a | |||||
| (+) → (+) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (+) → (-) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (-) → (+) | 1 (3.1) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | |||||
| (-) → (-) | 31 (96.9) | 7 (100.0) | 6 (85.7) | 2 (100.0) | 16 (100.0) | |||||
| pYAP (cytoplasmic) | 1.000 | n/a | 1.000 | n/a | 1.000 | |||||
| (+) → (+) | 2 (6.2) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (6.2) | |||||
| (+) → (-) | 3 (9.4) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 2 (12.5) | |||||
| (-) → (+) | 2 (6.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (12.5) | |||||
| (-) → (-) | 25 (78.1) | 7 (100.0) | 5 (71.4) | 2 (100.0) | 11 (68.8) | |||||
| pYAP (nuclear) | 0.500 | n/a | 0.500 | n/a | n/a | |||||
| (+) → (+) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (+) → (-) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (-) → (+) | 2 (6.2) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 0 (0.0) | |||||
| (-) → (-) | 30 (93.8) | 7 (100.0) | 5 (71.4) | 2 (100.0) | 16 (100.0) | |||||
Impact of YAP and phospho-YAP expression on patient prognosis
The impacts of YAP expression and phospho-YAP expression on clinical outcomes were investigated by univariate analysis. This analysis revealed that nuclear YAP positivity (P = 0.008) and nuclear phospho-YAP positivity (P = 0.003) were significantly associated with shorter overall survival (Figure 2 and Table 5).
Figure 2.
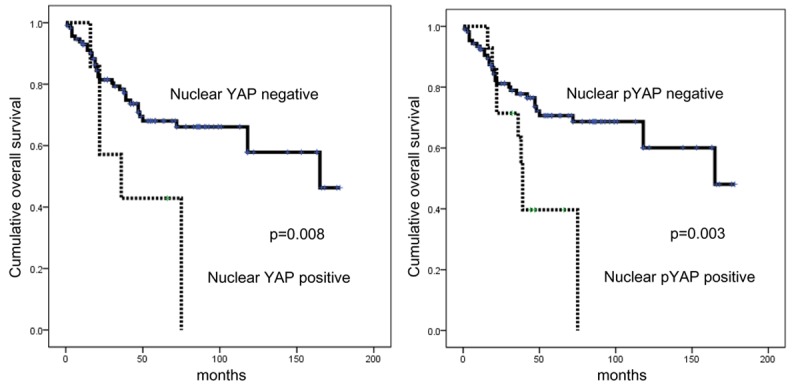
Impacts of YAP and phospho-YAP expression on patient prognosis in metastatic breast cancer.
Table 5.
Univariate analysis of the impacts of YAP and phospho-YAP expression in metastatic breast cancer on overall survival by the log-rank test
| Parameter | Total n = 122 (%) | Bone metastasis n = 29 (%) | Brain metastasis n = 38 (%) | Liver metastasis n = 12 (%) | Lung metastasis n = 43 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean survival, months (95% CI) | P-value | Mean survival, months (95% CI) | P-value | Mean survival, months (95% CI) | P-value | Mean survival, months (95% CI) | P-value | Mean survival, months (95% CI) | P-value | |
| YAP (cytoplasmic) | 0.624 | 0.432 | 0.873 | 0.501 | 0.415 | |||||
| Negative | 110 (92-128) | 73 (47-98) | 107 (81-133) | 85 (59-110) | 152 (125-178) | |||||
| Positive | 120 (97-142) | 86 (46-127) | 74 (49-99) | 57 (22-91) | 125 (94-157) | |||||
| YAP (nuclear) | 0.008 | 0.205 | 0.198 | n/a | n/a | |||||
| Negative | 120 (105-135) | 88 (58-117) | 112 (89-134) | n/a | n/a | |||||
| Positive | 45 (24-66) | 50 (21-79) | 36 (13-60) | n/a | n/a | |||||
| Stromal YAP | 0.965 | 0.892 | n/a | n/a | n/a | |||||
| Negative | 114 (99-130) | 79 (52-106) | n/a | n/a | n/a | |||||
| Positive | 55 (35-74) | 48 (22-73) | n/a | n/a | n/a | |||||
| pYAP (cytoplasmic) | 0.507 | 0.951 | 0.777 | 0.501 | 0.360 | |||||
| Negative | 111 (95-128) | 79 (53-105) | 108 (84-132) | 85 (59-110) | 135 (108-162) | |||||
| Positive | 111 (89-134) | 26 (7-45) | 71 (39-102) | 57 (22-91) | 132 (110-154) | |||||
| pYAP (nuclear) | 0.003 | 0.205 | 0.028 | n/a | n/a | |||||
| Negative | 123 (108-139) | 88 (58-117) | 126 (105-147) | n/a | n/a | |||||
| Positive | 47 (34-60) | 50 (21-79) | 40 (28-51) | n/a | n/a | |||||
| Stromal pYAP | 0.267 | 0.645 | n/a | n/a | n/a | |||||
| Negative | 112 (97-128) | 77 (51-103) | n/a | n/a | n/a | |||||
| Positive | 76 (57-95) | 54 (25-84) | n/a | n/a | n/a | |||||
Discussion
