Abstract
Objectives: Our previous study has shown that downregulation of FOLR1 by siRNA partially reversed taxol-resistant phenotype in taxol-resistant nasopharyngeal carcinoma cell lines. We aim to gain further insight into the molecular mechanisms of this process and identify the differentially expressed genes after FOLR1 downregulation. Method: The global gene expression profile was identified and analyzed using the Affymetrix HG-U133 Plus 2.0 array. Results: There was a significant dysregulation in the global gene expression of the FOLR1-suppressed taxol-resistant nasopharyngeal carcinoma cell lines. There were 41 upregulated genes and 109 downregulated genes. QRT-PCR validation of the selected differentially expressed genes demonstrated there was a good correlation with the microarray analysis. There was a significant deregulation of expression in the apoptosis-related genes such as BIRC3, PRKX, TNFRSF10A and involved in Viral carcinogenesis, MAPK signaling pathways after FOLR1 was downregulated. Conclusion: The suppression of FOLR1 by RNA interference altered gene expression profile of taxol-resistant nasopharyngeal carcinoma cell lines. The apoptosis-related genes and the gene alterations in viral carcinogenesis, MAPK signaling pathways might be important in FOLR1 siRNA-induced taxol-resistant reversal.
Keywords: Gene expression profiling, FOLR1, taxol resistance, nasopharyngeal carcinoma, signaling pathway, small interfering RNA
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most important head and neck cancers in China. Taxol is one of the widely used chemotherapeutic drugs against NPC [1-3]. Despite its initial effectiveness as a cancer therapeutic agent, in many cases, treatment failure often occurs due to development of acquired resistance. Therefore, it is important to understand the molecular mechanisms responsible for the development of drug resistance.
In a previous study, we reported that higher FOLR1 expression plays an important role in taxol resistance, and silencing FOLR1 expression partially reverses taxol-resistant phenotype [4], the precise mechanism is different from the role of FOLR1 in methotrexate resistance in melanoma and KB cells [5], because the FOLR1 protein transports methotrexate but not taxol.
The signal process of FOLR1 on taxol resistance remains to be determined. In the present study, we further analyzed the global gene expression profiles in CNE-1/taxol, 5-8F/taxol and HNE-2/taxol cells after FOLR1-siRNA treatment.
Materials and methods
Cell cultures
The taxol-resistant cell lines CNE-1/Taxol, 5-8F/Taxol and HNE-2/Taxol were stablished previously in our laboratory by exposing parental cells to gradually increasing concentrations of taxol [6]. The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C for 48 h before use.
RNA interference for inhibiting FOLR1 expression in taxol-resistant NPC cells
FOLR1-specific siRNA (sense: AUAUAGGUAGGAAACAUCCUUAUGG; antisense: CCAUAAGGAUGUUUCCUACCUAUAU), and the corresponding nonsilencing negative control siRNA were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
The CNE-1/Taxol, 5-8F/Taxol and HNE-2/Taxol cells were grown in RPMI media and plated at 2×105 cells/well in 6-well plates. The cells were incubated in medium without antibiotics for 16 hours and then replaced with OPTM 1 medium without serum for 1 hour. A mixture of 200 nM siRNA (FOLR1) and HiPerfect transfection reagent (Qiagen, Valencia, CA) was added to each well for 4 hours, after which RPMI media containing 10% serum were added. A transfection of negative siRNA served as a negative control. Cells were harvested for RNA isolation and purification 24 hours later, and the silencing of the FOLR1 gene was examined.
