Abstract
Polymorphisms of apolipoprotein B (ApoB), apolipoprotein A1 (ApoA1) gene and ApoB/ApoA1 Ratio were associated with lipid metabolism disorders in previous reports. The aim of this study assess whether variation of ApoB, ApoA1 gene are associated or not with alcohol-induced osteonecrosis of femoral head (ONFH). In a case-control study, we genotyped 4 single-nucleotide polymorphisms (SNPs) in ApoB and ApoA1 genes in 209 alcohol-induced ONFH patients and 300 healthy control subjects in Han Chinese population using χ2 test and genetic model analysis. The analysis revealed that the frequencies of ApoB and ApoA1 genotypes were significantly different in alcohol-induced ONFH patients than in controls. We identified rs1042034, rs676210 and rs673548 in ApoB gene were associated with decreased risk of alcohol-induced ONFH using recessive model analysis (odds ratio [OR], 0.44; 95% confidence interval [CI], 0.19-0.99; P = 0.042), the OR, CI, P value of three SNPs were the same after adjusted for gender + age. We also identified rs632153 in ApoA1 gene was associated with increased risk of alcohol-induced ONFH using allele model (OR, 1.83; 95% CI, 1.16-2.88; P = 0.008) and log-additive model (adjusted OR, 1.77; 95% CI, 1.00-3.14; P = 0.046), analysis respectively. Haplotype analysis demonstrated no difference between ApoB and alcohol-induced ONFH. Polymorphisms of the ApoB and ApoA1 gene are associated with alcohol-induced ONFH in the Han Chinese population.
Keywords: ApoB, ApoA1, alcohol, osteonecrosis of femoral head, gene polymorphisms, case-control study
Introduction
Osteonecrosis of the femoral head (ONFH) is a devastating bone disease in which patients experience progressive collapse of the femoral head caused by anomalies in the fibrinolytic system and a disturbance in the supply of blood [1]. It is assumed that the whole population of osteonecrosis is about 7 million people and reached from 150 to 200 thousand new cases of ONFH are annually found in China [2]. Osteonecrosis is divided into traumatic and non-traumatic one. The etiologies of non-traumatic osteonecrosis are numerous and some of common associations and the well accepted include corticosteroid use, alcohol abuse, systemic lupus erythematous, haemoglobinopathies, bone marrow transplantations, and chemotherapy [3-5].
One of the possible causes of non-traumatic ONFH is occlusion of vessels responsible for blood supply of the femoral head because of lipid metabolism abnormality [6]. Heavy alcohol intaking and excessive use of corticosteroids may result in lipid metabolism abnormality in general populations [7]. Apolipoproteins B (ApoB) and apolipoproteins A1 (ApoA1) are the major structural and functional protein constituents of the high density lipoprotein (HDL) and triglyceride-rich lipoproteins, respectively. When considering vascular pathology, ApoB and ApoA1 are important factors because elevated blood ApoB and ApoA1 levels reflect the status of enhanced lipid transport to the peripheries, including bone tissue [8,9].
Studies showed that the incidence of coronary heart disease was associated with abnormal ApoB and ApoA1 levels resulting from polymorphisms of ApoB and ApoA1 gene [10]. Previous studies indicated that rs1042034, rs676210 and rs673548 in ApoB and rs632153 in ApoA1 gene were associated with lipid metabolism abnormality related diseases, such as cerebrovascular disease events, acute lung injury and atherosclerosis [11-13]. However, the relationship between ApoB, ApoA1 gene and alcohol-induced ONFH has been less systematically studied. The aim of this study to investigate possible correlations between polymorphisms of ApoB and ApoA1 genes and alcohol-induced ONFH in Han Chinese population.
Materials and methods
Ethics statement
This protocol was adhered to the principles of the Declaration of Helsinki and was ratified by the Ethical Committee of Zhengzhou Traditional Chinese Medicine Traumatology Hospital. All candidate subjects signed informed consent.
