Abstract
Ki-67 and caspase indices are two very important prognostic variables for breast cancer. The aim of this study was to compare the immunoexpressions of these two prognostic variables at the center, the periphery of the tumor in comparison with each other, and additional parameters in 53 breast cancer specimens. It has been shown that the increase of caspase immunoexpression either at the periphery or the center of the tumor correlated with the increase of Ki-67 immunoexpression at the same areas. There was no statistically significant difference between caspase and Ki-67 immunoexpression at the center or the periphery of the tumor. No statistically significant correlation was found between immunoexpressions of caspase and Ki-67 with the tumor grade, stage, lymph node metastasis, lymphovascular or perineural invasion. The two very important prognostic variables of breast cancer (caspase and Ki-67) were evenly distributed in the center and the periphery of the tumor. It is evident that there is no need for a specific locational sampling when these two variables are considered. In accordance with this information, the differences in sampling due to observer diversity may be prevented.
Keywords: Ki-67, caspase, breast cancer, immunohistochemistry
Introduction
Invasive breast carcinoma is the most common carcinoma among women. It is responsible for 3% of all cancers in women globally and 7% of cancers in women in affluent countries. While a variety of imaging modalities are used for preoperative radiological diagnosis of breast cancer, distinguishing between malignant and benign lesions is still based on histological studies [1].
A wide variety of prognostic factors have been revealed. They are: invasive carcinoma versus in situ disease, presence of distant metastases and lymph node metastases, tumor size, locally advanced disease, and inflammatory carcinoma. Histological subtype, histological grade, estrogen and progesterone receptors, HER2/neu immunoexpression, lymphovascular invasion, response to neoadjuvant therapy, gene expression profiling, and proliferative rate are other prognostic and predictive factors [1-8].
The degree of tumor proliferation is one of the most important prognostic factors in breast carcinoma. Proliferation can be measured by mitotic counts, immunohistochemical detection of cellular proteins produced during the cell cycle (e.g., cyclins or Ki-67), flow cytometry (as the S-phase fraction), or the thymidine-labeling index [3,9,10]. If proliferation is to be used as a prognostic factor, it is important for pathology reports to use a standard technique. Until the reliability of new methods is confirmed, the current standard proliferation assay should be Ki-67 immunohistochemistry, given its simplicity and wide availability [10]. Ki-67 is a reproducible, easily assessed proliferation marker and a reliable substitute for mitotic counts [11]. Apoptosis is a genetically programmed cell death that functions primarily to eliminate senescent or altered cells that are useless or harmful in a multicellular organism [12]. Specific changes in nuclear architecture (such as the segmentation of the nucleus) occur; however, these nuclear features are not always easily interpreted [12-14]. Apoptosis relies on cysteine proteases called caspases. Caspases are synthesized as performs, and become activated by cleavage at aspartate residues. Because caspases cleave and activate each other, the protease cascade amplifies, ensuring proper apoptotic cell death [12].
This study was conducted to determine the differences of immunohistochemical expression of Ki-67 and caspase, by means of location (center and periphery) in the tumor. The necessity for a locational sampling was questioned. The correlation between the Ki-67 and caspase immunoexpression and the tumor grade, stage, mitotic activity, P53, estrogen (ER), progesterone (PR) hormone receptor and cerbB2 status was also investigated.
Materials and methods
Breast carcinoma cases were retrieved from the files of the pathology department and 53 invasive ductal carcinoma cases operated between 2009 and 2014 were examined in this study. For each case, paraffin embedded blocks and slides were retrieved, 2 blocks for each case were selected. The center and the periphery of the tumor were classified as follows; infiltrated areas were classified as “periphery” and the area containing solely the tumor cells were classified as the “center”. The histological subtypes of the tumors which were determined according to World Health Organization (WHO) classification and grades of the tumors which were determined according to Elston-Ellis histological grading system were recorded as reported in the original pathology reports, and were reevaluated by two pathologists (DF/HK). Tumors were staged according to the TNM 2010 staging system [1].
Immunohistochemistry
For immunohistochemical examination, a Ki-67 + Caspase-3 Multiplex Cocktail (Ki-67 + Caspase-3 Prediluted Multiplex Cocktail (4-Step), Biocare Medical, PPM 240 DS AA, USA) was performed. In this method, 3 μm sections were deparaffinized with xylene and ethanol. Intrinsic peroxidase activity in tissue was blocked by treatment with Biocare Peroxidase. For antigen retrieval, the slides were heated with antigen retrieval solution in a microwave oven. The sections were washed two times in phosphate buffered saline (PBS) for 5 minutes. After protein blockage, the sections were incubated with the anti-body for 30 minutes at room temperature and washed two times in PBS for 5 minutes. Then the sections were incubated with Biocare MACH 2 Double Stain 2 for 30 minutes, Biocare Betazoid DAB for 5 minutes, and Biocare Warp Red for 5 minutes. Mayer’s hematoxylin was used to counterstain the slides.
