Abstract
To investigate the inhibition effect of polyethylene glycol interferon α-2b and imatinib alone or combination on imatinib-resistant GIST cell lines, and to explore the possible mechanism. Imatinib resistant GIST cell lines (GIST-R) were exposured to either Peg-IFNα-2b or imatinib alone or combination. The proliferative inhibition rates and the combination index (CI) values of GIST-R cells were detected by MTT assay. The apoptotic rates of GIST-R cells were detected by flow cytometry. The expression levels of phospho-mammalian target of rapamycin (p-mTOR), and Bcl-2 of GIST-R cells were analyzed by Western blot. GIST-R cells presents remarkable resistance to imatinib, and the resistance index (RI) were (P<0.05). And The proliferative inhibition rate and the apoptotic rate of GIST-R cells in combination of Peg-IFNα2b and Imatinib group were higher than those in Peg-IFNα-2b or imatinib alone group (P<0.05). The CI value of Peg-IFNα-2b and imatinib was less than each alone, which had a synergistic effect (CI=0.63). As compared with the control (GIST-R cells without any treatment), the expression levels of p-mTOR and Bcl-2 proteins of GIST-R cells in combination of Peg-IFNα-2b and imatinib group were decreased (P<0.01). The combination of Peg-IFNα-2b and imatinib generats a synergistic effect in GIST-R cells, and reversal of drug resistance. This effect may be related with apoptosis and down-regulation of the expression of p-mTOR.
Keywords: GIST, drug resistance, polyethylene glycol interferonα-2b, imatinib, combination, sensibilization
Introduction
The gastrointestinal stromal tumor (GIST) is the most common mesenchymal tissue endogenous tumor. It accounts for 1.1% of the malignant tumors of the gastrovascular system, of which, 80% to 90% are mutated in the fibroblast growth factor receptor gene, KIT, 5% to 10% are mutated in the blood platelet endogenous growth factor receptor, and another 5% to 10% are mutated in the wild type KIT and PDGFRα gene [1]. Imatinib mesylate (IM) has been the first recommended for GIST therapy. Gleeve can inhibit selectively the combination of KIT, BCR-ABL and PDGFR. IM to the ATP binding site in the tyrosine kinase (PTK) functional domain in cytoplasm, interdict the signal transduction to the phosphate group from ATP to the protein substrate, inhibit the cell proliferation and recover the normal apoptosis. But almost all of the patients for whom the initial therapy was effective will present progress of the state of an illness after less than 20 months, and produce the acquired drug-resistance [2]. The main mechanism on the acquired drug-resistance of the gastrointestinal stromal tumors to the Imatinib is that the secondary mutation of the KIT or PDGFRα gene may result in the alteration of the protein conformation and the impediment to the combination of with IM [3,4]. Peg-IFNα-2b is one of the covalent conjugate of the recombinant human interferon α-2b and polyethylene glycol monomethyl oxygen radicals, which has longer plasma half-life and better hypotoxicity and tolerance effect resistance, is mainly used for the therapy of the chronic hepatitis. So we intended to investigate the inhibition effect of Peg-IFNα-2b and imatinib on imatinib-resistant GIST cell lines, and also to explore the possible mechanism.
Materials and methods
Materials
Collected the fresh specimens from 5 cases of patients receiving biopsy in the Second Xiang-yak Hospital from December, 2013 to February, 2014. There into, the patients included three cases of male and two cases of female; whose average age was 53 years old; the patients were administrated orally Imatinibe Mesylate (IM) for 11 months averagely. The inclusion criteria to the cases was that: The past c-kit gene detection conducted to the patients indicated that the exon 11 occurred mutation of the drug susceptibility, and the focus progressed or local recurred after the treatment by oral administration of IM, then the c-kit gene detection conducted again indicated the secondary mutation, of which the mutation domain centered on the exon 13, 14 and 17. All of the patients signed the treatment informed consent and this study was approved by the ethics committee for the clinical trial on medicine. The GIST-T1 cell line was purchased from the Shenzhen Biowit Biotechnology Company. Imatinibe Mesylate (IM) is the product manufactured by NVS of Switzerland. The Annexin V-FITCA apoptosis Detection Kit was provided by the Nan Keygentec Biotechnology Limited Company. The rabbit anti human p-mTOR and β-actin polyclonal antibody were provided by American Cell Signal Technology Company.