Quantitative real-time PCR
Real-time PCR was performed using a SYBR-green-containing PCR kit (GenePharma, Shanghai, China). The primers for qRT-PCR to detect mRNA were synthesized and purified by Shanghai GenePharma. The primers of FOLR1 were: 5’-GAATGCCTGCTGTTCTACCA-3’ (forward); and 5’-CCACCCTTCTAACAGCGT-3’ (reverse). The primers of β-actin, used as internal control, were: 5’-CAGAGCCTCGCCTTTGCCGA-3’ (forward); and 5’-ACGCCCTGGTGCCTGGGGCG-3’ (reverse). All RT-PCR was performed on the BIO-RAD IQ5 Multicolor Real-time PCR detection System (Bio-Rad, Hercules, CA). The 2–∆∆CT method was used to calculate the relative expression of mRNA.
Microarray hybridization and analysis
cDNA microarray was hybridized using HG-U133 Plus 2.0 array (Affymetrix, Santa Clara, CA), and the array slides scanned with a GeneChip Scanner 3000 (Affymetrix, Santa Clara, CA, US); the raw data were normalized by the MAS 5.0 algorithm using the GeneSpring Software version 11.0 (Agilent technologies, Santa Clara, CA, US).
Statistical analysis
All experiments were performed at least 3 times. Data are means ± SD. Statistical comparisons were performed with the Student t test. A P value of less than .05 was considered statistically significant.
Results
Global gene expression profile in FOLR1 siRNA-treated taxol-resistant NPC cells
Global gene expression was analyzed by comparing the transcriptome profiles of the FOLR1 siRNA- and negative siRNA-treated cells (CNE-1/Taxol, 5-8F/Taxol and HNE-2/Taxol). The microarray analysis showed that the FOLR1 was downregulated 54.89-fold in the CNE-1/Taxol cells, 3.58-fold in the 5-8F/Taxol cells, and 11.15-fold in the HNE2/Taxol cells.
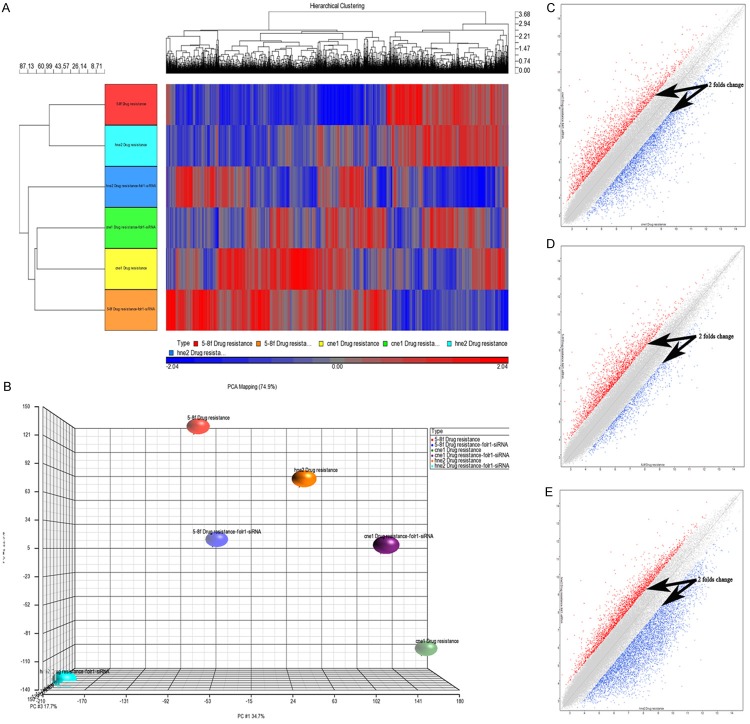
Microarray analysis of CNE-1/Taxol, 5–8F/Taxol and HNE-2/Taxol cells show that 150 differentially expressed genes out of 54,614 probes had >;2-fold changes in expression between FOLR1 siRNA-transfected and control cells, including 41 genes upregulated and 109 genes downregulated. Hierarchical clustering was analyzed to distinguish arrays with similar expression patterns and for visualization using a color scale. There was an obvious difference in expression patterns of genes between the FOLR1 siRNA and negative siRNA treatment cells (Figure 1A). Principal component analysis indicated that the changes in the taxol-resistant NPC cells gene expression profile could be accounted for primarily by the FOLR1 siRNA treatment (Figure 1B). Two-dimensional scatterplot analysis of gene expression values for all genes on the FOLR1 siRNA-transfected cells and control cells from microarray are shown in Figure 1C-E.
