Abstract
Objective: The process of epithelial-mesenchymal transition (EMT) clearly contributes to cancer metastasis. The aim of this study was to investigate the expression of the EMT-related transcription repressor ZEB1 and the expression of EMT-associated markers (E-cadherin, β-catenin and N-cadherin) in cervical squamous cell carcinoma. In addition, the role of ZEB1 and these EMT-associated markers in the progression and metastasis of cervical squamous cell carcinoma was explored. Methods: The expression of ZEB1, E-cadherin, β-catenin and N-cadherin was evaluated in 81 specimens of cervical squamous cell carcinoma by immunohistochemistry; the clinicopathological significance of these markers was then analyzed. Results: 1) Of the 81 samples, 37 cases (45.7%) were positive for ZEB1, and nuclear expression of ZEB1 in tumor cells was positively associated with the differentiation status of the tumor tissue (P < 0.05), vascular invasion (P < 0.05) and lymph node metastasis (P < 0.05). 2) The loss of E-cadherin and β-catenin expression in tumor cells and the acquisition of N-cadherin expression were positively associated with the differentiation status of the tumor tissue (P < 0.05) and with the occurrence of vascular invasion (P < 0.05). 3) A significant negative correlation was observed between ZEB1 and E-cadherin expression (Spearman = -0.636, P < 0.05) and between ZEB1 and β-catenin expression (Spearman = -0.417, P < 0.05). Moreover, a significant positive correlation was observed between ZEB1 and N-cadherin expression (Spearman = 0.557, P < 0.05). Conclusions: These results emphasize the role of EMT in cervical squamous cell carcinoma. The upregulation of ZEB1 is associated with the abnormal expression of E-cadherin, β-catenin and N-cadherin, which might promote the progression and metastasis of cervical squamous cell carcinoma.
Keywords: ZEB1, E-cadherin, β-catenin, N-cadherin, cervical squamous cell carcinoma, lymph nodes metastasis
Introduction
Cervical carcinoma is the second most prevalent female cancer worldwide, and squamous cell carcinoma (SCC) accounts for approximately 80% of the cases of cervical carcinoma [1]. Metastatic spread of cervical carcinoma cells to distant areas, such as the pelvic lymph nodes, is the primary cause of treatment failure and subsequent death in patients with cervical carcinoma [2]. Therefore, an understanding of the mechanism of the progression and metastasis of cervical carcinoma is an important issue with respect to the treatment of patients with this disease.
Epithelial cells can convert into mesenchymal cells via a multiple-step process termed epithelial-mesenchymal transition (EMT). The phenotypic transition from an epithelial to a mesenchymal-like state represents one important mechanism of epithelial plasticity. During EMT, epithelial tumor cells downregulate cell-cell adhesion systems, lose their polarity, and acquire a mesenchymal phenotype. This transformation increases the migratory capacity of the cells. The concept of EMT was first recognized in the field of embryology, but has recently been reported to play a vital role in the progression and metastasis of epithelial tumors [3,4].
The induction of EMT is mediated by key transcription factors within the cells, including Slug, Snail, Twist, ZEB1 and ZEB2. These factors are activated in response to different signaling pathways, which allow them to orchestrate intracellular changes that regulate the expression of epithelial and mesenchymal markers [5-7]. Over the past few years, ZEB1 has increasingly been considered to be an important contributor to the process of malignant cancer progression. ZEB1 is encoded by the TCF8 gene and is a vertebrate homologue of the ZFH gene family of zinc finger/homeodomain proteins. It interacts with the regulatory regions of responsive target genes by binding to the sequence motif CAGGTG/A [8,9]. A significant amount of insight as to the physiological functions of ZEB1 was obtained by experiments in model organisms. It was found that ZEB1 acts as a master repressor of “epithelialness” via the repression of E-cadherin and other epithelial markers. In cancer, it has been reported that the upregulation of the expression of ZEB1 plays an important role in the progression and metastasis in renal clear cell carcinomas [10], endometroid cancer [11], invasive breast cancer [12] and lung adenocarcinomas [13].