Study population
A total of 209 unrelated patients with alcohol-induced ONFH and 300 control subjects were consecutively enrolled at Zhengzhou Traditional Chinese Medicine Traumatology Hospital from June 2014 to March 2015. Alcohol-induced ONFH was defined by the consumption of more than 400 ml of pure ethanol per week [14]. ONFH was diagnosed by clinical examination and radiographic analysis. Anteroposterior (AP) and frog view X-rays of both hips were done in all of the patients. Magnetic resonance imaging (MRI) was performed to confirm the diagnosis of ONFH in patients without X-ray changes. ONFH was present in one hip in 53 patients and in both hips in 156 patients (312 hips). Exclusion criteria: (i) Primary disease in serious condition which required hormone replacement therapy. (ii) Drugs use which can affect the liver enzyme and lipid metabolism in patients. (iii) It did not meet the diagnostic criteria of osteonecrosis or patients with traumatic osteonecrosis and other hip diseases. (iv) Those who did not agree to participate in this study.
The control group consisted of 300 healthy individuals selected between July 2014 and March 2015 based on medical examination at Zhengzhou Traditional Chinese Medicine Traumatology Hospital. They did not have osteonecrosis and other related diseases. The controls were all Han Chinese, and lived in Zhengzhou city or the surrounding area. Subjects with excessive use of corticosteroids, alcohol consumption, chronic diseases of kidney, heart, liver and brain were excluded.
Genotyping
Genomic DNA was extracted from whole blood using an extraction kit (GoldMag, China) and stored at -20°C. DNA concentration was measured by spectrometry (DU530 UV/VIS spectrophotometer, Beckman Instruments, Fullerton, CA, USA). Using public databases (dbSNP; http://www.ncbi.nlm.nih.gov/SNP/, HAPMAP; http://www.Hapmap.org/index.html.en), a total of 4 single nucleotide polymorphisms (SNPs) in the ApoB and ApoA1 gene were selected on the basis of their location, allele frequencies, and disease relevance. We designed Multiplexed SNP Mass EXTEND assay by Sequenom MassARRAY Assay Design 4.0 Software [15]. The Sequenom MassARRAY RS1000 uses a standard protocol to make recommendations for each SNP [16]. Data analyses and management were conducted by Sequenom Typer 4.0 Software [17].
Statistical analysis
Statistical analyses were performed using Microsoft Excel and SPSS 16.0 statistical packages (SPSS, Chicago, IL). All p values in this study were two-sided. A P < 0.05 was considered the threshold for statistical significance. The genotype frequencies of each SNP were checked using the Hardy-Weinberg equilibrium (HWE) in control subjects. The genotype frequencies of cases and controls were calculated using χ2 test [18,19]. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were determined using unconditional logistic regression analysis with adjustment for gender and age [20]. The four genetic models (Dominant, Recessive, Codominant and Log-additive) were applied using PLINK software (http://pngu.mgh.harvard.edu/purcell/plink/) to assess the association of SNP with the risk of alcohol-induced ONFH. Finally, we performed haplotype interaction analysis on the genes containing the significant SNPs. The linkage disequilibrium structure was examined using Haploview 4.2.
Transcriptional prediction
We used the PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) to predict protein function of non-synonymous SNPs (nsSNPs) in ApoB gene coding regions. Each variant was given a score based on the impact of its mutation on protein function. PolyPhen-2 results were divided into five categories: probably benign (0.000-0.999), borderline (1.000-1.249), potentially damaging (1.250-1.449), possibly damaging (1.500-1.999) and probably damaging (≥ 2.000).
Results
The primers of the four selected SNPs are shown in Table 1, which were designed by Sequenom MassARRAY Assay Design 4.0 Software [15]. Four SNPs in the two genes were analyzed in this study. Chromosomal position, gene, role, allele A/B, odds ratio (OR), 95 % confidence interval (95% CI), p value and call rate (%) are shown in Table 2. The rs632153 was associated with increased risk of alcohol-induced ONFH risk by allele model analysis (OR, 1.83; 95% CI, 1.16-2.88; P = 0.008). Average call rate of SNPs was 99.63% (range from 98.31% to 100%). The genotype distribution was in Hardy-Weinberg equilibrium in control group.
Table 1.