Figure 1.
Results of the gene chip microarray analysis. A. Visual display of the cluster analysis for the FOLR1 siRNA transfected and control cells. B. Principal component analysis. The closer the dots, the more similar the gene expression profiles are; the farther apart the dots, the greater the difference is. C-E. Two-dimensional scatter plot analysis of gene expression values for all genes on the FOLR1 siRNA-transfected CNE-1/taxol, 5-8F/taxol, HNE-2/taxol cells and control cells. Dots outside the 2× difference lines, indicated by black arrows, represent differentially expressed genes. Red dots represent genes upregulated with a >;2-fold difference, blue dots represent genes downregulated with a >;2-fold difference.
Pathway analysis of the differentially expressed genes
By mapping the differentially expressed genes in CNE-1/Taxol, 5-8F/Taxol and HNE-2/Taxol cells to KEGG pathways, the results showed that differentially expressed genes mainly concentrated on 28 kinds of signaling pathways. Our interest was focused mainly on the genes associated with apoptosis, viral carcinogenesis and involved in MAPK signaling pathway. The differentially expressed genes related to apoptosis, viral carcinogenesis and the MAPK signaling pathway are shown in Table 1.
Table 1.
The differentially expressed genes related to apoptosis, viral carcinogenesis and from MAPK signaling pathway in CNE-1/Taxol, 5-8F/Taxol and HNE-2/Taxol cells of FOLR1 downregulation
| Gene symbol | Access | Gene description | Fold change (CNE-1/Taxol) | Fold change (5-8F/Taxol) | Fold change (HNE-2/Taxol) |
|---|---|---|---|---|---|
| Apoptosis-related genes | |||||
| BIRC3 | NM_001165 | baculoviral IAP repeat-containing 3 | -2.25 | -2.23 | -7.83 |
| IL1A | NM_000575 | interleukin 1, alpha | -9.25 | -6.41 | -4.37 |
| PRKX | NM_005044 | protein kinase, X-linked | -2.08 | -4.60 | -5.67 |
| TNFRSF10A | NM_003844 | tumor necrosis factor receptor superfamily, member 10a | 2.06 | 2.27 | 5.48 |
| Viral carcinogenesis | |||||
| C3 | NM_000064 | complement component 3 | -4.48 | -3.72 | -3.35 |
| EGR2 | NM_000399 | early growth response 2 | -6.79 | -2.27 | -3.80 |
| IL6ST | NM_001190981 | interleukin 6 signal transducer | -2.10 | -3.23 | -13.41 |
| NRAS | NM_002524 | neuroblastoma RAS viral (v-ras) oncogene homolog | -2.03 | -2.84 | -6.09 |
| PRKX | NM_005044 | protein kinase, X-linked | -2.08 | -4.60 | -5.67 |
| MAPK signaling pathway | |||||
| DUSP1 | NM_004417 | dual specificity phosphatase 1 | -10.32 | -3.55 | -4.42 |
| FOS | NM_005252 | FBJ murine osteosarcoma viral oncogene homolog | -26.81 | -6.26 | -11.10 |
| HSPA1A | NM_005345 | heat shock 70 kDa protein 1A | 10.00 | 2.03 | 5.05 |
| IL1A | NM_000575 | interleukin 1, alpha | -9.25 | -6.41 | -4.37 |
| NRAS | NM_002524 | neuroblastoma RAS viral (v-ras) oncogene homolog | -2.03 | -2.84 | -6.09 |
| PDGFA | NM_002607 | platelet-derived growth factor alpha polypeptide | 2.07 | 5.33 | 6.31 |
| PRKX | NM_005044 | protein kinase, X-linked | -2.08 | -4.60 | -5.67 |
Validation of the differentially expressed genes by quantitative real-time PCR
To validate the microarray data, we focused on genes related to apoptosis, viral carcinogenesis and involved in MAPK signaling pathway. Six differentially expressed genes, which might contribute to FOLR-siRNA induced taxol-resistant reversal, were selected and measured using quantitative PCR. The fold change ratios were determined between the FOLR siRNA- and negative siRNA-treated cells. The real-time PCR expression pattern of the 6 selected differentially expressed genes listed in Table 2 was in agreement with the microarray analysis (Figure 2).