Extraction and culturing of the passage acquired drug-resistant GIST cells
The GISTs cells were cultured using the human cancer cell primary culture kit. Cut the tissue specimens from the five cases of GISTs patients into pieces and prepared them into pasty, and added the Hank’s solution; centrifuged; added the Hank’s solution and 10× diluted cell dispersing material, let the mixture to react for 2 hours, and observed the dispersing state of the cell aggregate under the optical microscope; added the RPMI-1640 cell culture fluid containing the FCS for terminating reaction; discarded the fat and fiber texture from the suspension and centrifuged; added equal Hank’s solution and RPMI-1640 culture fluid, filtrated the mixture through the filter screen; collected the cell suspension, centrifuged for recovering the precipitation; suspended the recovered cell in the pre-culture solution PCM-1 again, and transferred the suspension into the cell culture bottle coated with type I collagen gel, and placed the bottles to culture for 24 h, and then discarded the hemolytic and the dead cell and yielded the cell precipitation by centrifuging. After conducted three times of cell passage operation, the drug-resistant cell line was named as GIST-R.
Detecting the cell activity by means of the MTT method and the half maximal inhibitory concentration of a substance (IC50)
Fetched the cell lines of GIST-T1 and GIST-R at the logarithmic phase respectively, digested them to be the cell suspension containing 3×104 cells per mL. After the adherence of the cell, the alone drug group was added Imatinib and Peg-IFNα-2b at different concentrations of 0.0, 2.5, 5, 10, 20, 40 μmol/L and 0.0, 1×103, 2×103, 4×103, 8×103 and 16×103 U/mL respectively. Placed in the 37°C, 5% CO2 incubator and culturing for 48 h, then added 20 mL of MTT into each holes (5 mg/mL), added 150 ml of DMSO into each hole, detected the absorbance value of each well (A) at wave length of 492 nm and calculated the average value. Calculated the resistance index (RI)=IC50 of the drug-resistant cell line/IC50 of the parental generation cell line. Three repeats were tested.
Assaying the apoptosis rate of the cell by means of the flow cytometry
Collected the GIST-R cell at logarithmic phase. The experimental grouping and medication were as following: the experiment consisted of the control group (which contained only cells and culture solution and not drug), IM administration group (at the concentration of 10 μmol/L), Peg-IFNα-2b administration group (the final concentration was 4×103 U/mL) and the drug combination group. After 24 h, digested the cells with pancreatin and collected the cells, added the combined buffer solution to suspend the cells again to prepare the cell suspension, adjusted the cell density to 1×106 cells/Ml. Drewg 100 μL of cell suspension, added 5 μL of Annexin V-FITC and 5 μL of propidium iodide (PI) to the suspension, blended these components, incubated the mixture away from light at the room temperature, added the combination 400 μL of buffer solution, analyzed them on the FCM immediately. Set the zero value using the unstained cell, tested the data for each tube loaded the sample for the percentage of the apoptotic cell using the Annexin V-FITC simple staining tube and the PI simple staining tube as the standard control. Three repeats were tested.
Assaying the protein expression level for the p-mTOR gene and Bcl-2 gene with Western blotting
Conducted the experiment with the GIST-R cell at the logarithmic phase, and the method of experimental grouping and medication were the same as those of section 1.2.3. After treating the cells for 24 h, collected the cells of each groups, and extracted the total protein (TP) of the cell. detected the expression of the p-mTOR protein and Bcl-2 protein by means of the Western blotting respectively: transferred the cell TP to the polyvinylidene difluoride (PVDF) film after 8% SDS-PAGE electrophoretic separation, blocked the protein with the TBST solution containing 0.5% skim milk powder, added the antibody for Mammalian Target Of Rapamycin (mTOR) resisting phosphorylation diluted at the ratio of 1:2000 and the antibody for Bcl-2 and β-actin (the internal reference) diluted at the ratio of 1:1000, incubated at 4°C for the night, rinsed three times with TBST, then added DAB for coloration; exposed under light for development. Three repeats were tested. Analyzed the gray value of the protein bands with the Image J software, showed the relative expression level of the aimed protein with the ratio of the gray value of the aimed protein bands to the gray value of internal reference β-actin protein.