One key feature of EMT is the downregulation of E-cadherin, a cell adhesion molecule that presents in the membrane of normal epithelial cells. E-cadherin binds to β-catenin to form a protein complex that is linked to the actin cytoskeleton. Impairment of the adherens junction components has been demonstrated to play an important role in the induction of EMT that occurs during development and tumorigenesis [8]. As another member of the cadherin family, N-cadherin is indicative of a mesenchymal phenotype. Aberrant expression of N-cadherin in the cytomembrane or cytoplasm is associated with the progression and metastasis of solid tumors, and the forced expression of N-cadherin has been shown to induce migration and increase the invasiveness of cancer cells [14,15]. It was reported that a process termed “cadherin switching” involves a decrease in E-cadherin and an increase in N-cadherin [16]. This leads to cytoskeletal reorganization with reductions in the levels of cytokeratins and the acquisition of vimentin and α-smooth muscle actin expression, which provide resistance to deformation and motility [17,18].
The way in which cervical carcinoma cells acquire the ability to invade surrounding tissue is not fully understood, but EMT likely plays a role [19]. Based on the expression of epithelial and mesenchymal markers that define the EMT process, observations of clinical tissue have shown that EMT is involved in the progression of malignant tumors and is associated with the invasive capability of cervical carcinoma cells [20].
Although the ability of ZEB1 to repress E-cadherin by binding to E-box sequences on its promoter is documented at the molecular level, studies that have focused on the clinical implications of ZEB1 and EMT-associated markers in cervical carcinogenesis are limited. In this study, we detected ZEB1, E-cadherin, β-catenin and N-cadherin in tumors from 81 patients with cervical squamous cell carcinoma, and evaluated their relationship to the clinicopathological features of the patients. We also analyzed the correlation of these markers. Moreover, the expression of ZEB1 and E-cadherin in the metastatic lesions was also explored.
Materials and methods
Patients and tissue samples
The paraffin-embedded tissues of 81 patients with cervical squamous cell cancer who were seen between January 2009 and December 2012 were selected from the Department of Pathology at the 1st Affiliated Hospital of Zhengzhou University. Clinical information was obtained from the case histories of the patients. All patients underwent a traditional hysterectomy with clearance of the pelvic cavity and lymphadenectomy. None of the patients received preoperative therapy such as chemotherapy and radiotherapy before operations or after operations. In addition, 10 cases of normal cervical tissue were obtained from patients who underwent hysterectomies for benign conditions, and used as negative controls. These patients did not have a history of cervical intraepithelial neoplasia or an abnormal Papanicolaou test. Approval for this study was granted by the Medical Ethics Committee of 1st Affiliated Hospital of Zhengzhou University.
Immunohistochemistry
Four-micrometer thick sections were prepared from the paraffin-embedded tissues. Immunohistochemistry was performed with a PV-6000 polymer detection system. The sections were deparaffinized in xylene, rehydrated in graded alcohol solutions, and then boiled in EDTA buffer (pH 9.0) for 2.5 minutes in an autoclave. They were incubated at 4°C overnight with an anti-ZEB1 antibody (Cell Signaling Technology, Beverly, MA, USA; mouse monoclonal antibody, 1:50), anti-E-Cadherin antibody (Beijing Zhong Shan Biotechnology Co.Ltd., Beijing, China; mouse monoclonal antibody; 1:50), an anti-N-Cadherin antibody (Beijing Zhong Shan Biotechnology Co.Ltd., Beijing, China; mouse monoclonal antibody; 1:50), and an anti-β-catenin antibody (Beijing Zhong Shan Bio-technology Co.Ltd., Beijing, China; mouse monoclonal antibody; 1:50). The peroxidase reaction was developed with 3,3’-diaminobenzidine (DAB). The PV-6000 system, secondary antibodies, and the DAB were obtained from Zhongshan Golden Bridge Biotechnology. The sections that were stained without the primary antibodies served as negative controls.
Immunohistochemical evaluation
The immunohistochemical evaluation was performed by two experienced pathologists who had no knowledge of the clinical status of the patients. Nuclear expression of ZEB1 was regarded as positive, while membranous and cytoplasmic expression of E-cadherin, β-catenin and N-cadherin was regarded as positive. The status of ZEB1 protein expression was assessed by an evaluation of the intensity and the percentage of the stained tumor cells [21,22]. The percentage of positive tumor cells was scored as follows: 1, 0% to 9% positivity; 2, 10% to 50% positivity; 3, more than 50% positivity. The staining intensity was scored as follows: 0, negative staining; 1, weak staining; 2, moderate staining; 3, intense staining. The final score was calculated by multiplying the percentage stained score with the staining intensity score. Scores less than or equal to 1 were categorized as negative, and scores greater than 1 were categorized as positive. Loss of expression of E-cadherin and β-catenin in the cell membrane was considered if more than 10% of the tumor cells were not stained. Cell membranous or cytoplasmic expression of N-cadherin was considered positive if more than 10% of the tumor cells were stained [28]. In cases of discrepancies, the slides were re-evaluated and discussed until a consensus was achieved.