Table 2.
Primer sequences for real-time polymerase chain reaction
| Gene symbol | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| BIRC3 | TACTGTATTAGCGAAAGGAA | CAATGTCATCTGTGGGAAG |
| PRKX | GGACTTTGGGTTCGCCAAGA | TTCCGTGGCCCTTGCTCT |
| TNFRSF10A | TCCAGCAAATGGTGCTGAC | GAGTCAAAGGGCACGATGTT |
| FOS | CCAACCTGCTGAAGGAGAAG | AGATCAAGGGAAGCCACAGA |
| DUSP1 | TGTGGGCAACATTCCTGTAA | CAAAGGATGGCACAGGATTT |
| EGR2 | TCGGATCTGCATGCGAAACT | TCGGATCTGCATGCGAAACT |
Figure 2.
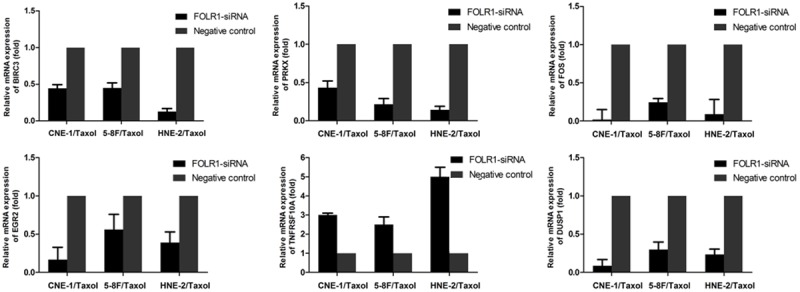
Validation of different genes in the FOLR1 siRNA and negative control groups by qRT-PCR. Quantitative RT-PCR analysis of 6 genes in FOLR1 siRNA-transfected taxol-resistant NPC cells compared with negative control cells. β-actin was used as a reference gene. The graphs depict the mean mRNA expression changes ± the s.d. of three independent amplifications.
Discussion
Gene expression profiling of FOLR1 siRNA-treated CNE-1/Taxol, HNE-2/Taxol and 5-8F/Taxol cells compared with negative siRNA-treated cells showed that there was a significant dysregulation in the global gene expression of the FOLR1-suppressed cells. Of the 6 differentially expressed genes related to apoptosis, viral carcinogenesis and involved in MAPK signaling pathway 100% showed concordance between the microarray data and the real-time RT-PCR data.
The BIRC3 (cIAP2), a member of the IAP family of apoptosis inhibitors, may enhance cancer cell survival have been discussed, BIRC3 may block apoptosis by impeding caspase-8 dependent autocrine TNF-α signaling [7,8]. Another research about platinum-resistance in ovarian cancer cells reported that cIAP2 inhibition significantly sensitized ovarian carcinoma cells to cisplatin, but the exact molecular mechanism was unclear [9]. We found that BIRC3 was all downregulated in the three FOLR1 siRNA- treated taxol-resistant NPC cells, but the transcript level of caspase-8 was not consistent in the three cell lines (-1.75-fold downregulation in CNE-1/Taxol cells, 2.86-fold upregulation in 5-8F/Taxol cells and 2.71-fold upregulation in HNE-2/Taxol cells). Interestingly, caspase-2 was all upregulated in our study (3.30-fold in CNE-1/Taxol cells, 2.37-fold in 5-8F/Taxol cells and 2.81-fold in HNE-2/Taxol cells).