The purpose of this study was to investigate the expression of YAP in different metastatic sites in metastatic breast cancer. Previous studies reported that YAP is expressed in 75% [6] and 45% [3] of all breast cancers. The subcellular localization of YAP also differs; nuclear YAP expression was observed in 6.2-26.5% and cytoplasmic YAP expression was observed in 4.7-15% of all patients with breast cancer [3,25]. Kinase-mediated phosphorylation of YAP results in its sequestration from the nucleus to cytoplasm, meaning that it accumulates in the nucleus (where we observed YAP). Therefore, in theory, IHC staining of YAP should reveal nuclear expression and phospho-YAP cytoplasmic expression. However, in this study, IHC staining of YAP demonstrated cytoplasmic expression and phospho-YAP nuclear expression. Our results are consistent with those of a previous study that reported nuclear and cytoplasmic expression of YAP in breast cancer [24].
We found that YAP expression varied according to the metastatic site. The expression levels of nuclear YAP and phospho-YAP were higher in bone and brain metastasis, whereas the expression levels of cytoplasmic YAP and phospho-YAP were higher in liver and lung metastasis. These metastatic site-specific differences in YAP expression have several potential explanations. First, these differences may reflect different cancer cell characteristics. In breast cancer, YAP expression has been reported to be related with ER, PR, and HER-2 positivity [3,25], all of which are known to be relevant to tumor biology. Metastatic breast cancer is known to exhibit distinct tumor biology according to the metastatic site; for instance, brain metastasis exhibits ER negativity, HER-2 overexpression, EGFR overexpression, and the basal subtype [11-13]. In contrast, bone metastasis exhibits lower histologic grade, ER positivity, and PR negativity [10,17,18]. As a result, these distinct cancer cell characteristics of the various metastatic sites may influence YAP expression. Another possible mechanism is microenvironment differences at the metastatic site. For instance, YAP has been reported to be involved in the cancer - stroma interaction. The actin cytoskeleton might reduce the phosphorylation of YAP, which is a key molecule in the Hippo signaling pathway, thereby stimulating nuclear translocation of activated YAP. This translocation could in turn drive the expression of downstream CCN growth factors, such as CCN1/CYR61 and CCN2/CTGF [26,27]. These factors could create different microenvironments at each metastatic site, thus resulting in different levels of YAP expression.
Cancer-associated fibroblasts (CAFs) are an essential factor in the stromal cellular component of breast cancer. Although CAFs consist of several different groups of cells, α-smooth muscle actin-expressing myofibroblasts have been shown to be the most important component [28]. CAFs may play a role in YAP expression and regulation. In primary breast cancer, YAP has been reported to be expressed both in tumors and in stroma [25]; we observed YAP stromal expression in 5-6% of all samples. In addition, different CAF phenotypes have been reported to be associated with different histological and molecular stroma subtypes [29]. Different CAF phenotypes might be associated with different metastatic sites, a finding that would support a role for YAP expression in metastatic breast cancer.
In this study, nuclear YAP positivity and nuclear phospho-YAP positivity were both associated with shorter overall survival. Our results are consistent with previous studies reporting that YAP expression is linked with poor prognosis in ovarian cancer [30], urinary bladder cancer [31], colorectal cancer [32], esophageal cancer [33], stomach cancer [34], and lung cancer [35]. Our results indicate that YAP might be a potential treatment target in metastatic breast cancer. The efficacy of targeting YAP in various carcinomas has been demonstrated in one study in which targeting YAP inhibited cancer proliferation [36]. Further studies are required to substantiate this hypothesis.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A1A1A05001209).
Disclosure of conflict of interest
None.