Primers used for this study
| SNP ID | 1st-PCR primer sequences | 2nd-PCR primer sequences | UEP sequences |
|---|---|---|---|
| rs1042034 | ACGTTGGATGATGAAGATTAAGGCATAGG | ACGTTGGATGATCCAAGATGAGATCAACAC | ATGAGATCAACACAATCTTCA |
| rs676210 | ACGTTGGATGATAGCTTGCCAAAAGTAGG | ACGTTGGATGTTTTCAAGTTCCTGACCTTC | AGTTCCTGACCTTCACATAC |
| rs673548 | ACGTTGGATGCTTTCAGTGCATTGTCCAG | ACGTTGGATGAAGAGCAATGAACATTAGGC | agGAACATTAGGCAAAAATACC |
| rs632153 | ACGTTGGATGAGGGACATGAGCAACCCTTC | ACGTTGGATGAGCTGTGCTCCTGGAGGCTG | AGGCTGCAGGGAAAAAT |
SNP, single-nucleotide polymorphism; PCR, polymorphism chain reaction; UEP, unextended mini-sequencing primer.
Table 2.
Basic SNP summary of all study participants
| SNP ID | Chromosome | Position | Gene | Role | Alleles A/B | OR (95% CI) | P value | Call rate (%) | |
|---|---|---|---|---|---|---|---|---|---|
| rs1042034 | 2 | 21225281 | ApoB | Exon 30 | T/C | 0.84 (0.63-1.12) | 0.245 | 99.31 | |
| rs676210 | 2 | 21231524 | ApoB | Exon 28 | G/A | 0.83 (0.62-1.11) | 0.245 | 99.45 | |
| rs673548 | 2 | 21237544 | ApoB | Intron | G/A | 0.84 (0.63-1.11) | 0.222 | 100.00 | |
| rs632153 | 11 | 116710239 | ApoA1 | Promoter | T/G | 1.83 (1.16-2.88) | 0.008* | 99.76 |
SNP, single-nucleotide polymorphism; A/B, minor/major alleles. OR, odds ratio; 95% CI, 95% confidence interval.
The minor allele of each SNP was assumed to be a risk factor and their minor alleles frequencies (MAF) were listed in Table 3. We further analyzed the association of SNPs and alcohol-induced ONFH risk using logistic test including dominant model, recessive model, codominant model and log-additive model. The genotype “T/T” in rs1042034 decreased alcohol-induced ONFH risk was revealed by the recessive model (crude OR, 0.45; 95% CI, 0.22-0.95; P = 0.027; adjusted for gender + age OR, 0.44; 95% CI, 0.19-0.99; P = 0.042). The genotype “G/G” in rs676210 decreased alcohol-induced ONFH risk was revealed by the recessive model (crude OR, 0.45; 95% CI, 0.22-0.94; P = 0.026; adjusted for gender + age OR, 0.44; 95% CI, 0.19-0.99; P = 0.042). The genotype “G/G” in rs673548 decreased alcohol-induced ONFH risk was revealed by the recessive model (crude OR, 0.45; 95% CI, 0.22-0.95; P = 0.027; adjusted for gender + age OR, 0.44; 95% CI, 0.19-0.99; P = 0.042). However, the genotype “G/T-T/T” of rs632153 increased the risk of alcohol-induced ONFH was shown by the dominant model (crude OR, 1.95; 95% CI, 1.20-3.15; P = 0.0065) and the genotype “G/T” of rs632153 increased the risk of alcohol-induced ONFH was shown by the codominant model (crude OR, 1.96; 95% CI, 1.20-3.19; P = 0.024). By adjusted for gender + age, the genotype “G/T-T/T” and “G/T” of rs632153 are no significant difference in dominant model and codominant model. The rs632153 increased risk of alcohol-induced ONFH was revealed by log-additive model (crude OR, 1.88; 95% CI, 1.18-2.99; P = 0.008; adjusted for gender + age OR, 1.77; 95% CI, 1.00-3.14; P = 0.046).
Table 3.