Claus Bender [10] reported that downregulation of PRKX using siRNA result in an enhanced therapeutic effect of Sunitinib in parental- and a strikingly increased effect in Sunitinib resistant-cell lines. Our microarray analysis showed that the PRKX was all downregulated in the three FOLR1 siRNA- treated taxol-resistant NPC cells, suggesting that PRKX was involved in FOLR1 siRNA induced taxol-resistant reversal. However the exact molecular mechanism remains unknown.
TNFRSF10A (also known as DR4, TRAIL-R1) is a transmembrane receptor that mediates TRAIL-induced apoptosis. TERRI B. HUNTER [11] reported that DR4’ ligand increased small cell lung cancer killing by paclitaxel through an apparent caspase-independent route involving activation/translocation of apoptosis inducing factor (AIF). Another study showed that retinoids sensitized cancer cells to TRAIL-induced apoptosis by upregulating expression of TRAIL-R1 [12]. Li et al [13] reported that patients with bladder cancer with either high DR4 or DR5 expression might benefit from epirubicin therapy and had a significantly longer postoperative recurrence-free rate than those with low expression. The upregulation of TNFRSF10A in our data suggesting that TNFRSF10A is involved in FOLR1 siRNA induced-apoptosis.
The FOS, also known as c-fos, of the classic MAPK signaling pathway was downregulated in our study. Blocking c-fos expression by small interfering RNA in human colon carcinoma cells was found to lead to a significant reduction in transforming growth factor β1 and as a consequence reduced tumor cell growth [14]. Strong FOS expression is also highly correlated with poor response to chemotherapy [15]. So, the downregulation of FOS following FOLR siRNA may play a role in the reversal of taxol resistance.
Mitogen-activated protein kinase phosphatases (MKPs), also known as dual-specificity phosphatases, regulate the activity of MAPKs (ERK, JNK and p38) by dephosphorylating both threonine and tyrosine residues and therefore deactivating them [16]. The MKP family is composed of 12 members, of which MKP1 (also referred to as DUSP1) is located in the nucleus and regulates the three MAPKs with different substrate preferences based on cell type and context [16,17]. C Montagut et al and others are providing increasing evidence for a role of MKP1 in acquisition of resistance to anti-cancer drugs; the molecular mechanism underlying MKP1-mediated resistance is in part due to the activation of JNK-driven apoptosis by several anti-tumor agents, such as taxanes, cisplatin, proteasome inhibitors and more recently anti-EGFR (epidermal growth factor receptor) drugs, high levels of MKP1 inhibit JNK-mediated apoptosis and counterbalance the cytotoxic effects of such drugs, leading to resistance to anti-tumor drugs [18-20]. The expression level of DUSP1 in our data is downregulated, suggesting that the FOLR1-siRNA induced apoptosis in taxol-resistant NPC cells is mediated by downregulating the DUSP1. More research is needed to verify the mechanism of DUSP1 in taxol resistance of NPC.
EGR2, which encodes a zinc finger transcription factor, belongs to EGR family. Recent researches revealed that the roles of EGR2 in various cell types are different. A study of Abhishek Chandra [21] about osteoprogenitor cell line reveals that the EGFR signaling activate the MAPK/ERK pathways to stimulate the expression of EGR2, which in turn lead to cell growth, and knocking down the expression of EGR2 attenuate the cell growth. Another research reported that macrophage colony-stimulating factor (M-CSF) could activate MEK/ERK and induces MEK-dependent expression of the immediate early gene EGR2, inhibition of either MEK1/2 or inhibition of EGR2 increased osteoclast apoptosis [22]. But in some other researches, the EGR2 serves as a pro-apoptotic gene which can be regulated by p53 in HCT116 cells [23] or miR-150 in gastric adenocarcinoma cell lines [24]. Whether downregulation of EGR2 contribute to the FOLR1 siRNA-induced apoptosis or not, further investigation is needed to verify that.