The immunostained sections were examined under a light microscope (Olympus BX53, Tokyo, Japan), and evaluated by 2 pathologists (DF/HK). Five hundred tumor cells were counted both at the center and at the periphery of the tumor (Figures 1, 2). The following cut-offs were applied for considering positive/negative staining: ≥ 14% for Ki-67 and > 3% for caspase.
Figure 1.
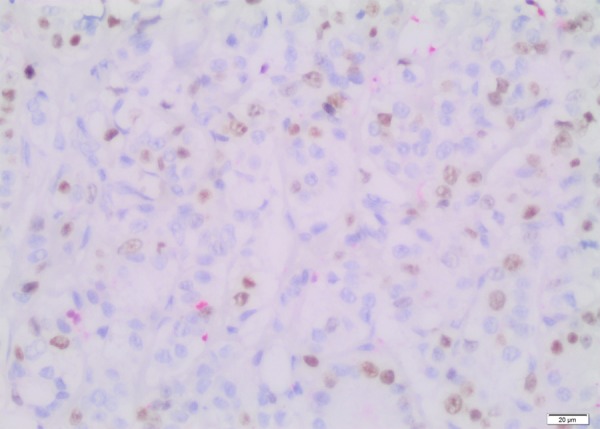
Nuclear staining with Ki-67 (Immunohistochemistry, × 200).
Figure 2.
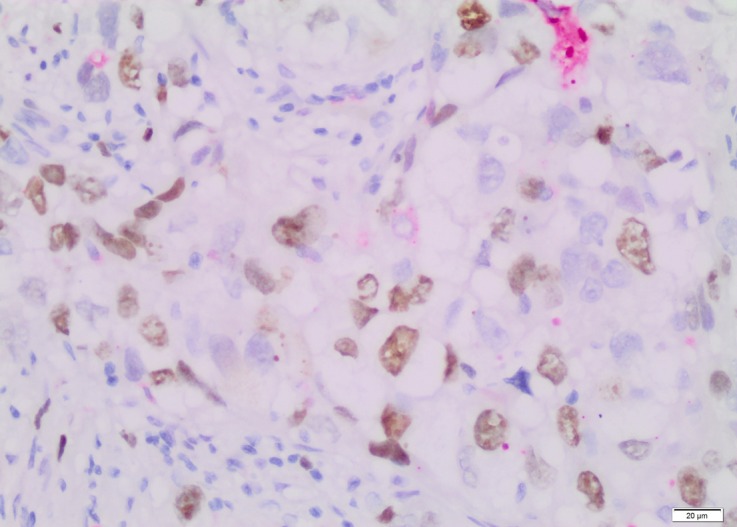
Cytoplasmic staining with caspase (Immunohistochemistry, × 200).
Statistical analysis
Statistical analysis was carried out using NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA). Statistical comparisons between groups were performed using the Kruskal Wallis and Mann Whitney U tests. We analyzed the correlation between immunoexpressions of Ki-67 and caspase in both the periphery and the center of the tumors. Statistical analyses were carried out both with and without cut-offs. The correlation between the immunoexpressions and the tumor stage (T1, T2, T3, T4), histological grade (1, 2, 3), lymph node metastasis (N0, N1, N2, N3), lymphovascular invasion, perineural infiltration, surgical margin invasion, estrogen and progesterone receptor, cerbB2 status, and p53 immunoreactivity were also studied by the Pearson correlation test. Values are given as mean ± SD and P value of < 0.05 was considered to be statistically significant.
Results
All patients were female, having unilateral breast cancer, neither had distant site metastasis nor neoadjuvant chemotherapy. The mean age of patients was 56.4 (range: 29-82) years. Out of the 53 cases, 24 (45.3%) were mastectomy specimens, 20 (37.7%) were lumpectomy specimens, 5 (9.4%) were breast-conserving surgery specimens, and 4 (7.6%) were simple mastectomy specimens (Table 1).
Table 1.