Statistical analyses
SPSS 19.0 was used for all statistical analysis. The chi-squared test was used to assess the association between ZEB1 expression and the clinicopathological characteristics of the patients as well as the correlation between the expression of ZEB1 and EMT-associated markers. P < 0.05 was considered to be significant.
Results
Clinicopathologic characteristics of the cases
A total of 91 patients were included as follows: 10 cases of normal cervical tissue and 81 cases of primary squamous cell cervical carcinoma (mean age, 48.1 years; range, 28-73 years). The histological differentiation and the FIGO stage of the tumors were determined based on the criteria of the World Health Organization. The invasive depth of the tumor was determined based on 1/2 of the cervical thickness. Vascular invasion by the primary lesions and positive node metastasis were found in 32 and 34 of the 81 cases, respectively. The principal clinicopathological characteristics are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of the cases
| Variable | No. of cases 81 | % | |
|---|---|---|---|
| Age (year) | ≤ 50 | 44 | 54.3 |
| > 50 | 37 | 45.7 | |
| FIGO stage | IA | 3 | 3.7 |
| IB | 32 | 39.5 | |
| IIA | 25 | 30.9 | |
| IIB | 20 | 24.7 | |
| III | 1 | 1.2 | |
| IV | 0 | 0.0 | |
| Invasive depth | ≤ 1/2 | 36 | 44.4 |
| > 1/2 | 45 | 55.6 | |
| Differentiation | Well | 14 | 17.3 |
| Moderate | 51 | 62.9 | |
| Poor | 16 | 19.8 | |
| Vascular invasion | Negative | 49 | 60.5 |
| Positive | 32 | 39.5 | |
| Lymph nodes | Negative | 47 | 58.0 |
| positive | 34 | 42.0 | |
Expression of ZEB1 and EMT-associated markers in cervical squamous cell carcinoma
The expression of ZEB1, E-cadherin, N-cadherin and β-catenin was analyzed. Negative staining for ZEB1 and N-cadherin was found in all 10 cases of normal cervical epithelium along with positive membranous staining for E-cadherin and β-catenin. In 81 cases of cervical squamous cell carcinoma, positive expression of ZEB1 was found in 45.7% (37/81). The loss expression of E-cadherin and β-catenin was 34.6% (28/81) and 19.8% (16/81), respectively. Aberrant expression of N-cadherin was 28.4% (23/81). Specifically, at the interface of the tumor parenchyma and the mesenchyma, intense positive expression of ZEB1 was found in the cell nuclei, along with definite loss of expression of E-cadherin and β-catenin in the cell membrane; in addition, up-regulated expression of N-cadherin was observed in the cytoplasm. The representative immunohistochemical images are shown in Figure 1A-E.
Figure 1.
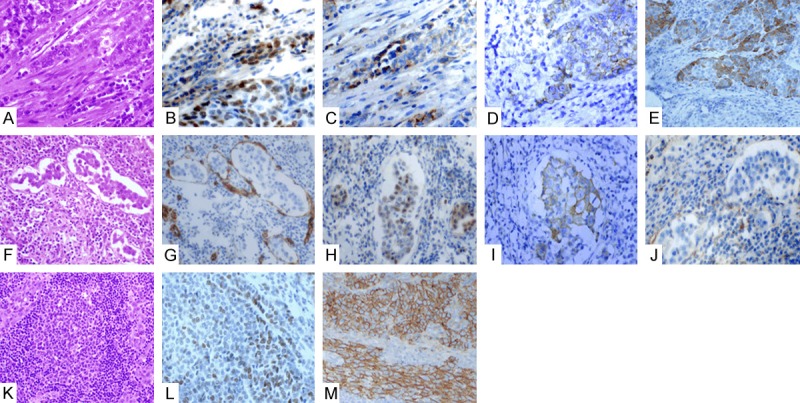
Expressions of ZEB1, E-cadherin, N-cadherin and β-catenin in tumor cells at the borders of tumor epithelium and stroma, in the vessels and in lymph nodes. At the interface of the tumor parenchyma and the mesenchyma (HE, A), intense positive expression of ZEB1 was found in the cell nuclei (B), along with definite loss of expression of E-cadherin (C) and β-catenin (E) in the cell membrane; in addition, up regulated expression of N-cadherin (D) was observed in the cytoplasm. The tumour thrombus in vessels (HE, F) (demonstrated by CD34 staining for the endothelial cells (G) showed the expression of ZEB1 in the cell nucleus (H), along with loss expression of E-cadherin (I) and β-catenin (J) in the membrane (I). Expression of ZEB1 (L) was downregulated in the metastatic lesions of lymph nodes (HE, K), and meanwhile the membranous expression of E-cadherin was regained to a degree (M).