In conclusion, the gene expression profile in taxol-resistant NPC cells of FOLR1 downregulation is shown. Apoptosis-related genes BIRC3, PRKX and TNFRSF10A, and alterations in the viral carcinogenesis, MAPK signaling pathways may be important during FOLR1 siRNA-induced taxol-resistant reversal. These findings provide information useful for combination targeted therapy in NPC. We need to identify the exact molecular mechanism for FOLR1 involved in taxol-resistance of NPC.
Acknowledgements
This study was supported by: the Hunan Provincial Innovation Foundation for Postgraduate (China) (grant number CX2013B125); the Nature Science Foundation of Hunan Province (China) (grant number 12JJ2053); the Science and Technology Project of Hunan Province (China) (grant number S2013F1023); and the Doctoral Foundation of Education Department (China) (grant number 20110162110023).
Disclosure of conflict of interest
None.
References
- 1.Leong SS, Wee J, Rajan S, Toh CK, Lim WT, Hee SW, Tay MH, Poon D, Tan EH. Triplet combination of gemcitabine, paclitaxel, and carboplatin followed by maintenance 5-fluorouracil and folinic acid in patients with metastatic nasopharyngeal carcinoma. Cancer. 2008;113:1332–1337. doi: 10.1002/cncr.23687. [DOI] [PubMed] [Google Scholar]
- 2.Hussain M, Gadgeel S, Kucuk O, Du W, Salwen W, Ensley J. Paclitaxel, cisplatin and 5-fuorouracil for patients with advanced or recurrent squamous cell carcinoma of the head and neck. Cancer. 1999;86:2364–2369. [PubMed] [Google Scholar]
- 3.Tan EH, Khoo KS, Wee J, Fong KW, Lee KS, Lee KM. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol. 1999;10:235–237. doi: 10.1023/a:1008390929826. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Tan G, Ma Y, Li H, He G. Inhibition of α folate receptor resulting in a reversal of taxol resistance in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2012;146:250–8. doi: 10.1177/0194599811426260. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-del-Campo L, Montenegro MF, Cabezas-Herrera J, Rodríguez-López JN. The critical role of alpha-folate receptor in the resistance of melanoma to methotrexate. Pigment Cell melanoma Res. 2009;22:588–600. doi: 10.1111/j.1755-148X.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- 6.Peng X, Li W, Tan G. Reversal of taxol resistance by cisplatin in nasopharyngeal carcinoma by upregulation thromspondin-1 expression. Anticancer Drugs. 2010;21:381–8. doi: 10.1097/CAD.0b013e3283363980. [DOI] [PubMed] [Google Scholar]
- 7.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Bruchim I, Graiver D, Evron Z, Oron-Karni V, Pasmanik-Chor M, Eitan R, Bernheim J, Levavi H, Fishman A, Flescher E. Platinum-resistance in ovarian cancer cells is mediated by IL-6 secretion via the increased expression of its target cIAP-2. J Mol Med (Berl) 2013;91:357–68. doi: 10.1007/s00109-012-0946-4. [DOI] [PubMed] [Google Scholar]
- 10.Bender C, Ullrich A. PRKX, TTBK2 and RSK4 expression causes Sunitinib resistance in kidney carcinoma- and melanoma-cell lines. Int J Cancer. 2012;131:E45–55. doi: 10.1002/ijc.26486. [DOI] [PubMed] [Google Scholar]
- 11.Hunter TB, Manimala NJ, Luddy KA, Catlin T, Antonia SJ. Paclitaxel and TRAIL Synergize to Kill Paclitaxel-resistant Small Cell Lung Cancer Cells through a Caspase-independent Me- chanism Mediated through AIF. Anticancer Res. 2011;31:3193–204. [PMC free article] [PubMed] [Google Scholar]
