Abstract
Vitronectin (Vn), a multifunctional adhesive protein, is found in association with tumor progression, angiogenesis and metastasis in a variety of (human) tumors. But no studies concerning its correlation to osteosarcoma prognosis were found. Hence, we aimed to investigate the prognostic value of Vitronectin (Vn) in osteosarcoma. Here, we studied the expression of VN in the tumor tissues from 67 patients with osteosarcoma and 20 patients with osteochondroma using immunohistochemistry and estimated the effects of VN expression in osteosarcoma on progression-free survival (PFS) and overall survival (OS) using the Kaplan-Meier curve and COX proportional hazards regression model. Increased expression of VN in osteosarcoma tissue compared to no VN expression in osteochondroma tissue was shown in immunohistochemical assay. No associations were observed between VN expression and osteosarcoma patients’ gender (P = 0.675), age (P = 0.813), tumor size (P = 0.436), histologic subtype (P = 0.0.543) or tumor location (P = 0.456). Univariate survival analysis demonstrated significant correlations of high VN expression with shorter PFS (P = 0.002) and OS (P = 0.001); multivariate survival analysis revealed high VN expression as a significant independent prognostic indicator for shorter PFS (HR 2.788, P = 0.003) and OS (HR2.817, P = 0.003). In conclusion, the high expression of VN in tumor cells independently indicated poor clinical prognosis in patients with osteosarcoma, other than large tumor size and non-neoadjuvant chemoradiotherapy, suggesting that VN may serve as a potential therapeutic target in osteosarcoma.
Keywords: Vitronectin, immunohistochemistry, osteosarcoma, prognosis
Introduction
Osteosarcoma is the most frequently seen primary malignant bone tumor and reaches its peak morbidity rate predominantly in childhood and adolescence. The prognosis of osteosarcoma in younger patients has improved markedly, mainly due to the introduction of adjuvant and neo-adjuvant chemotherapy [1]. However, there still lacks favorable clinical outcome for patients with overt metastasis or recurrent disease. Unfortunately, increase in survival rate has not been witnessed in the last decade. As a result, it is indispensable to precisely recognize the key molecular mechanisms of tumor invasion and metastasis as well as resistance to chemoradiotherapy to improve prognosis for patients with malignant osteosarcoma.
It is well known that the tumor microenviroment plays an important role during the progression from primary tumor growth to metastasis [2]. Extracellular matrix (ECM) is one important component of the microenviroment containing a variety of matricellular proteins, which have structural and biological roles [3]. Extracellular matrix (ECM) glycoproteins participate in angiogenesis in many physiological and pathological processes, including ischemic vascular diseases, inflammation, tumor growth and metastasis [4,5]. Thus, its synthesis and degradation are associated with tumor invasion and metastasis.
Vitronectin (VN) is an important ECM glycoprotein that presents in plasma and is localized into the ECM of various tissues. Most plasma VN is an inactive monomer [4]. ECM VN, which is present as an active multimeric form that binds to various ligands such as integrins, plasminogen activator inhibitor-1 and urokinase receptors [6-8], is an adhesive protein that mediates the interactions between cells and their microenvironment. Integrin is recognized to be the primary receptor for VN. Integrin αvβ3-VN interaction promotes hypoxia resistance and tumor cell invasion through a mechanism that involves integrin-linked kinase (ILK) [9]. VN-binding integrins (both on tumor and endothelial cells), together with uPAR system, are essential for tumor progression and neovascularization [10-12]. The role of VN in carcinoma is well-documented. VN plays a role in promoting disease progression of several cancers including human breast cancer, melanoma, hepatocellular carcinoma and colorectal adenocarcinoma etc. [13-16].
In osteosarcoma lines, migration (haptotaxis) and invasive potentiation (haptoinvasion) of osteosarcoma (MG-63) cells can be mediated by vitronectin, and this process could be inhibited by high molecular weight kininogen (HK) [17]. Vitronectin improved the adhesive potency of osteosarcoma (MG-63) cells via directly associating with integrin αvβ3 and αvβ5 [18]. Integrin αvβ3 expression accelerated the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells through adhesion to vitronectin [19]. The above studies indicated VN plays a crucial role in the invasion and metastasis of osteosarcoma cell lines. Whereas, it is worth noting that the relationship between VN and osteosarcoma prognosis has not been tapped into. Besides, considering the features of osteosarcoma early metastasis and the mechanism where VN promotes the invasion and metastasis of tumor cells, investigating the correlation between VN expression and osteosarcoma survival rate is a novel and significant attempt. In the present study, VN expression in osteosarcoma tissues was examined and the influence of its expression on PFS and OS in patients with osteosarcoma was evaluated, which would assist in judging whether the inhibition of VN could work as therapeutic target for osteosarcoma.