Association of the clinicopathologic characteristics with the expression levels of ZEB1, E-cadherin, β-catenin and N-cadherin in primary cervical carcinoma
The nuclear expression of the ZEB1 protein was compared with the clinicopathologic features of the patients in order to investigate the possible relationships. None of the 14 well-differentiated samples showed any nuclear expression of ZEB1, but the expression of ZEB1 was significantly increased to 81.3% of cells (13/16) in poorly differentiated samples (P = 0.000). Definite vascular invasion (presence of tumor thrombi in the vascular space surrounded by endothelial cells) was observed in 32 of 81 samples. Nuclear expression of ZEB1 was significantly higher in samples (21/32, 65.6%) with vascular invasion than in those (12/49, 24.5%) without vascular invasion (P = 0.001). Our data also demonstrated the significant difference in ZEB1 expression in cervical squamous cell carcinoma between groups with negative lymph node metastasis (15/47, 31.9%) and positive lymph node metastasis (22/34, 64.7%, P = 0.004). The positive expression of ZEB1 was not associated with the FIGO stage or with the invasive depth of the tumors. Additionally, we found that the expression of all three EMT-related markers in the tumor cells was associated with the differentiation status and the expression of E-cadherin and β-catenin was associated with vascular invasion, but all the three markers were not associated with lymph node metastasis. The main immunohistochemical results are summarized in Table 2, and representative immunohistochemical images are shown in Figures 1F, 1J, 2.
Table 2.
Expression of ZEB1, E-cadherin, N-cadherin and β-catenin in primary cervical squamous cell carcinoma and their association with the clinicopathologic characters
| No. of cases | ZEB1 | ZEB1 | N-cadherin | β-catenin | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Variable | 81 | Negative (No.) 44 | Positive (No.) 37 | Loss (No.) 28 | Normal (No.) 53 | Negative (No.) 58 | Positive (No.) 23 | Loss (No.) 16 | Normal (No.) 65 |
| Differentiation | |||||||||
| Well | 14 | 14 | 0 | 0 | 14 | 14 | 0 | 0 | 14 |
| Moderate | 51 | 27 | 24 | 13 | 38 | 42 | 9 | 4 | 47 |
| Poor | 16 | 3 | 13 | 15 | 1 | 2 | 14 | 12 | 4 |
| P-value | 0.000* | 0.000* | 0.000* | 0.000* | |||||
| FIGO stage | |||||||||
| IA | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 3 |
| IB | 32 | 19 | 13 | 9 | 23 | 26 | 6 | 4 | 28 |
| IIA | 25 | 13 | 12 | 13 | 12 | 14 | 11 | 8 | 17 |
| IIB | 20 | 8 | 12 | 6 | 14 | 14 | 6 | 4 | 16 |
| III | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P-value | 0.255 | 0.187 | 0.200 | 0.330 | |||||
| Invasive depth | |||||||||
| ≤nva | 36 | 19 | 17 | 13 | 23 | 25 | 11 | 8 | 28 |
| >1/2 | 45 | 25 | 20 | 15 | 30 | 33 | 12 | 8 | 37 |
| P-value | |||||||||
| Vascular invasion | |||||||||
| Negative | 49 | 35 | 14 | 12 | 37 | 36 | 13 | 6 | 43 |
| Positive | 32 | 11 | 21 | 17 | 15 | 19 | 13 | 10 | 22 |
| P-value | 0.001* | 0.009* | 0.187 | 0.037* | |||||
| Lymph node | |||||||||
| Negative | 47 | 32 | 15 | 13 | 34 | 35 | 12 | 9 | 38 |
| Positive | 34 | 12 | 22 | 15 | 19 | 23 | 11 | 7 | 27 |
| P-value | 0.004* | 0.127 | 0.608 | 0.715 | |||||
P < 0.05 was regarded as statistically significant.
Figure 2.