Statistical treatment
Expressed the numerical value ± the standard deviation (mean ± SD), conducted the statistical analysis with SPSS11.5 software. In the statistical comparing with the pair t-test, P<0.05 meant that the difference is with the statistical significance.
Results
Effect of the administration of Peg-IFNα-2b and Imatinib to the cell proliferation of GIST-T1 and GIST-R
The assay result of the MTT method showed that: alone administrating with Peg-IFNα-2b hadn’t significant inhibition effect to the proliferation of the GIST-T1 and GIST-R cell lines of uniformly; Imatinib was drug-resistant at high degree to the GIST-R cell line, of which the proliferation inhibition effect was lower than to the sensitive GIST-T1 cell line, the RI is 46.14 (Table 1). The inhibition effect to the proliferation of the GIST-R cell caused by Peg-IFNα-2b and Imatinib respectively were significantly higher than each alone drug group, and the resistance index (RI=14.79) were significantly less than the Imatinib group (Table 1). By calculating the CI value of each drug concentration group, it was found that the CI value of the drug combination groups at different obvious drug concentration were uniformly 0.79 and which indicated the significant synergistic effect (Table 2).
Table 1.
The IC50 and RI of Peg-IFNα-2b and imatinib alone or combination on GIST-R cell lines
| Drugs | GIST-T1 IC50 (μmol/L) | GIST-R IC50 (μmol/L) | RI |
|---|---|---|---|
| IM | 0.75 | 34.65 | 46.14 |
| Peg-IFNα 2b | - | - | - |
| Peg-IFNα 2b+IM | 0.68 | 10.06 | 14.79 |
Table 2.
The CI of Different concentrations of Peg-IFNα-2b and imatinib on GIST-R cell lines
| Peg-IFNα 2b+IM c/(U/ml+μmol/L) | CI |
|---|---|
| 1×103+2.5 | 1.23 |
| 2×103+5.0 | 0.95 |
| 4×103+10 | 0.73 |
| 8×103+20 | 0.42 |
| 16×103+40 | 0.64 |
Peg-IFNα-2b and Imatinib promoted the apoptosis of the GIST-R cell
The assay result of the FCM method (Figure 1) showed that, the apoptosis rate of the GIST-R cell in the control group was 2.70%±0.62%, that of the GIST-R cell in the Imatinib group and Peg-IFNα-2b group were 9.5%±1.57% and 5.66%±0.76% respectively and that of the GIST-R cell in the drug combination group was 16.06%±3.24%. The cell apoptosis rate in the drug combination administration group was greater than the drug alone administration groups, and the difference was with statistical significance (P<0.05). The above result indicated that Peg-IFNα-2b and Imatinib had obvious effect of promoting the apoptosis of GIST-R cell.
Figure 1.