Patient characteristics and immunohistochemical results
| Age | Grade | pT | N | LVI | PNI | F/SM invasion | ER% | PR% | cerbB2 (0-3) | P53% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | 3 | 2 | 1 | + | - | - | 0 | 0 | 1 | 0 |
| 2 | 59 | 3 | 3 | 0 | - | - | - | 0 | 0 | 1 | 0 |
| 3 | 55 | 2 | 1 | 0 | + | - | - | 0 | 0 | 0 | 97.4 |
| 4 | 54 | 2 | 2 | 0 | - | - | - | 90 | 20 | 0 | 0 |
| 5 | 49 | 1 | 1 | 0 | - | - | - | 10 | 0 | 3 | 0 |
| 6 | 58 | 1 | 2 | 0 | - | - | - | 0 | 0 | 3 | 0 |
| 7 | 70 | 3 | 2 | 0 | + | - | - | 90 | 60 | 0 | 0 |
| 8 | 56 | 3 | 2 | 1 | + | - | - | 0 | 0 | 0 | 0 |
| 9 | 78 | 3 | 2 | 1 | + | - | - | 0 | 0 | 0 | 0 |
| 10 | 47 | 3 | 2 | 0 | + | - | - | 0 | 0 | 2 | 0 |
| 11 | 56 | 2 | 2 | 0 | + | - | - | 0 | 0 | 0 | 0 |
| 12 | 53 | 3 | 2 | 0 | + | - | - | 1 | 0 | 1 | 9.5 |
| 13 | 29 | 2 | 3 | 3 | + | + | - | 90 | 15 | 0 | 5 |
| 14 | 36 | 3 | 2 | 3 | + | - | - | 70 | 40 | 0 | 1 |
| 15 | 45 | 3 | 2 | 1 | + | - | - | 0 | 40 | 2 | 0 |
| 16 | 62 | 2 | 3 | 2 | + | - | - | 0 | 0 | 3 | 0 |
| 17 | 77 | 1 | 1 | 0 | - | - | - | 90 | 0 | 2 | 0 |
| 18 | 65 | 2 | 1 | 0 | - | - | - | 90 | 0 | 0 | 0 |
| 19 | 69 | 2 | 1 | 0 | - | - | - | 90 | 90 | 0 | 0.3 |
| 20 | 73 | 1 | 1 | 1 | - | + | - | 90 | 90 | 1 | 0 |
| 21 | 64 | 2 | 2 | 3 | + | + | - | 90 | 30 | 0 | 0 |
| 22 | 44 | 3 | 2 | 2 | + | - | - | 90 | 70 | 0 | 0 |
| 23 | 73 | 1 | 1 | 0 | - | - | - | 0 | 0 | 3 | 0 |
| 24 | 82 | 2 | 2 | 0 | + | - | - | 90 | 10 | 1 | 1 |
| 25 | 49 | 3 | 3 | 1 | + | - | - | 70 | 10 | 0 | 98 |
| 26 | 47 | 2 | 1 | 0 | - | + | - | 70 | 0 | 0 | 0 |
| 27 | 61 | 2 | 2 | 3 | + | + | - | 60 | 90 | 0 | 28 |
| 28 | 41 | 2 | 2 | 2 | + | - | - | 40 | 10 | 1 | 3 |
| 29 | 63 | 3 | 4 | 0 | + | + | + | 70 | 0 | 0 | 0 |
| 30 | 72 | 2 | 2 | 0 | + | - | - | 0 | 0 | 0 | 83.2 |
| 31 | 72 | 1 | 1 | 0 | - | - | - | 90 | 90 | 1 | 0 |
| 32 | 59 | 2 | 2 | 0 | - | - | - | 70 | 0 | 0 | 0 |
| 33 | 49 | 2 | 2 | 1 | + | + | - | 70 | 30 | 0 | 3 |
| 34 | 67 | 2 | 2 | 0 | - | - | - | 0 | 80 | 0 | 8.3 |
| 35 | 57 | 2 | 2 | 1 | + | + | - | 30 | 10 | 0 | 0 |
| 36 | 56 | 2 | 1 | 1mic | + | + | - | 80 | 90 | 0 | 0 |
| 37 | 54 | 2 | 1 | 1 | + | - | - | 90 | 10 | 0 | 0 |
| 38 | 40 | 2 | 2 | 0 | - | - | - | 90 | 0 | 0 | 0 |
| 39 | 73 | 2 | 4 | 2 | + | + | + | 40 | 0 | 0 | 0 |
| 40 | 75 | 2 | 2 | 0 | + | - | - | 60 | 50 | 0 | 0 |
| 41 | 40 | 2 | 1 | 1 | + | - | - | 60 | 80 | 0 | 0 |
| 42 | 74 | 3 | 3 | 3 | + | - | - | 70 | 90 | 0 | 0 |
| 43 | 30 | 3 | 1 | 0 | - | - | - | 0 | 0 | 2 | 0 |
| 44 | 57 | 3 | 2 | 1mic | - | - | + | 30 | 10 | 3 | 2 |
| 45 | 44 | 3 | 2 | 0 | + | - | + | 0 | 0 | 0 | 0 |
| 46 | 51 | 3 | 2 | 1 | + | - | - | 0 | 0 | 0 | 0 |
| 47 | 45 | 1 | 1 | 0 | - | - | + | 40 | 90 | 0 | 5 |
| 48 | 56 | 3 | 2 | 2 | + | + | - | 70 | 80 | 0 | 0 |
| 49 | 43 | 2 | 2 | 2 | + | - | + | 90 | 10 | 2 | 4 |
| 50 | 76 | 1 | 1 | 1 | + | + | - | 0 | 20 | 3 | 0 |
| 51 | 48 | 2 | 1 | 2 | + | + | - | 100 | 100 | 0 | 0 |