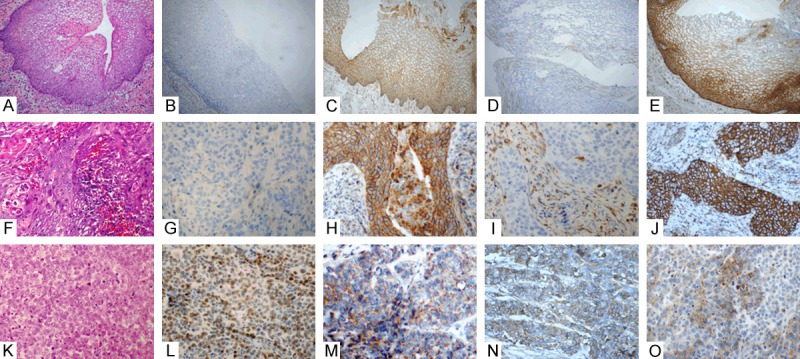
Expressions of ZEB1, E-cadherin, N-cadherin and β-catenin in normal cervical epithelium, well-differentiated and poorly-differentiated squamous cell carcinoma. Negative staining for ZEB1 (B, G) and N-cadherin (D, I) in normal cervical epithelium (HE, A) and well-differentiated squamous cell carcinoma (HE, F), along with positive membranous staining for E-cadherin (C, H) and β-catenin (E, J). Positive staining for ZEB1 (L) and N-cadherin (N) in poor-differentiated squamous cell carcinoma (HE, K), along with loss expression of membranous staining for E-cadherin (M) and β-catenin (O) (×400).
Differential expression of ZEB1 and E-cadherin between primary cervical squamous cell carcinomas and their corresponding metastatic lesions in lymph nodes
In our study, 47 of the 81 cases were primary cervical squamous cell carcinomas with negative pelvic lymph node metastasis, while 34 had positive pelvic lymph nodes metastasis. In regard to the primary lesions, a significant difference was observed between these two groups with respect to tissue differentiation, but no difference was observed with respect to age, tumor invasive depth, vascular invasion and FIGO stage.
Hematoxylin-eosin staining did not reveal any remarkable morphological differences between the two groups, and the tumor grade and differentiation status of the tumor tissues were similar. The expression of ZEB1 and E-cadherin in both primary and corresponding metastases was then compared. As shown in Table 3, the nuclear expression of ZEB1 was observed in 64.7% (22/34) of primary tumors, and its expression rate decreased to 38.2% (13/34) in the metastatic lesions (P = 0.03). Moreover, E-cadherin was re-expressed in 73.5% (25/34) of the metastatic lesions, but no significant difference was observed between the primary and metastatic lesions (P = 0.131). Representative immunohistochemical images are shown in Figure 1K-M.
Table 3.
Expression of ZEB1 and E-cadherin in primary cervical squamous cell carcinomas and their corresponding metastases lesions in lymph nodes
| Variable | Primary lesion (No. of cases) | Metastatic lesion (No. of cases) | p-Value | |
|---|---|---|---|---|
|
| ||||
| 34 | 34 | |||
| ZEB1 | Negative | 12 | 21 | 0.03* |
| Positive | 22 | 13 | ||
| E-cadherin | Loss | 15 | 9 | 0.131 |
| Normal | 19 | 25 | ||
P < 0.05 was regarded as statistically significant.
Correlation among the immunohistochemical expression patterns of ZEB1, E-cadherin, β-catenin and N-cadherin
A correlation analysis demonstrated a significant negative correlation between ZEB1 and E-cadherin expression (Spearman = -0.636, P = 0.000) and between ZEB1 and β-catenin expression (Spearman = -0.417, P = 0.000). Additionally, a significant positive correlation was also observed between ZEB1 and N-cadherin expression (Spearman = 0.557, P = 0.000). The data are summarized in Table 4.
Table 4.
Correlation among the immunostaining of ZEB1, E-cadherin, N-cadherin and β-catenin
| Variable | No. of cases | ZEB1 | Spearman | P-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Negative | Positive | |||||
| E-cadherin | Loss | 28 | 3 | 25 | -0.636 | 0.001* |
| Normal | 53 | 41 | 12 | |||
| N-cadherin | Negative | 58 | 42 | 16 | 0.557 | 0.001* |
| Positive | 23 | 2 | 21 | |||
| β-catenin | Loss | 16 | 2 | 14 | -0.417 | 0.001* |
| Normal | 65 | 42 | 23 | |||
P < 0.05 was regarded as statistically significant.