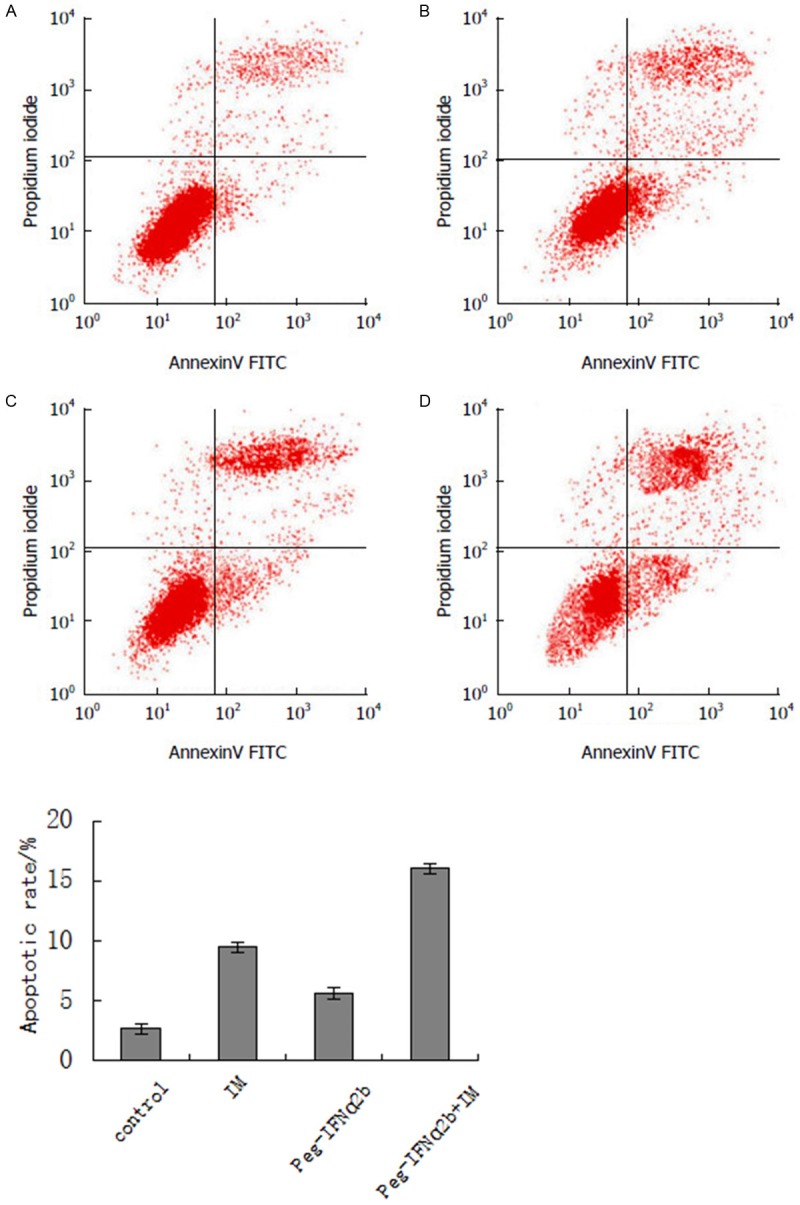
The apoptosis of GIST-R cells was detected by FCM assay. A: Control group; B: IM group; C: Peg-IFNα-2b group; D: Peg-IFNα-2b and IM group.
Protein expression of p-mTOR and Bcl-2 after treating the GIST-R cell with Peg-IFNα-2b and Imatinib
The assay result of the Western blotting method (Figure 2) showed that, compared with the control group, the p-mTOR protein expression level in the GIST-R cell of the Imatinib treatment group was significantly down-regulated, where the difference was with statistical significance (P<0.05), the p-mTOR protein expression level in the GIST-R cell of the Peg-IFNα-2b treatment group was significantly up-regulated, where the difference was with statistical significance (P<0.05); the GIST-R protein expression level in the PC9/GR cell of the drug combination treatment group was significantly down-regulated and lower than that in the singe drug group of Imatinib, where the difference was with statistical significance (P<0.05). At the same time, compared with the control group and singe drug group of Peg-IFNα-2b and Imatinib respectively, the Bcl-2 protein expression level in the GIST-R cell of the Peg-IFNα-2b and Imatinib combination group where the difference was with statistical significance (P<0.05). The result indicated that the combination treatment of Peg-IFNα-2b and Imatinib down-regulated the expression level of the p-mTOR protein and Bcl-2 protein in the Imatinib resistant line significantly.
Figure 2.