Association of SNPs with alcohol-induced osteonecrosis of femoral head risk based on logistical tests
| SNP ID | MAF case | MAF control | Model | Genotype | OR (95% CI) | p value | OR (96% CI)** | p value** |
|---|---|---|---|---|---|---|---|---|
| rs1042034 | 0.239 | 0.272 | Dominant | T/C-T/T | 0.95 (0.67-1.36) | 0.78 | 1.20 (0.79-1.84) | 0.39 |
| Recessive | T/T | 0.45 (0.22-0.95) | 0.027* | 0.44 (0.19-0.99) | 0.042* | |||
| Codominant | T/T | 0.47 (0.22-0.99) | 0.078 | 0.50 (0.22-1.17) | 0.033 | |||
| Log-additive | --- | 0.85 (0.64-1.13) | 0.26 | 0.97 (0.70-1.34) | 0.85 | |||
| rs676210 | 0.239 | 0.274 | Dominant | G/A-G/G | 0.93 (0.65-1.33) | 0.7 | 1.20(0.79-1.84) | 0.4 |
| Recessive | G/G | 0.45 (0.22-0.94) | 0.026* | 0.44 (0.19-0.99) | 0.042* | |||
| Codominant | G/G | 0.46 (0.22-0.98) | 0.08 | 0.50 (0.22-1.17) | 0.033 | |||
| Log-additive | --- | 0.84 (0.63-1.11) | 0.22 | 0.97 (0.70-1.34) | 0.84 | |||
| rs673548 | 0.239 | 0.273 | Dominant | A/G-G/G | 0.94 (0.66-1.34) | 0.72 | 1.19 (0.78-1.83) | 0.41 |
| Recessive | G/G | 0.45 (0.22-0.95) | 0.027* | 0.44 (0.19-0.99) | 0.042* | |||
| Codominant | G/G | 0.46 (0.22-0.99) | 0.081 | 0.50 (0.22-1.16) | 0.035 | |||
| Log-additive | --- | 0.84 (0.64-1.12) | 0.23 | 0.96 (0.69-1.34) | 0.83 | |||
| rs632153 | 0.108 | 0.062 | Dominant | G/T-T/T | 1.95 (1.20-3.15) | 0.0065* | 1.76 (0.99-3.16) | 0.053 |
| Recessive | T/T | 1.43 (0.09-23.04) | 0.8 | 7.25 (0.04-NA) | 0.41 | |||
| Codominant | G/T | 1.96 (1.20-3.19) | 0.024* | 1.73 (0.96-3.11) | 0.13 | |||
| Log-additive | --- | 1.88 (1.18-2.99) | 0.008* | 1.77 (1.00-3.14) | 0.046* |
OR, odds ratio; 95% CI, 95% confidence interval; MAF, minor alleles frequencies.
P < 0.05, statistical significance.
Adjusted for gender + age.
Two blocks were detected in ApoB gene by haplotype analysis (Figure 1). However, none of significant results were identified. We just found rs1042034, rs676210 and rs673548 were very strong linkage disequilibrium.
Figure 1.

Linkage disequilibrium analysis of the ApoB gene. LD is displayed by standard color schemes with bright red for very strong LD (LOD > 2, D’ = 1), pink red (LOD > 2, D’ < 1) and blue (LOD < 2, D’ = 1) for intermediate LD, and white (LOD < 2, D’ < 1) for no LD.
PolyPhen-2 results scores of 0.000 for 4338C>T and 0.999 for 2739A>G. PolyPhen-2 utilized two models (HumDiv and HumVar), in which the latter is more rigorous in its false discovery rate. In general, the HumVar dataset was used to predict protein function (Figure 2).
Figure 2.
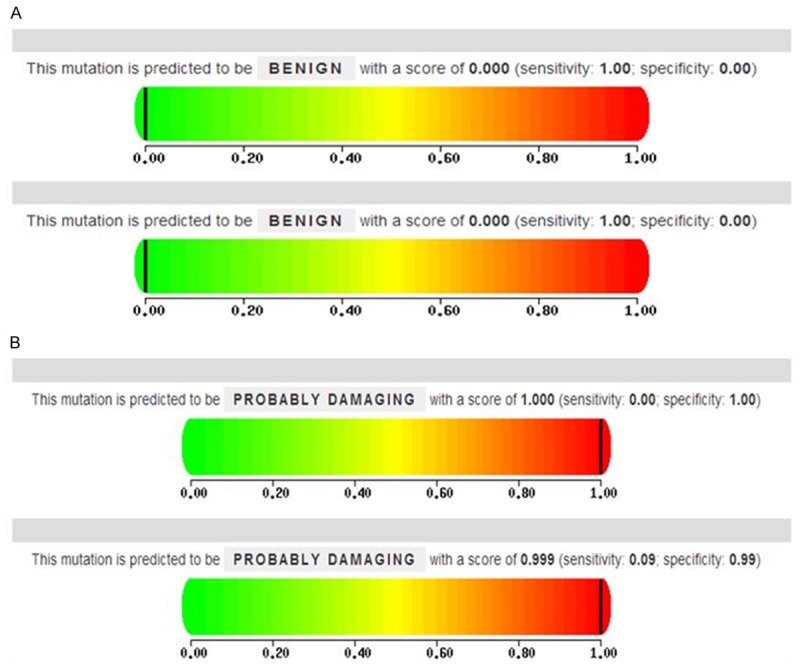
Predicted protein function of the 4338C>T and 2739A>G. A. Prediction of the 4338C>T. B. Prediction of the 2739A>G.