Discussion
In this study, we observed that the nuclear expression of ZEB1 was significantly up-regulated in cervical squamous cell carcinoma compared with normal cervical epithelium. The abnormal expression of ZEB1 was associated with the differentiation status as well as the occurrence of vascular invasion and lymph node metastasis of the cervical squamous cell carcinoma tissues. Altered expression of the EMT-associated markers E-cadherin, β-catenin and N-cadherin in cervical squamous cell carcinoma was also observed, and the expression of these three markers was related to the differentiation status and the presence of vascular invasion. The correlation analysis showed a significant correlation between the expression of ZEB1 and the expression of these three EMT-associated markers.
In contrast to normal cervical epithelium and well-differentiated cervical squamous cell carcinoma, ZEB1 was aberrantly upregulated in moderately and poorly differentiated cervical squamous cell carcinoma. It was previously reported that ZEB1 is frequently overexpressed in undifferentiated endometrial carcinomas and in highly aggressive endometrial cancers, such as endometrioid adenocarcinomas of FIGO grade 3, uterine serous carcinomas, and malignant mixed Müllerian tumors [23]. In prostate carcinomas, high ZEB1 expression was significantly correlated with a high Gleason score [24], which is evaluated according to the differentiation of the tumor tissues. In breast cancer, ZEB1 was especially up-regulated in triple-negative breast cancer and in basal-like breast cancer, which are both associated with an aggressive tumor phenotype compared with other types of breast carcinomas [12]. All of these data suggest that nuclear expression of ZEB1 is associated with an aggressive tumor phenotype. With the exception of ZEB1, we observed that the loss of expression of the epithelial markers E-cadherin and β-catenin was also associated with the differentiation status of our cases, along with the abnormal cytoplasmic expression of N-cadherin. A possible reason for this may be that, as an important regulator of cell polarity and differentiation in carcinomas, the nuclear expression of ZEB1 causes the tumor cells to lose their epithelial characteristics to a certain degree. They then in turn acquire mesenchymal characteristics, such as a polygonal shape or a decreasein cell adhesion, which leads to greater malignancy and aggression of the tumors. The acquisition of these mesenchymal characteristics also contributes to the malignant dedifferentiation of tumors and to additional metastases.
As expected, in our study, ZEB1 expression was significantly upregulated in cervical squamous cell carcinomas with vascular invasion and lymph node metastasis. Carcinoma cells leave the primary site, intravasate into the blood or lymphatic vessels, disseminate into a secondary site, and ultimately grow as metastatic foci. The initial stage of these processes is now believed to be associated with EMT, which is regulated by the activation of nuclear transcriptional regulators. ZEB1 was found to be highly expressed in bone-metastatic squamous cell lung cancer tissues and cell lines compared with those that were non-metastatic [25]. In gastric carcinoma tissue, a larger scope of invasion was correlated with a higher expression of ZEB1, and the suppression of ZEB1 expression in vitro could significantly reduce the invasive and metastatic ability of the AGS gastric carcinoma cell line [26]. The overexpression of ZEB1 in osteosarcoma was also found to be related to the metastasis and invasion of osteosarcoma [27]. In a recent study of cervical cancers, ZEB1 and another EMT transcription factor, SNAI1, were also observed to be up-regulated in cancers with lymph node metastasis [28]. Our results were consistent with the results of these reports, and indicated that nuclear expression of ZEB1 can increase the motility of cervical squamous cell carcinoma cells. Nuclear expression of ZEB1 also endows the tumor cells with an increased ability to invade distant sites. Additionally, we observed that ZEB1 was especially expressed at a strong intensity in the epithelial tumor cells at the edge of the tumor cell invasive area; this expression was accompanied by reduced membranous expression of E-cadherin and β-catenin and upregulated membranous or cytoplasmic expression of N-cadherin. In addition, a better correlation was observed between the expression of ZEB1 and the expression of these EMT-associated markers. The tumor-host interface is deemed to be the invasive front of solid tumors and often displays different molecular and morphologic characteristics from the more superficial parts of the same tumor. Tumor cells in this area is more easily regulated by EMT, may detach from the primary tumor mass to invade distant sites, and plays more important roles in tumor invasion and metastasis [29,30]. To our knowledge, it was reported that ZEB1 was immunohistochemically detected at the tumor-host interface in colorectal cancer and breast cancers [8]. We believe that the interface of the parenchyma and the tumor stroma contains more prognostic information than do the central and superficial parts of the same tumor, and thus is a favoredsite of observation within tumors.