Materials and methods
Ethics statement
This study was approved by the Ethics Com-mittee of Fujian Medical University. Written informed consents were taken from all the participants and ethical guidelines under Declaration of Helsinki were followed.
Clinical data
Specimens were obtained from archived, paraffin-embedded tissue sections from 67 patients with osteosarcoma in the First Affiliated Hospital of Fujian Medical University between January 1st 2006 and December 31st 2012 as experimental group. 20 osteochondroma tissue samples were acquired from homochronous surgical specimens and utilized as control group. Histopathological diagnosis of all specimens was confirmed by two trained pathologists. In the experimental group, thirty-eight patients (56.7%) were male and twenty-nine patients (43.3%) were female. The age at diagnosis ranged from 11 to 75 years (median age 20 years). Forty-one patients received neo-adjuvant chemotherapy, and the remainder underwent operation without any preoperative therapy. Upon completion of the surgery, all patients received normative post-operative chemotherapy using methotrexate, cisplatin and doxorubicin. Following chemotherapy, the patients were undergoing lung checkup through X-ray or CT scans every 3 months during the first 3 years after chemotherapy, every 4 months during the fourth and fifth years and every 6 months thereafter. The X-ray or CT scans were applied for the evaluation of the development of local recurrence and distant metastases.
All patients were followed up until December 2014 either by phone call or outpatient visit. The period of time between the date of surgical excision and first tumor progression (or death, or the latest follow-up) was determined as Progression-free survival (PFS). Overall survival (OS) was calculated according to the time between surgery and death (or the latest follow-up). All dead cases died of osteosarcoma, other than other diseases.
Protein expression of vitronectin in osteosarcoma
Tissues were obtained from patients during surgical excision. Paraffin-embedded specimens were cut into 4 μm thick slices, which were baked for 1-2 h in an incubation of 60-65°C to prevent slice detachment. Next, xylol and alcohol were used for dewaxation and hydration. During antigen retrieval process, these slices were placed in citrate buffer (pH = 6.0) in an autoclave sterilizer for approximately 2 min. To block the endogenous enzymes, they were rinsed in PBS and immersed in 3% H2O2 for 12 min once cooling to room temperature. Then, the specimens were incubated for 2 h at room temperature with an antibody against vitronectin (EP781Y, rabbit monoclonal antibody, Abcam, UK) at a dilution of 1:200. Next, the sections were exposed to polyperoxidase-anti-rabbit/mouse IgG (Maixin Biotechnology Co, Fuzhou, China) for 30 min following the manufacturer’s instruction. With the help of diaminobenzidine detection kit (Maixin Biotechnology Co) under light microscope, immunoreaction was identified. Hematoxylin was employed to counter stain the slices and gelatin to cover them. Phosphate buffer solution (0.01 M, pH 7.2), rather than the primary antibody, was used as negative control, and known positive human lung tissue as positive control.
Assessment of vitronectin expression in osteosarcoma patient specimens
Two pathologists participated in the assessment of tumor tissue specimens independently. Brown granules occurring in the nucleus or cytoplasm of tumor cells were determined as positive vitronectin expression under light microscope. Cells with no positive staining were rated as ‘0’, ‘±’ if only a few scattered positive cells were present, ‘+’ if a cluster(s) of positively stained cells (≤ 30% within a visual field) were present and ‘++’ if there were cluster(s) of positively stained cells (> 30% within a visual field) were present. For the statistical analysis in the osteosarcoma cells lines, no positive expression or the positive expression rates lower than 10% were determined as weak expression, stained neoplastic cells of higher than 10% positive expression were considered as strong expression.
Statistical analysis
SPSS 22.0 statistical software was utilized for statistical analyses. The correlations between vitronectin expression and clinical variables (gender, age, tumor size, histologic subtybe and tumor location) were analyzed using Chi-square test. How vitronectin expression related with PFS and OS was indicated by log-rank tests and presented as Kaplan-Meier curves. In multivariate analysis, the COX proportional hazards model and stepwise regression analysis were adopted to estimate the influences of vitronectin expression on PFS and OS. Besides, hazard ratios (HR), together with the corresponding 95% confidence intervals (CI), were calculated. P value\0.05 (two-sided) was proved to be statistically significant.