| 52 | 33 | 3 | 2 | 0 | + | - | - | 90 | 90 | 0 | 0 |
| 53 | 57 | 3 | 2 | 0 | + | + | - | 90 | 70 | 0 | 0 |
The mean values of Ki67 TP (tumor periphery) (9.1 ± 7.6) were found to be higher than Ki67 TC (tumor center) (7.4 ± 6.5); a positive correlation was found between the groups and the correlation was statistically significant (R = 0.822, P = 0.0001). Also, the mean values of caspase TP (2.9 ± 2.4) were higher than caspase TC (2.1 ± 1.6) and a positive correlation was found between the groups and the correlation was statistically significant (R = 0.365, P = 0.007). The Ki67 immunoexpression in TC was correlated with caspase immunoexpression at the same area, and this correlation was found to be statistically significant (R = 0.341, P = 0.013) (Table 2).
Table 2.
The immunoexpressions of caspase and Ki-67 at the center and the periphery of the tumor
| Immunoexpressions | Ki67 TC% | Ki67 TP% | Caspase TC% | Caspase TP% | |
|---|---|---|---|---|---|
| Ki67 TC% | r | 0.822 | 0.227 | 0.356 | |
| p | 0.0001 | 0.102 | 0.009 | ||
| Ki67 TP% | r | 0.822 | 0.236 | 0.341 | |
| p | 0.0001 | 0.089 | 0.013 | ||
| Caspase TC% | r | 0.227 | 0.236 | 0.365 | |
| p | 0.102 | 0.089 | 0.007 | ||
| Caspase TP% | r | 0.356 | 0.341 | 0.365 | |
| p | 0.009 | 0.013 | 0.007 |
TC: tumor center, TP: tumor periphery.
Among the study group, 6 (11.3%) patients had tumor positivity on posterior surgical margin. When the mean Ki67 TC (13.02 ± 9.67) and Ki 67 TP (14 ± 7.07) immunoexpressions were compared to mean Ki67 TC (5.71 ± 6.79) and Ki67 TP (6.89 ± 9.01) immunoexpressions of patients with negative margin status, we found statistically significant difference between the groups regarding to the same areas (P = 0.029 and 0.015, respectively). Also, the caspase TC (3 ± 0.89) and caspase TP (5.1 ± 2.53), values of patients with positive surgical margins were compared to caspase TC (1.96 ± 1.69) and Ki67 TP (2.75 ± 2.24) values of patients with negative surgical margin status, a statistically significant difference was found between the groups regarding the same areas (P = 0.014 and 0.007, respectively) (Table 3).
Table 3.
The correlation between caspase and Ki-67 immunoexpressions and the tumor positive surgical margin group, both at the center and the periphery of the tumor
| Immunoexpressions | Surgical margin (-) | Surgical margin (+) | P | |
|---|---|---|---|---|
| Ki67 TC% | Mean ± SD | 5.71 ± 6.79 | 13.02 ± 9.67 | 0,029 |
| Median (IQR) | 3 (1-7) | 9.5 (5.78-25) | ||
| Ki67 TP% | Mean ± SD | 6.89 ± 9.01 | 14 ± 7.07 | 0,015 |
| Median (IQR) | 3 (1-9) | 15 (7.5-20.5) | ||
| Caspase TC% | Mean ± SD | 1.96 ± 1.69 | 3 ± 0.89 | 0,014 |
| Median (IQR) | 1.3 (1-2) | 3 (2-4) | ||
| Caspase TP% | Mean ± SD | 2.75 ± 2.24 | 5.1 ± 2.53 | 0,007 |
| Median (IQR) | 2.3 (1-3.7) | 4.5 (3.45-6.25) |
TC: tumor centre, TP: tumor periphery, SD: standard deviation, IQR: interquartile range.