In this study, we also observed the differential expression of ZEB1 and E-cadherin between the primary and the metastatic lesions. Interestingly, the nuclear expression of ZEB1 was downregulated in the metastatic lesions compared with the primary tumors, and a portion of the tumor cell nests in lymph nodes re-expressed E-cadherin in the membrane. Similarly, in one study of primary cutaneous squamous cell carcinomas, Twist, another EMT regulator, was found to be associated with an increased metastatic risk and was attenuated in lymph node metastases [31]. However, according to several studies, the expression levels of EMT-associated markers were significantly higher in lymph node metastases compared with primary tumors of the lung [32] and breast [33]. The author explained that the contradictory results between different studies may be due to the activation of organ-specific pathways. Another possible explanation is that epithelial and mesenchymal cells migrate together, and that the preponderance of epithelial markers in metastatic lesions might reflect a favorable growth rate for epithelial cells compared with mesenchymal cells [34]. An alternative hypothesis proposes that cancer cells undergo EMT in order to gain a migratory mesenchymal phenotype that enables them to migrate from the primary tumor to colonize distant sites, where they then undergo mesenchymal-to-epithelial transition (MET). This process enables the growth of metastatic tumors to form with the same epithelial characteristics as the parent tumor. During growth, the re-expression of E-cadherin has been suggested to play a central role in the establishment of metastases. It has been proposed that this MET conversion may be common once the tumor cells have seeded in the metastatic organ [35]. It is also possible that during malignant progression, different inductive tumor microenvironments within the primary and metastatic sites are involved and that they regulate the cycling dynamics of EMT/MET. Moreover, it was demonstrated that in squamous cell carcinoma, some cells that have undergone EMT can reverse this process through MET, but this ability is not shared by all cancer cells that have undergone EMT [36]. Thus far, little information has been discovered as to the signals that drive MET in cancer, and therefore, the phenomenon of MET needs to be further explored in additional cases and tumor types.
In summary, the overexpression of ZEB1 and the downregulation of E-cadherin and β-catenin, along with the upregulation of N-cadherin expression are closely related to the differentiation status and the invasive capacity of cervical carcinoma. In cervical carcinoma tissue, ZEB1 is highly expressed, which may further enhance its regulation of the expression of E-cadherin, β-catenin and N-cadherin. This may cause an increase in malignancy and invasiveness of cervical cancer cells. This study further demonstrates the important role of ZEB1, E-cadherin, β-catenin and N-cadherin in cervical carcinoma, which provides a theoretical basis for the role of gene therapy in the metastasis of cervical carcinoma. Next, we will further explore the possible molecular mechanism of ZEB1 up regulation in the metastasis of cervical squamous cell carcinoma.
Acknowledgements
Supported by the National Natural Science Youth Foundation of China (81401936); The youth foundation of 1st Affiliated Hosp, Zhengzhou University.
Disclosure of conflict of interest
None.
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Mountzios G, Soultati A, Pectasides D, Pectasides E, Dimopoulos MA, Papadimitriou CA. Developments in the systemic treatment of metastatic cervical cancer. Cancer Treat Rev. 2013;39:430–43. doi: 10.1016/j.ctrv.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Sleeman JP, Thiery JP. SnapShot: The epithelial-mesenchymal transition. Cell. 2011;145:162, e1. doi: 10.1016/j.cell.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res. 2013;5:187–195. doi: 10.2147/CMAR.S35171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.oez-Novoa JM, Nieto MA. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 9.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber KL, Doucet M, Price JE. Renal cell carcinoma bone metastasis: epidermal growth factor receptor targeting. Clin Orthop Relat Res. 2003;415:S86–94. doi: 10.1097/01.blo.0000093050.96273.35. [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Spoelstra NS, Jean A, Howe E, Torkko KC, Clark HR, Darling DS, Shroyer KR, Horwitz KB, Broaddus RR, Richer JK. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol. 2008;21:912–923. doi: 10.1038/modpathol.2008.82. [DOI] [PubMed] [Google Scholar]
- 12.Karihtala P, Auvinen P, Kauppila S, Haapasaari KM, Jukkola-Vuorinen A, Soini Y. Vimentin, zeb1 and Sip1 are up-regulated in triple-negative and basal-like breast cancers: association with an aggressive tumour phenotype. Breast Cancer Res Treat. 2013;138:81–90. doi: 10.1007/s10549-013-2442-0. [DOI] [PubMed] [Google Scholar]