References
- 1.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 3.Tufail R, Jorda M, Zhao W, Reis I, Nawaz Z. Loss of Yes-associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2012;131:743–750. doi: 10.1007/s10549-011-1435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 5.Zhi X, Zhao D, Zhou Z, Liu R, Chen C. YAP promotes breast cell proliferation and survival partially through stabilizing the KLF5 transcription factor. Am J Pathol. 2012;180:2452–2461. doi: 10.1016/j.ajpath.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer. 2012;48:1227–1234. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Abali H, Celik I. High incidence of central nervous system involvement in patients with breast cancer treated with epirubicin and docetaxel. Am J Clin Oncol. 2002;25:632–633. doi: 10.1097/00000421-200212000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Colleoni M, O’Neill A, Goldhirsch A, Gelber RD, Bonetti M, Thurlimann B, Price KN, Castiglione-Gertsch M, Coates AS, Lindtner J, Collins J, Senn HJ, Cavalli F, Forbes J, Gudgeon A, Simoncini E, Cortes-Funes H, Veronesi A, Fey M, Rudenstam CM. Identifying breast cancer patients at high risk for bone metastases. J. Clin. Oncol. 2000;18:3925–3935. doi: 10.1200/JCO.2000.18.23.3925. [DOI] [PubMed] [Google Scholar]
- 11.Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, Gutteridge E, Robertson JF, Hornbuckle J, Cheung KL. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol (R Coll Radiol) 2004;16:345–349. doi: 10.1016/j.clon.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20:864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 13.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 14.Lorincz T, Toth J, Badalian G, Timar J, Szendroi M. HER-2/neu genotype of breast cancer may change in bone metastasis. Pathol Oncol Res. 2006;12:149–152. doi: 10.1007/BF02893361. [DOI] [PubMed] [Google Scholar]
- 15.Nicolson GL. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988;7:143–188. doi: 10.1007/BF00046483. [DOI] [PubMed] [Google Scholar]
- 16.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–572. [PubMed] [Google Scholar]
- 17.Hasebe T, Imoto S, Yokose T, Ishii G, Iwasaki M, Wada N. Histopathologic factors significantly associated with initial organ-specific metastasis by invasive ductal carcinoma of the breast: a prospective study. Hum Pathol. 2008;39:681–693. doi: 10.1016/j.humpath.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Wei B, Wang J, Bourne P, Yang Q, Hicks D, Bu H, Tang P. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol. 2008;39:1809–1815. doi: 10.1016/j.humpath.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 22.Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010;41:107–112. doi: 10.1016/j.humpath.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlug EJ, van de Ven RA, Vermeulen JF, Bult P, van Diest PJ, Derksen PW. Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer. Cell Oncol (Dordr) 2013;36:375–384. doi: 10.1007/s13402-013-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SK, Jung WH, Koo JS. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol. 2014;7:3224–3234. [PMC free article] [PubMed] [Google Scholar]
- 26.Quan T, Xu Y, Qin Z, Robichaud P, Betcher S, Calderone K, He T, Johnson TM, Voorhees JJ, Fisher GJ. Elevated YAP and Its Downstream Targets CCN1 and CCN2 in Basal Cell Carcinoma: Impact on Keratinocyte Proliferation and Stromal Cell Activation. Am J Pathol. 2014;184:937–43. doi: 10.1016/j.ajpath.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy P, Deguchi M, Cheng Y, Hsueh AJ. Actin cytoskeleton regulates Hippo signaling. PLoS One. 2013;8:e73763. doi: 10.1371/journal.pone.0073763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 29.Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015;149:727–741. doi: 10.1007/s10549-015-3291-9. [DOI] [PubMed] [Google Scholar]
- 30.Jeong W, Kim SB, Sohn BH, Park YY, Park ES, Kim SC, Kim SS, Johnson RL, Birrer M, Bowtell DS, Mills GB, Sood A, Lee JS. Activation of YAP1 Is Associated with Poor Prognosis and Response to Taxanes in Ovarian Cancer. Anticancer Res. 2014;34:811–817. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ, Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, Zeng YX, Zhou FJ, Xie D. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013;13:349. doi: 10.1186/1471-2407-13-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, Wen W, Zhu Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Yeo MK, Kim SH, Kim JM, Huang SM, Kim MR, Song KS, Kim KH. Correlation of expression of phosphorylated and non-phosphorylated Yes-associated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a Korean population. Anticancer Res. 2012;32:3835–3840. [PubMed] [Google Scholar]
- 34.Song M, Cheong JH, Kim H, Noh SH. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827–3834. [PubMed] [Google Scholar]
- 35.Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Teng L. YAP/TAZ for cancer therapy: opportunities and challenges (review) Int J Oncol. 2015;46:1444–1452. doi: 10.3892/ijo.2015.2877. [DOI] [PubMed] [Google Scholar]