The Ki67 TC and Kİ67 TP immunoexpressions were compared with age, ER, PR, cerbB2 status and p53 values of the patients. We didn’t find any statistically significant correlation (P > 0.05). Also, no statistically significant correlation was observed when the caspase TC values were compared with age, cerbB2 status and p53 values of the patients (P > 0.05). However, the caspase TC values had negative correlation with ER and PR expressions (R = -0.433 and P = 0.001; R = -0.355 and P = 0.009, respectively). Only, the immunoexpression of caspase TP and ER showed statistically significant negative correlation (R = -0.299, P = 0.03). We observed no statistically significant correlation between caspase TP values and age, PR, cerbB2 and p53 values of the patients (P > 0.05) (Table 4).
Table 4.
The correlation of caspase and Ki-67 immunoexpressions with age, ER, PR, cerbB2 and p53 at the center and at the periphery of the tumor
| Ki67 TC% | Ki67 TP% | Caspase TC% | Caspase TP% | ||
|---|---|---|---|---|---|
| Age | R | -0.193 | -0.248 | -0.105 | -0.242 |
| P | 0.166 | 0.073 | 0.454 | 0.081 | |
| ER% | R | -0.198 | -0.057 | -0.433 | -0.299 |
| P | 0.156 | 0.684 | 0.001 | 0.03 | |
| PR% | R | -0.133 | -0.141 | -0.355 | -0.212 |
| P | 0.344 | 0.313 | 0.009 | 0.127 | |
| cerbB2 | R | -0.202 | -0.139 | 0.089 | -0.11 |
| P | 0.148 | 0.322 | 0.526 | 0.435 | |
| P53% | R | -0.147 | -0.136 | 0.136 | 0.057 |
| P | 0.295 | 0.331 | 0.333 | 0.687 |
TC: tumor center, TP: tumor periphery, ER: estrogen receptor, PR: progesterone receptor.
The tumor grades (I-II-III) of the patients were compared with Ki67 TC and Ki67 TP values and no statistically significant difference was observed (P = 0.123, P = 0.892, respectively). Also, the tumor grades were compared with caspase TC and caspase TP values and no statistically significant difference was found between the groups (P = 0.321, P = 0.455, respectively).
The pT stages (T1 to T4) of the patients were compared with Ki67 TC and Ki67 TP values and no statistically significant difference was found (P = 0.790, P = 0.803, respectively). The PT stages were compared with caspase TC and caspase TP values and we observed no statistically significant difference (P = 0.510, P = 0.296, respectively).
Also, the comparison of lymph node status of the patients and Ki67 TC, Ki67 TM, caspase TC and caspase TP values showed no statistically significant difference (P = 0.710, P = 0.792, P = 0.801, P = 0.845, respectively). The presence of perineural involvement within the tumors were compared with Ki67 TC, Ki67 TP, caspase TC and caspase TM immunoexpressions; no statistically significant difference was shown (P = 0.278, P = 0.277, P = 0.303, P = 0.602, respectively). The presence of lymphovascular involvement within the tumors were compared to Ki67 TC, Ki67 TP, caspase TC and caspase TM immunoexpression; we also observed no statistically significant difference (P = 0681, P = 0.638, P = 0.48, P = 0.354, respectively) (Table 5).
Table 5.
The correlation of Caspase and Ki-67 immunoexpressions with Grade, Stage, Lymphovascular Involvement and Perineural Infiltration
| Grade I | Grade II | Grade III | p | T1 | T2 | T3 | T4 | p | N0 | N1 | N2 | N3 | p | LVI (-) | LVI (+) | p | PI (-) | PI (+) | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki67 TM% | Mean ± SD | 4.38 ± 3.85 | 5.16 ± 6.13 | 9.13 ± 9.31 | 0.123 | 5.63 ± 6.89 | 7.24 ± 8.42 | 3.86 ± 1.41 | 6.05 ± 5.59 | 0.790 | 6.75 ± 6.89 | 8.58 ± 10.33 | 4.31 ± 3.41 | 2.8 ± 1.79 | 0.710 | 6.56 ± 6.6 | 6.53 ± 7.88 | 0.681 | 7.34 ± 8.17 | 4.3 ± 4.27 | 0.278 |