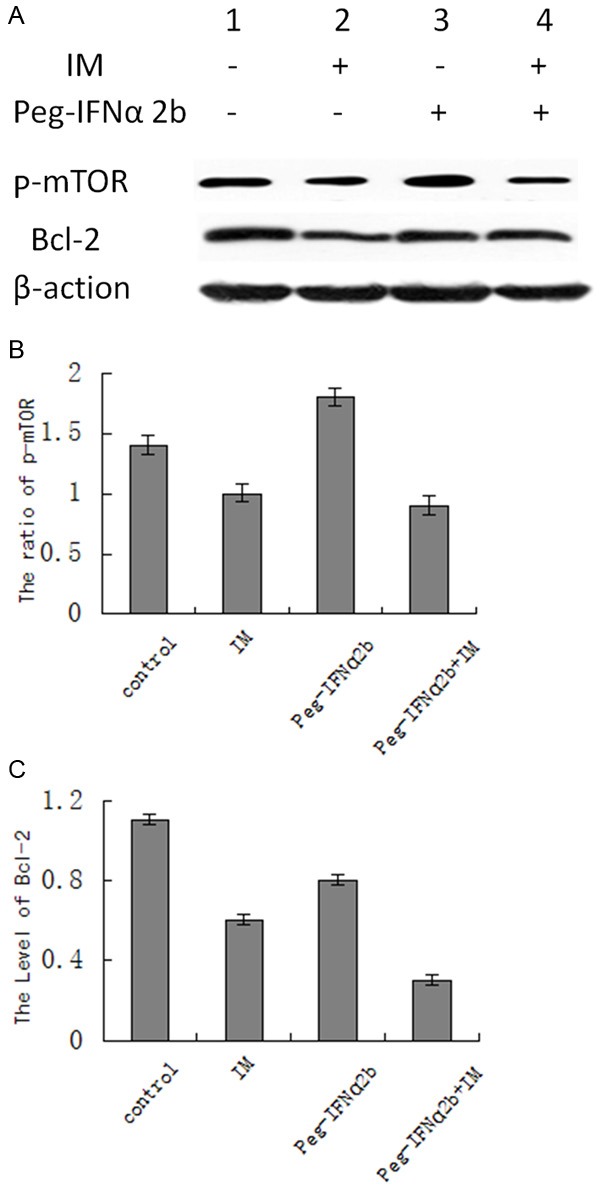
The expression levels of p-mTOR and Bcl-2 of GIST-R cells in different treatment groups were analyzed by Western. A: Protein blotting stripe. B: The relative content of mTOR protein. C: The relative content of Bcl-2 protein. 1: Control group; 2: IM group; 3: Peg-IFNα-2b group; 4: Peg-IFNα-2b and IM group.
Discussion
It was pointed out by the study on the two international famous clinical trials USS0033 and EORTC62005 following the Imatinib resistance that, if the disease progress, the patients of 400 mg/d dose group were distributed to the high dose group of 800 mg/d [5,6]. But it was found in the clinical practice that the tolerance of Chinese in the 800 mg/d Imatinib treatment group was rather poor. In order to resolve the drug resistance problem of GIST to IM, many kinds of new type molecular targeted drug for treating GIST had been developed one after the other, such as sunitinib [7], dasatinib [8], nilotinib [9] and sorafnib [10], and so on. Because of the diversity in mutation and the different sensibility of the drug to different mutation, the combination of the new type molecular targeted drug and IM may extend the scope of the targeted therapy, but the evaluation to the clinical safety and feasibility were still at early clinical trial stage at present.
Along with the increasing importance to the immunotherapy, the stem cells which can enhance the antineoplastic immunity itself to help to eradicate the derivative clone and possesses the ability of self-renewal became the new pattern concerned. IFNα may combine the autoimmunity and the adaptation acquired immunity by means of promoting the maturity of Dendritic Cell (DC), antigen presentation, stimulating the generating and gathering of the Th1, and improving the rate of the cloning and amplification and survival rate of DC. IFNα-2bpossesses extensive antiviral, antineoplastic and immunoregulation effect, which may treat the hepatitis B and hepatitis C, the chronic granulocytic leukemia (CGC), the hairy cell leukemia, the follicular lymphoma, the renal cell carcinoma, the melanin and the Kaposi’s sarcoma [11]. The treatment result of to the patients with chronic stage chronic myelogenous leukemia with the combination of Gleevec 600 mg/d and pegylated interferons α-2b indicated that the treatment effect was the overlap of that of the two single drug treatments [12,13]. Chen [14,15] reported six cases of III/IV phase GIST patients treating with the combination of Peg-IFNα-2b and IM, and found that compared with the classical trial (B2222) treating GIST with IM alone, the clinical period of validity for combination treatment was extended; the reuse Peg-IFNα-2b in the disease progressive stage caused by the IM resistance reversed the drug-resistance, and induced the second PR phenomenon. Another report stated that IM could act on the DC and generate the NK cell dependent anticancer effect. The IFNγ level of the NK cell can indicate the time to live (TTL) of the treatment to the GIST patients with IM [16]. If the IFNγ level in the Peripheral Blood (PB) is very high, then the treatment of GIST may be sufficiently effective [17].