Discussion
Non-traumatic ONFH is an intractable disease that is pathophysiologically characterized by ischemic necrosis of the femoral head and deterioration of hip joint function. Many ONFH cases develop in association with alcohol consumption and steroid treatment are also contributing factors. Hypercoagulability, lipid metabolism abnormality and vascular endothelial damage have all been reported as causes of ONFH [4,21]. Regarding lipid metabolism abnormality, it is considered that the transport of lipids from central to peripheral tissues is enhanced by elevated serum LDL/HDL ratio, and resultant fat embolism leads to the inhibition of circulation within the bone marrow. Some studies have associated ONFH with serum ApoB/ApoA1 ratio and LDL/HDL ratio [22]. The aim of this study assess polymorphisms of ApoB, ApoA1 gene are associated with alcohol-induced ONFH.
ApoB and alcohol-induced ONFH
The ApoB gene, which is mapped to chromosome 2p24.1, contains three SNPs (genotype “T/T” of rs1042034, genotype “G/G” of rs676210 and genotype “G/G” of rs673548) associated with decreased risk of alcohol-induced ONFH. ApoB is almost the only protein component of low-density lipoprotein cholesterol (LDL-C), and is also a component of chylomicrons, lipoprotein(a), very low-density lipoprotein cholesterol (VLDL-C) and metabolic remnants of chylomicrons and VLDL-C [23]. The rs1042034 of ApoB gene is a missense resulting in Ser4338Asn. It is in strong linkage disequilibrium (LD) with a second missense, rs676210, which results in Ser4338Asn as well as intronic SNP rs673548. In a previous study, the rs676210 minor allele (A) was associated with total cholesterol, lower triglyceride and low-density lipoprotein cholesterol levels and with higher high-density lipoprotein (HDL) cholesterol levels, with a P < 5×10-8, in comparison with major allele (G) carriers. In another earlier report, rs676210 was found to associate with triglycerides, VLDL-related fractions, and mean VLDL/LDL ratio [24]. It reported that gene-by-environment interactions between SNPs in APOB gene and dietary cholesterol intake showed the prediction of total cholesterol level [13].
Previous reports that a higher frequency of 7623TT or CT of the ApoB gene was observed in steroid-induced ONFH cases than in referent patients (P = 0.033), resulting in an elevated odds ratio that was statistically significant (adjusted OR = 6.37, 95% CI = 1.53-26.5, P = 0.011) in the Japanese population. A higher value of ApoB/ApoA1 ratio was observed in cases (P = 0.045) [9]. However, these results were inconformity with our results. In our study, rs1042034, rs676210 and rs673548 were very strong linkage disequilibrium (Figure 1). The rs1042034 and rs676210 of ApoB gene located at coding exon region. By predicting protein function, we found mutation of rs676210 in ApoB may affect protein express (Figure 2). The same ApoB gene showed different results in ONFH. The rs1042034 and rs676210 whether have reality biological functions, the alcohol, corticosteroid and ethnic difference whether affect ApoB gene express. Therefore, additional research is merited to examine the biological functions of ApoB gene.
ApoA1 and alcohol-induced ONFH
The ApoA1 gene, which is mapped to chromosome 11q23.3, contains a SNP (genotype “T/G” of rs632153) associated with increased risk of alcohol-induced ONFH. Previous studies found that at the start site of human ApoA1 gene transcription, G/A polymorphism existed at upstream -75 bp regions, this polymorphism was located at GC-rich region in ApoA1 gene promoter, and the GC-rich region was the regulatory elements of ApoA1 gene transcription [25]. ApoA1 is important in removing excess cholesterol from tissues and incorporating it into high-density lipoprotein cholesterol (HDL-C) for reverse transport to the liver, thus manifesting antiatherogenic effects [23]. Food eaten while drinking, liver or pancreatic disorders, dietary habits and other factors should be taken into account when investigating the relation to lipid metabolism [26]. Hyperlipidemia affected the microcirculation of the femoral head to result in femoral necrosis from multiple links, such as influencing bone fat embolism, affecting the formation of bone micro-thrombosis and affecting blood coagulation solvent systems [27].