| Mean (IQR) | 2.5 (1.38-8.25) | 3 (1-7) | 4.75 (2.1-14.13) | 2.4 (1-9.75) | 3.2 (1.3-11.25) | 4.5 (2.4-5) | 6.05 (1.58-8) | 4 (1-11) | 3.1 (1.43-16) | 3 (1.4-7) | 3 (1-4.5) | 4 (1.5-10.5) | 3 (1.05-9.25) | 4 (1.5-11) | 2.55 (1-7.75) | ||||||
| Ki67 TP% | Mean ± SD | 4.7 ± 3.32 | 7.96 ± 10.26 | 8.55 ± 9.06 | 0.892 | 8.23 ± 11.53 | 7.52 ± 7.97 | 3.2 ± 1.64 | 8.5 ± 7.78 | 0.803 | 7.02 ± 6.52 | 10.69 ± 14.04 | 7.29 ± 7.45 | 3.5 ± 2.45 | 0.792 | 7.62 ± 7.44 | 7.73 ± 9.8 | 0.638 | 8.6 ± 9.96 | 5.15 ± 5.25 | 0.277 |
| Mean (IQR) | 4 (1.4-8.5) | 4 (2-9) | 4 (1.25-16) | 4 (1.25-9) | 4 (1.5-11) | 4 (1.5-4.5) | 8.5 (2.25-11) | 4 (2-11) | 2.8 (2-19) | 5 (1-14) | 4 (1-5.75) | 6.5 (1.5-9) | 3 (2-11) | 4 (2-11) | 3 (1-7.25) | ||||||
| Caspase TM% | Mean ± SD | 1.88 ± 1.55 | 1.77 ± 1.27 | 2.56 ± 2.02 | 0.321 | 1.89 ± 1.41 | 2.16 ± 1.85 | 2.1 ± 1.67 | 3 ± 0 | 0.510 | 1.95 ± 1.34 | 2.72 ± 2.4 | 1.77 ± 1.22 | 1.44 ± 0.44 | 0.801 | 2.12 ± 1.45 | 2.06 ± 1.75 | 0.480 | 2.28 ± 1.84 | 1.54 ± 0.7 | 0.303 |
| Mean (IQR) | 1.5 (1-2.75) | 1.3 (1-2) | 2 (1-3) | 1.35 (1-2.3) | 1.6 (1-2.55) | 1.5 (1-3.5) | 3 (2.25-2.75) | 1.7 (1-2.4) | 1.5 (1-4.25) | 1 (1-3) | 1.5 (1-1.85) | 1.7 (1-3) | 1.45 (1-2.08) | 2 (1-3) | 1.35 (1-1.78) | ||||||
| Caspase TP% | Mean ± SD | 2.81 ± 3.32 | 3.01 ± 2.6 | 3.11 ± 1.65 | 0.455 | 2.66 ± 2.44 | 2.8 ± 1.44 | 2.6 ± 1.92 | 4.5 ± 0.71 | 0.296 | 3.41 ± 2.96 | 2.65 ± 1.74 | 2.71 ± 1.38 | 2.36 ± 0.96 | 0.845 | 3.85 ± 3.63 | 2.62 ± 1.34 | 0.354 | 3.19 ± 2.65 | 2.52 ± 1.22 | 0.602 |
| Mean (IQR) | 1 (1-4.38) | 3 (1.2-3.85) | 3 (2-4) | 1.7 (1-4) | 3 (1.9-3.55) | 2 (1-4.5) | 4.5 (3-4.25) | 3 (1-4.5) | 2.5 (1-3.18) | 3 (1-4) | 2.3 (1.5-3.25) | 4 (1-5) | 2.8 (1.5-3.5) | 3 (1-4) | 2.15 (1.75-3.25) |
LVI: Lymphovascular Involvement, PI: Perineural Invasion, T: T stage, N: N stage.
Discussion
Breast cancer is a group of very heterogeneous diseases that can be demonstrated at the histological, molecular, and clinical levels [15]. Due to the differences in biological behaviors of breast cancer, a wide variety of prognostic factors have been revealed. They are: invasive carcinoma versus in situ disease, distant metastases, lymph node metastases, tumor size, locally advanced disease, and inflammatory carcinoma. Histological subtype, histological grade, estrogen and progesterone receptors, HER2/neu, lymphovascular invasion, response to neoadjuvant therapy, gene expression profiling and proliferative rate are other prognostic and predictive factors [1-8].
The degree of tumor proliferation is one of the most important prognostic factors in breast carcinoma. Proliferation can be measured by mitotic counts (as part of histological grading), immunohistochemical detection of cellular proteins produced during the cell cycle (e.g., cyclins, Ki-67), flow cytometry (as the S-phase fraction), or the thymidine-labeling index [3,9,10]. Ki-67 is a reproducible, easily assessed proliferation marker and a reliable substitute for mitotic counts [11]. Proliferation marker Ki-67 is an antibody, which is located in the nucleus and can be detected at all phases of the cell cycle except the G0 phase [16-19].