- 13.Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, Zeng C, Baron A, Bemis L, Erickson P, Wilder E, Rustgi A, Kitajewski J, Gabrielson E, Bremnes R, Franklin W, Drabkin HA. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U S A. 2003;100:10429–10434. doi: 10.1073/pnas.1734137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 15.Wells A, Chao YL, Grahovac J, Wu Q, Lauffenburger DA. Epithelial and mesenchymal phenotypic switchings modulate cell motility in metastasis. Front Biosci (Landmark Ed) 2011;16:815–837. doi: 10.2741/3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariotti A, Perotti A, Sessa C, Rüegg C. N-cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs. 2007;16:451–465. doi: 10.1517/13543784.16.4.451. [DOI] [PubMed] [Google Scholar]
- 17.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MY, Shen MR. Epithelial-mesenchymal transition in cervical carcinoma. Am J Transl Res. 2012;4:1–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-Mesenchymal Transitionin Cervical Cancer: Correlation with Tumor Progression, Epidermal Growth Factor Receptor Overexpression, and Snail Up-Regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 21.Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB, Richer JK. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66:3893–3902. doi: 10.1158/0008-5472.CAN-05-2881. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, Xu J, Xue R, Liu Y, Yu H. Overexpression of AIB1 correlates inversely with E-cadherin expression in pancreatic adenocarcinoma and may promote lymph node metastasis. Int J Clin Oncol. 2014;19:319–324. doi: 10.1007/s10147-013-0549-2. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Pérez L, López-García MÁ, Díaz-Martín J, Biscuola M, Castilla MÁ, Tafe LJ, Garg K, Oliva E, Matias-Guiu X, Soslow RA, Palacios J. ZEB1 overexpression associated with E-cadherin and microRNA-200 downregulation is characteristic of undifferentiated endometrial carcinoma. Mod Pathol. 2013;26:1514–1524. doi: 10.1038/modpathol.2013.93. [DOI] [PubMed] [Google Scholar]
- 24.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O’Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Zhang N, Wang Y, Xu M, Liu N, Pang X, Cao J, Ma N, Pang H, Liu L, Zhang H. Zinc finger E-box binding homeobox 1 promotes invasion and bone metastasis of small cell lung cancer in vitro and in vivo. Cancer Sci. 2012;103:1420–1428. doi: 10.1111/j.1349-7006.2012.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia B, Liu H, Kong Q, Li B. Overexpression of ZEB1 associated with metastasis and invasion in patients with gastric carcinoma. Mol Cell Biochem. 2012;366:223–229. doi: 10.1007/s11010-012-1299-6. [DOI] [PubMed] [Google Scholar]
- 27.Shen A, Zhang Y, Yang H, Xu R, Huang G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J Surg Oncol. 2012;105:830–834. doi: 10.1002/jso.23012. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Li S, Huang K, Zhang Q, Wang J, Li X, Hu T, Wang S, Yang R, Jia Y, Sun H, Tang F, Zhou H, Shen J, Ma D, Wang S. The nuclear protein expression levels of SNAI1 and ZEB1 are involved in the progression and lymph node metastasis of cervical cancer via the epithelial-mesenchymal transition pathway. Hum Pathol. 2013;44:2097–2105. doi: 10.1016/j.humpath.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis. 1998;4:70–77. doi: 10.1111/j.1601-0825.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Sah P, Sharma SS, Radhakrishnan R. Molecular changes in invasive front of oral cancer. J Oral Maxillofac Pathol. 2013;17:240–247. doi: 10.4103/0973-029X.119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toll A, Masferrer E, Hernández-Ruiz ME, Ferrandiz-Pulido C, Yébenes M, Jaka A, Tuneu A, Jucglà A, Gimeno J, Baró T, Casado B, Gandarillas A, Costa I, Mojal S, Peña R, de Herreros AG, García-Patos V, Pujol RM, Hernández-Muñoz I. Epithelial to mesenchymal transition markers are associated with an increased metastatic risk in primary cutaneous squamous cell carcinomas but are attenuated in lymph node metastases. J Dermatol Sci. 2013;72:93–102. doi: 10.1016/j.jdermsci.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Dong W, Shen H, Mu X, Li Z, Lin X, Liu Y, Du J. A comparison of Twist and E-cadherin protein expression in primary non-small-cell lung carcinoma and corresponding metastases. Eur J Cardiothorac Surg. 2011;39:1028–1032. doi: 10.1016/j.ejcts.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Markiewicz A, Ahrends T, Wełnicka-Jaśkiewicz M, Seroczyńska B, Skokowski J, Jaśkiewicz J, Szade J, Biernat W, Zaczek AJ. Expression of epithelial to mesenchymal transition-related markers in lymph node metastases as a surrogate for primary tumor metastatic potential in breast cancer. J Transl Med. 2012;10:226. doi: 10.1186/1479-5876-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ocaña OH, Córcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]