Previous reports that -75 G > A polymorphism of ApoA1, AA genotype frequency was significantly higher in patients with steroid-induced osteonecrosis than that in control subjects, resulting in an elevated odds ratio that was statistically significant (OR = 3.932, 95% CI = 3.0847-5.0123, P <0.0001) in the Chinese population [2]. Combined with our study, it provided an evidence that polymorphisms of ApoA1 gene increased the risk of non-traumatic osteonecrosis (alcohol and steroid-induced). Alternatively, relationship between ApoA1 gene and non-traumatic osteonecrosis is of great interest and warrants further investigation.
The limitations must be mentioned in our study. First, we collected all the samples that were from Han Chinese population who lived in Zhengzhou city or around. Substantial population of confounding factors, which may cause type I error (false positive) for association study. Second, the sample size is not large enough for association studies and a large sample size is required to confirm these findings in further study.
In conclusion, polymorphisms of the ApoB and ApoA1 gene are associated with alcohol-induced ONFH in the Han Chinese population.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81160228, 81260284). We are also grateful to the clinicians and other hospital staff who contributed to the blood sample and data collection for this study.
Disclosure of conflict of interest
None.
References
- 1.Kim H, Cho C, Cho Y, Cho S, Yoon K, Kim K. Significant associations of PAI-1 genetic polymorphisms with osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2011;12:160. doi: 10.1186/1471-2474-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin JM, Liu Z, Zhao SC, Guo YJ, Liu ZT. Relationship between the Apolipoprotein AI, B gene polymorphism and the risk of non-traumatic osteonecrosis. Lipids Health Dis. 2014;13:149. doi: 10.1186/1476-511X-13-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, Yang G, Shi X, Levine A, Iqbal J, Yaroslavskiy BB, Isales C, Blair HC. ACTH protects against glucocorticoid- induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 5.Seamon J, Keller T, Saleh J, Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone. 2011;49:1005–1009. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Powell C, Chang C, Gershwin ME. Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin Rev Allergy Immunol. 2011;41:102–113. doi: 10.1007/s12016-010-8217-z. [DOI] [PubMed] [Google Scholar]
- 8.Lind L, Vessby B, Sundstrom J. The apolipoprotein B/AI ratio and the metabolic syndrome independently predict risk for myocardial infarction in middle-aged men. Arterioscler Thromb Vasc Biol. 2006;26:406–410. doi: 10.1161/01.ATV.0000197827.12431.d0. [DOI] [PubMed] [Google Scholar]
- 9.Hirata T, Fujioka M, Takahashi KA, Arai Y, Asano T, Ishida M, Kuribayashi M, Akioka K, Okamoto M, Yoshimura N, Satomi Y, Nishino H, Fukushima W, Hirota Y, Nakajima S, Kato S, Kubo T. ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J Orthop Sci. 2007;12:199–206. doi: 10.1007/s00776-007-1110-9. [DOI] [PubMed] [Google Scholar]
- 10.Huang G, Zhong H, Re HM, Mao HW, Niu Q, Chi YH. Coalition of DNA polymorphisms of ApoB and ApoAI genes is related with coronary artery disease in Kazaks. J Geriatr Cardiol. 2012;9:33–37. doi: 10.3724/SP.J.1263.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makela KM, Traylor M, Oksala N, Kleber ME, Seppala I, Lyytikainen LP, Hernesniemi JA, Kahonen M, Bevan S, Rothwell PM, Sudlow C, Dichgans M Wellcome Trust Case Control Consortium 2 (WTCCC2) Delgado G, Grammer TB, Scharnagl H, Markus HS, Marz W, Lehtimaki T. Association of the novel single-nucleotide polymorphism which increases oxidized low-density lipoprotein levels with cerebrovascular disease events. Atherosclerosis. 