Many studies have investigated the significance of the Ki-67 proliferation index. It has been shown that the Ki-67 proliferation index increases significantly in malignant breast tumors compared to benign lesions. There is a statistically significant relationship between the risk of recurrence and death in breast carcinoma and high Ki-67 expression and a positive correlation between the Ki-67 proliferation index and tumor stage, resistance to treatment, and the Elston-Ellis histological grading system [5,11,16,20-29]. Cheang et al. determined that the Ki-67 index and HER2 expression could be used to determine the risk of recurrence and death in patients with hormone receptor positive breast carcinoma treated with tamoxifen and chemotherapy. Assessment of Ki-67 has been controversial because some studies have used 10% or 20% cut-points, whereas others dichotomized around the mean or median value [22]. This lack of consistency is due to the difficulty in determining a standard threshold in daily practice. Some authors state that choosing the cut-off point for immunohistochemistry depends on the clinical objective: if Ki-67 is used to exclude patients with slowly proliferating tumors from chemotherapeutic protocols, a cut-off of 10% will help to avoid overtreatment. In contrast, if Ki-67 is used to identify patients sensitive to chemotherapy protocols, it is preferable to set the cut-off at 25% [16,30].
The caspases constitute a family of cysteine proteases-peptidases that use a cysteine residue as the catalytic nucleophile, that share an exquisite specificity for cleaving target proteins at sites next to aspartic acid residues. The concerted action of caspases is responsible for apoptosis, a specific form of programmed cell death that is essential for embryonic development and the pathology of many diseases. In addition to apoptosis, a subgroup of the caspase family is involved in inflammation, where they act as pro-cytokine activators. The apoptotic caspases are classified as initiators or executioners, depending on their point of entry into the apoptotic cascade [33].
Apoptosis is a highly regulated cell suicide mechanism that is important for many biological processes, including embryonic development, response of tumors to cancer chemotherapy, and the pathogenesis of neurodegenerative diseases. Specific changes in nuclear architecture, such as the segmentation of the nucleus, have been the most specific cytological markers of apoptosis. These nuclear features are not always easily interpreted, especially in routinely processed paraffin sections of archival tissues [12-14,31]. The balance between programmed cell death (apoptosis) and cell proliferation determines tumor growth, and any alteration between these two may be a key element in the uncontrolled expansion of malignant tumors [32].
Numerous studies have investigated the role the apoptotic pathway plays in determining the response of solid tumors to chemotherapy. Meyn et al. reported that cyclophosphamide treatment of mice bearing the murine mammary adenocarcinoma Mca-4 increased the apoptotic index from 2.5% at baseline to more than 20% immediately after treatment. This degree of apoptosis was also found after treatment with cysplatin, doxorubicin, and ionizing radiation [25]. Other authors subsequently reported that taxanes also initiate early cell death after treatment. In an in vivo study, three of four murine mammary adenocarcinomas showed a significant increase in apoptosis over baseline (e.g., from 1.2% to 23.7%) shortly after treatment with paclitaxel. Furthermore, the baseline and paclitaxel induced levels of apoptosis statistically correlated with a delay in tumor growth, whereas the peak percentage of cells displaying mitotic arrest did not [34].
The aim of this study was to focus on the differences in cell behavior between the two locations of the tumor by immunohistochemical analysis of two very important prognostic variables of breast cancer: caspase and Ki-67. We investigated both variables in the center and the periphery of the tumor and compared them with each other and some other prognostic factors. This produced two important results: 1. It was demonstrated that both caspase and Ki-67 had a positive correlation in either the center or the periphery of the tumor. 2. There was no statistically significant difference between caspase and Ki-67 immunoexpression at the center and the periphery of the tumor, and expression of these markers did not correlate with the tumor’s histological grade, stage, lymph node metastasis, lymphovascular, and perineural invasion status.
Conclusion
The positive correlation between caspase and Ki-67 may help researchers use these variables interchangeably. It is clear that these two variables are evenly distributed in the center and the periphery of the tumor. Their correlation with the tumor grade, stage, lymph node metastasis, lymphovascular and perineural invasion also did not differ either at the center or the periphery of the tumor. Both the peripheral infiltrative areas and the center of the tumor demonstrated similar results of caspase and Ki-67 immunoexpression. It is evident that there is no need for a specific locational sampling when these two variables are considered.
Disclosure of conflict of interest
None.