We cultured the drug-resistance primary cell of the GISTs patient caused by the secondary mutation of the C-kit gene and the Imatinib sensitive GIST-T1 and the Imatinib sensitive GIST- T1 cell line to simulate the acquired drug-resistance course of the gastrointestinal stromal tumor with alone Peg-IFNα-2b or Imatinib or the combination intervention by these two components. The experiment result demonstrated the high drug-resistant of Imatinib to the GIST-R cell line, verified the drug-resistance of the GIST-R cell line; the Peg-IFNα-2b alone administration had not obvious proliferation inhibition effect to the GIST-T1 and GIST-R cell line while the GIST-R inhibition rate of combination of Peg-IFNα-2b and Imatinib to the GIST-R cell line was higher than the Imatinib alone administration, and the resistance index (RI) decreased and which demonstrated obvious synergy sensibilization effect (CI=0.79). Furthermore, the assay result of FCM demonstrated that, the combination administration of Peg-IFNα-2b and Imatinib had obvious effect of promoting the apoptosis. Thus it was illustrated at the cell level that, Peg-IFNα 2b might enhance the inhibition effect of Imatinib to the drug-resistance cell line and promote their apoptosis.
The secondary drug-resistance of GIST was mainly related to the secondary mutation of c-kit/PDGFRA, while the downstream signal transduction path PI3K/Akt/mTOR should be the main activation path [18]. Phosphorylated mTOR exerts the function of centric regulate and control point in the proliferation, survival and apoptosis. The result of this study demonstrated that, compared with the control group, the p-mTOR protein expression level in the GIST-R cell of the Peg-IFNα-2b treatment group was up-regulated and that of the Imatinib treatment group was down-regulated, while that level in the PC9/GR cell of the drug combination treatment group was down-regulated to lower than the level of the Imatinib alone treatment group. Their downstream signal paths were not completely same. the author speculated that, when Peg-IFNα 2 combines Imatinib, the signal transduction may be enhanced and the targeting of the treatment of Imatinib may be improved, which may result in the up-regulated expression level of the p-mTOR protein in the GIST-R cell line when alone Peg-IFNα-2b administration and the down-regulated expression level of the p-mTOR protein in the GIST-R cell line when Peg-IFNα-2b and Imatinib combination administration. These result uniformly indicated that the Peg-IFNα-2b might enhance the sensitivity to Imatinib of the cell and thus reverse the drug-resistance by means of influencing the activity of the PI3K/Akt/mTOR path.
All of the genes in the Bcl-2 family are the proto oncogenes related to the apoptosis, of which the expressed product, Bcl-2 protein is an inhibitor of apoptosis protein (IAP) with the effect of inhibiting the apoptosis, of which the expression may down-regulate the sensitivity of the cancer cell to apoptosis [19]. The study result demonstrated that, Peg-IFNα-2b and Imatinib can down-regulate the expression level of the Bcl-2 protein respectively at different degree, and the combination of Peg-IFNα-2b and Imatinib can down-regulate the expression level of the Bcl-2 protein in the GIST-R cell. This result showed that the enhancement of the apoptosis of the GIST-R cell in the combination of Peg-IFNα-2b and Imatinib administration might be related to the down-regulated expression of the Bcl-2 protein.
This study result demonstrated that, the combination of Peg-IFNα-2b and Imatinib administration generated excellent inhibiting effect to the secondary Imatinib-resistant GISTs cell line, Peg-IFNα-2b had obvious synergetic sensibilization effect and reversed the drug-resistance, furthermore, this effect might be related to affecting the activity of the PI3K/Akt/mTOR path, the down-regulation of the expression level of the p-mTOR protein and the induction to the apoptosis. The Peg-IFNα-2b and Imatinib combination administration may be considered as a medicine choice for Imatinib resistant GISTs cells, and may provide certain experimental basis for the clinical treatment to the Imatinib resistant GIST patients, of which the clinical application is awaiting the further evidence-based medicine verification with large sample.