- 12.Dhandapani L, Yue P, Ramalingam SS, Khuri FR, Sun SY. Retinoic acid enhances TRAIL induced apoptosis in cancer cells by upregulating TRAIL receptor 1 expression. Cancer Res. 2011;71:5245–54. doi: 10.1158/0008-5472.CAN-10-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Jin X, Li J, Jin X, Yu J, Sun X, Chu Y, Xu C, Li X, Wang X, Kakehi Y, Wu X. Expression of TRAIL, DR4, and DR5 in Bladder Cancer: Correlation With Response to Adjuvant Therapy and Implications of Prognosis. Urology. 2012;79:968, e7–15. doi: 10.1016/j.urology.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Pandey MK, Liu G, Cooper TK, Mulder KM. Knockdown of c-Fos suppresses the growth of human colon carcinoma cells in athymic mice. Int J Cancer. 2012;130:213–22. doi: 10.1002/ijc.25997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakar S, Mihalov M, Chachlani NA, Ghosh L, Johnstone H. Correlation of c-fos, p53 and PCNA expression with treatment outcome in osteosarcoma. J Surg Oncol. 2000;73:125–6. doi: 10.1002/(sici)1096-9098(200002)73:2<125::aid-jso14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–61. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 17.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 18.Workman P, de Bono J. Targeted therepeutics for cancer treatment: major progress towards personalized molecular medicine. Curr Opin Pharmacol. 2008;8:359–62. doi: 10.1016/j.coph.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Rojo F, González-Navarrete I, Bragado R, Dalmases A, Menéndez S, Cortes-Sempere M, Suárez C, Oliva C, Servitja S, Rodriguez-Fanjul V, Sánchez-Pérez I, Campas C, Corominas JM, Tusquets I, Bellosillo B, Serrano S, Perona R, Rovira A, Albanell J. Mitogen-activated protein kinase phosphatase-1 in human breast cancer independently predicts prognosis and is repressed by doxorubicin. Clin Cancer Res. 2009;15:3530–9. doi: 10.1158/1078-0432.CCR-08-2070. [DOI] [PubMed] [Google Scholar]
- 20.Montagut C, Iglesias M, Arumi M, Bellosillo B, Gallen M, Martinez-Fernandez A, Martinez-Aviles L, Cañadas I, Dalmases A, Moragon E, Lema L, Serrano S, Rovira A, Rojo F, Bellmunt J, Albanell J. Mitogen-activated protein kinase phosphatase-1(MKP-1) impairs the response to anti-epidermal growth factorreceptor (EGFR) antibody cetuximab in metastatic colorectal cancer patients. Br J Cancer. 2010;102:1137–44. doi: 10.1038/sj.bjc.6605612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Lan S, Zhu J, Siclari VA, Qin L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J Biol Chem. 2013;288:20488–98. doi: 10.1074/jbc.M112.447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley EW, Ruan MM, Oursler MJ. Novel pro-survival functions of the Kruppel-like transcription factor Egr2 in promotion of macrophage colony-stimulating factor-mediated osteoclast survival downstream of the MEK/ERK pathway. J Biol Chem. 2008;283:8055–64. doi: 10.1074/jbc.M709500200. [DOI] [PubMed] [Google Scholar]
- 23.Yokota I, Sasaki Y, Kashima L, Idogawa M, Tokino T. Identification and characterization of early growth response 2, a zinc-finger transcription factor, as a p53-regulated proapoptotic gene. Int J Oncol. 2010;37:1407–16. doi: 10.3892/ijo_00000792. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K, Ren G, Su T, Pan Y, Feng B, Xue Z, Wang X, Fan D. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun. 2010;392:340–5. doi: 10.1016/j.bbrc.2009.12.182. [DOI] [PubMed] [Google Scholar]