References
- 1.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver M. Invasive breast carcinoma: Introduction and general features. In: Colditz G, Chia KS, editors. WHO Classification of Tumours of the Breast. 4th edion. Lyon: IARC; 2012. pp. 14–31. [Google Scholar]
- 2.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Ann Oncol. 2008;19:614–622. doi: 10.1093/annonc/mdm481. [DOI] [PubMed] [Google Scholar]
- 3.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 2007;20:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 4.Cserni G, Vörös A, Liepniece-Karele I, Bianchi S, Vezzosi V, Grabau D, Sapino A, Castellano I, Regitnig P, Foschini MP, Zolota V, Varga Z, Figueiredo P, Decker T, Focke C, Kulka J, Kaya H, Reiner-Concin A, Amendoeira I, Callagy G, Caffrey E, Wesseling J, Wells C. Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast. 2014;23:259–263. doi: 10.1016/j.breast.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Railo M, Nordling S, von Boguslawsky K, Leivonen M, Kyllönen L, von Smitten K. Prognostic value of Ki-67 immunolabelling in primary operable breast cancer. Br J Cancer. 1993;68:579–583. doi: 10.1038/bjc.1993.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;15:2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Chevallier B, Heintzmann F, Mosseri V, Dauce JP, Bastit P, Graic Y, Brunelle P, Basuyau JP, Comoz M, Asselain B. Prognostic value of estrogen and progesterone receptors in operable breast cancer. Results of a univariate and multivariate analysis. Cancer. 1988;15:2517–2524. doi: 10.1002/1097-0142(19881215)62:12<2517::aid-cncr2820621211>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF International Ki-67 in Breast Cancer Working Group. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;16:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beresford MJ, Wilson GD, Makris A. Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res. 2006;8:216. doi: 10.1186/bcr1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R, Chen HJ, Wei B, Zhang HY, Pang ZG, Zhu H, Zhang Z, Fu J, Bu H. Reproducibility of the Nottingham modification of the Scarff-Bloom-Richardson histological grading system and the complementary value of Ki-67 to this system. Chin Med J (Engl) 2010;5:1976–1982. [PubMed] [Google Scholar]
- 12.Coutinho-Camillo CM, Lourenço SV, Nishimoto IN, Kowalski LP, Soares FA. Caspase expression in oral squamous cell carcinoma. Head Neck. 2011;33:1191–1198. doi: 10.1002/hed.21602. [DOI] [PubMed] [Google Scholar]
- 13.Wyllie AH. Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992;11:95–103. doi: 10.1007/BF00048057. [DOI] [PubMed] [Google Scholar]
- 14.Sanders EJ. Methods for detecting apoptotic cells in tissues. Histol Histopathol. 1997;12:1169–1177. [PubMed] [Google Scholar]
- 15.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 16.De Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;21:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;15:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 18.Cattoretti G, Becker MH, Key G, Duchrow M, Schlüter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 19.Walker RA. Immunohistochemical markers as predictive tools for breast cancer. J Clin Pathol. 2008;61:689–696. doi: 10.1136/jcp.2006.041830. [DOI] [PubMed] [Google Scholar]
- 20.Wintzer HO, Zipfel I, Schulte-Mönting J, Hellerich U, von Kleist S. Ki-67 immunostaining in human breast tumors and its relationship to prognosis. Cancer. 1991;15:421–428. doi: 10.1002/1097-0142(19910115)67:2<421::aid-cncr2820670217>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–334. doi: 10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;20:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura R, Osako T, Okumura Y, Tashima R, Toyozumi Y, Arima N. Changes in the ER, PgR, HER2, p53 and Ki-67 biological markers between primary and recurrent breast cancer: discordance rates and prognosis. World J Surg Oncol. 2011;17:131. doi: 10.1186/1477-7819-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;23:245–262. doi: 10.1677/ERC-10-0136. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;30:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 26.Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjällskog ML, Blomqvist C. Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology. 2007;51:491–498. doi: 10.1111/j.1365-2559.2007.02798.x. [DOI] [PubMed] [Google Scholar]
- 27.De Censi A, Guerrieri-Gonzaga A, Gandini S, Serrano D, Cazzaniga M, Mora S. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22:582–587. doi: 10.1093/annonc/mdq427. [DOI] [PubMed] [Google Scholar]
- 28.Domagala W, Markiewski M, Harezga B, Dukowicz A, Osborn M. Prognostic significance of tumor cell proliferation rate as determined by the MIB-1 antibody in breast carcinoma: its relationship with vimentin and p53 protein. Clin Cancer Res. 1996;2:147–154. [PubMed] [Google Scholar]
- 29.Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors-a surrogate marker? Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 30.Spyratos F, Ferrero-Pous M, Trassard M, Hacene K, Phillips E, Tubiana-Hulin M, Le Doussal V. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94:2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 31.Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry-using antibodies to cleaved caspase 3. J Histochem Cytochem. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- 32.Chang J, Ormerod M, Powles TJ, Allred DC, Ashley SE, Dowsett M. Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma. Cancer. 2000;89:2145–2152. [PubMed] [Google Scholar]
- 33.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz TA, Davis DW, McConkey DJ, Symmans WF, Valero V, Jhingran A, Tucker SL, Pusztai L, Cristofanilli M, Esteva FJ, Hortobagyi GN, Sahin AA. Chemotherapy-induced apoptosis and Bcl-2 levels correlate with breast cancer response to chemotherapy. Cancer J. 2003;9:33–41. doi: 10.1097/00130404-200301000-00007. [DOI] [PubMed] [Google Scholar]