2014;234:214–217. doi: 10.1016/j.atherosclerosis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Hao J, He XD. Haplotype analysis of ApoAI gene and sepsis-associated acute lung injury. Lipids Health Dis. 2014;13:79. doi: 10.1186/1476-511X-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DS, Burt AA, Ranchalis JE, Jarvik ER, Rosenthal EA, Hatsukami TS, Furlong CE, Jarvik GP. Novel gene-by-environment interactions: APOB and NPC1L1 variants affect the relationship between dietary and total plasma cholesterol. J Lipid Res. 2013;54:1512–1520. doi: 10.1194/jlr.P035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, Choi SJ, Hong JM, Lee WK, Baek JI, Kim SY, Park EK, Kim SY, Kim TH, Kim UK. Association of a polymorphism in the intron 7 of the SREBF1 gene with osteonecrosis of the femoral head in Koreans. Ann Hum Genet. 2009;73:34–41. doi: 10.1111/j.1469-1809.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- 15.Trembizki E, Smith H, Lahra MM, Chen M, Donovan B, Fairley CK, Guy R, Kaldor J, Regan D, Ward J, Nissen MD, Sloots TP, Whiley DM. High-throughput informative single nucleotide polymorphism-based typing of Neisseria gonorrhoeae using the Sequenom MassARRAY iPLEX platform. J Antimicrob Chemother. 2014;69:1526–1532. doi: 10.1093/jac/dkt544. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009;2:2–12. doi: 10.1002/0471142905.hg0212s60. [DOI] [PubMed] [Google Scholar]
- 17.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, Wang M, Feng W, Zander T, MacConaill L, Lee JC, Nicoletti R, Hatton C, Goyette M, Girard L, Majmudar K, Ziaugra L, Wong KK, Gabriel S, Beroukhim R, Peyton M, Barretina J, Dutt A, Emery C, Greulich H, Shah K, Sasaki H, Gazdar A, Minna J, Armstrong SA, Mellinghoff IK, Hodi FS, Dranoff G, Mischel PS, Cloughesy TF, Nelson SF, Liau LM, Mertz K, Rubin MA, Moch H, Loda M, Catalona W, Fletcher J, Signoretti S, Kaye F, Anderson KC, Demetri GD, Dummer R, Wagner S, Herlyn M, Sellers WR, Meyerson M, Garraway LA. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 18.Kochl S, Niederstatter H, Parson W. DNA extraction and quantitation of forensic samples using the phenol-chloroform method and real-time PCR. Methods Mol Biol. 2005;297:13–30. doi: 10.1385/1-59259-867-6:013. [DOI] [PubMed] [Google Scholar]
- 19.Adamec C. [Example of the Use of the Nonparametric Test. Test X2 for Comparison of 2 Independent Examples] . Cesk Zdrav. 1964;12:613–619. [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glueck CJ, Freiberg R, Tracy T, Stroop D, Wang P. Thrombophilia and hypofibrinolysis: pathophysiologies of osteonecrosis. Clin Orthop Relat Res. 1997:43–56. [PubMed] [Google Scholar]
- 22.Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, Iwamoto Y. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits. Rheumatology (Oxford) 2001;40:196–201. doi: 10.1093/rheumatology/40.2.196. [DOI] [PubMed] [Google Scholar]
- 23.Savas Erdeve S, Simsek E, Dallar Y, Biyikli Z. Utility of ApoB/ApoA1 ratio for the prediction of cardiovascular risk in children with metabolic syndrome. Indian J Pediatr. 2010;77:1261–1265. doi: 10.1007/s12098-010-0217-8. [DOI] [PubMed] [Google Scholar]
- 24.Paniagua JA, Lopez-Miranda J, Perez-Martinez P, Marin C, Vida JM, Fuentes F, Fernandez de la Puebla RA, Perez-Jimenez F. Oxidized-LDL levels are changed during short-term serum glucose variations and lowered with statin treatment in early Type 2 diabetes: a study of endothelial function and microalbuminuria. Diabet Med. 2005;22:1647–1656. doi: 10.1111/j.1464-5491.2005.01703.x. [DOI] [PubMed] [Google Scholar]
- 25.Dawar R, Gurtoo A, Singh R. Apolipoprotein A1 gene polymorphism (G-75A and C+83T) in patients with myocardial infarction: a pilot study in a north Indian population. Am J Clin Pathol. 2010;134:249–255. doi: 10.1309/AJCPKPTXQ3QN1IFG. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo K, Hirohata T, Sugioka Y, Ikeda M, Fukuda A. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988:115–123. [PubMed] [Google Scholar]
- 27.Phemister DB. The classic: repair of bone in the presence of aseptic necrosis resulting from fractures, transplantations, and vascular obstruction. Clin Orthop Relat Res. 2008;466:1021–1033. doi: 10.1007/s11999-008-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