Conclusions
The combination of Peg-IFNα-2b and imatinib generates a synergistic effect in GIST-R cells. This effect may be related with apoptosis and down-regulation the expression of p-mTOR.
Disclosure of conflict of interest
None.
References
- 1.Agaimy A, Wunsch PH, Hostaedter F, Blaszyk H, Rümmele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–120. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 3.Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, Leversha MA, Jeffrey PD, Desantis D, Singer S, Brennan MF, Maki RG, DeMatteo RP. Acquired resistance to imatinib in gastrointestinal stromal tumor Occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 4.McLean SR, Gana Weisz M, Hartzoulakis B, Frow R, Whelan J, Selwood D, Boshoff C. Imatinib binding and c-KIT inhibition is abrogated by the cKIT kinase domain l missense mutation Val654 Ala. Mol Cancer Ther. 2005;4:2008–2015. doi: 10.1158/1535-7163.MCT-05-0070. [DOI] [PubMed] [Google Scholar]
- 5.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CD, Crowley JJ, Borden EC. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J. Clin. Oncol. 2008;26:626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 6.Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR EORTC Soft Tissue and Bone Sarcoma Group; the Italian Sarcoma Group; Australasian Gastrointestinal Trials Group. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751–7. doi: 10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, Cherrington JM, Mendel DB. Contribution of individual argents to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006;5:1280–1289. doi: 10.1158/1535-7163.MCT-03-0156. [DOI] [PubMed] [Google Scholar]
- 8.Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, Bokemeyer C, Deininger MW, Druker BJ, Heinrich MC. Dasatinih (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits thekinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 9.Braconi C, Bracci R, Cellerino R. Molecular targets in gastrointestinal stromal tumors (GIST) therapy. Curr Cancer Drug Targets. 2008;8:359–366. doi: 10.2174/156800908785133169. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pham Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 11.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 12.Bukowski RM, Tendler C, Cutler D, Rose E, Laughlin MM, Statkevich P. Treating cancer with PEG Intron: pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer. 2002;95:389–96. doi: 10.1002/cncr.10663. [DOI] [PubMed] [Google Scholar]
- 13.Nicolini FE, Hayette S, Legros L, Rousselot P, Maloisel F, Tulliez M, Guerci A, Charbonnier A, Prébet T, Rigal-Huguet F, Chabane K, Magaud JP, Paillet C, Pivot C, Michallet M. Pegylated IFN-alpha2a combined to imatinib mesylate 600mg daily can induce complete cytogenetic and molecular responses in a subset of chronic phase CML patients refractory to IFN alone or to imatinib 600 mg daily alone. Leuk Res. 2011;35:80–6. doi: 10.1016/j.leukres.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Chen LL, Chen X, Choi H, Sang H, Chen LC, Zhang H, Gouw L, Andtbacka RH, Chan BK, Rodesch CK, Jimenez A, Cano P, Jones KA, Oyedeji CO, Martins T, Hill HR, Schumacher J, Willmore C, Scaife CL, Ward JH, Morton K, Randall RL, Lazar AJ, Patel S, Trent JC, Frazier ML, Lin P, Jensen P, Benjamin RS. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol Immunother. 2012;61:1113–24. doi: 10.1007/s00262-011-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borg C, Terme M, Taïeb J, Ménard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, Le Cesne A, Chung-Scott V, Lazar V, Tchou I, Crépineau F, Lemoine F, Bernard J, Fletcher JA, Turhan A, Blay JY, Spatz A, Emile JF, Heinrich MC, Mécheri S, Tursz T, Zitvogel L. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–88. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ménard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taïeb J, Delahaye NF, Flament C, Emile JF, Le Cesne A, Zitvogel L. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in astrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–9. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 17.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 18.Reichardt P. Novel approaches to imatinib-and sunitinib-resistant GIST. Curr Oncol Rep. 2008;10:344–349. doi: 10.1007/s11912-008-0053-4. [DOI] [PubMed] [Google Scholar]
- 19.Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl-2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]