Results
Topology of vitronectin expression in osteosarcoma tissues
Immunohistochemical analysis demonstrated that vitronectin expression, primarily localized in osteosarcoma cell nuclear or cytoplasm, was found in 50 out of 67 (74.6%) cases. In the osteosarcoma cells, VN immunoreactivity was scored as ‘-’ in 25.4% (n = 17), ‘+/-’ in 22.4% (n = 15), ‘+’ in 32.8% (n = 22) and ‘++’ in 19.4% (n = 13) of the samples. Among the 67 osteosarcoma tissue samples, we discovered strong expression (Figure 1A) in 35 samples (52.2%) and weak vitronectin expression (Figure 1B) in 32 samples (47.8%). Apart from carcinoma cells, we also detected immunohistochemical staining in vascular endothelial cells (Figure 1C). However, there was no significant vitronectin staining observed in osteochondroma tissue samples.
Figure 1.
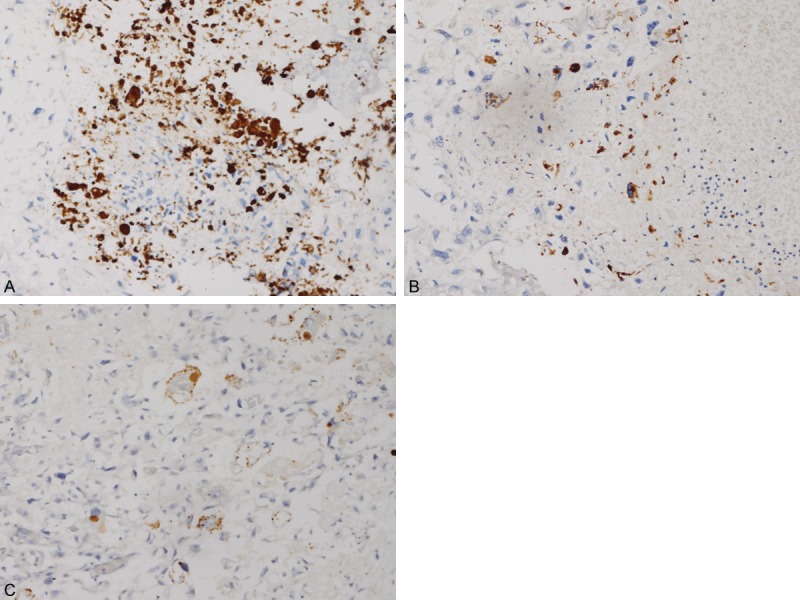
Immunohistochemical staining of vitronectin in osteosarcoma tissues. A. Strong for vitronectin staining in osteosarcoma, × 200; B. Weak for vitronectin staining in osteosarcoma, × 200; C. Staining is observed in the nucleus of vascular endothelial cells, × 200.
Associations between vitronectin expression and clinicopathological characteristics
Of 67 osteosarcoma samples, 38 patients (56.7%) were male and 29 patients (43.3%) were female. Median age at diagnosis was 20 years old (range 11-75). Median tumor size at diagnosis was 7 cm (range 2-17). Histologic subtype classification indicated there were 62 cases of conventional osteosarcoma and 5 cases of special osteosarcoma. With regard to tumor location, 43 cases were located in the tibia or femur and 24 in other locations. In order to estimate the effect of clinical characteristics on vitronectin expression, we analyzed the relationships between vitronectin expression and clinical factors, including gender, age, tumor size, histologic subtype, and tumor location. However, our findings suggested that there were no statistically noticeable correlations between vitronectin expression and patient gender (P = 0.675), age (P = 0.813), tumor size (P = 0.436), histologic subtype (P = 0.543) or tumor location (P = 0.456) (Table 1).
Table 1.
Relationships between Vitronectin expression and clinicopathological characteristics
| Clinicopathologic data | Case number | Vitronectin | P Value (Chi-square test) | |
|---|---|---|---|---|
|
| ||||
| Weak | Strong | |||
| Gender | ||||
| Male | 38 | 19 | 19 | 0.675 |
| Female | 29 | 13 | 16 | |
| Age (years) | ||||
| < 18 | 24 | 11 | 13 | 0.813 |
| ≥ 18 | 43 | 21 | 22 | |
| Tumor size | ||||
| < 5 | 16 | 9 | 7 | 0.436 |
| ≥ 5 | 51 | 23 | 28 | |
| Histologic subtype | ||||
| Conventional | 62 | 30 | 32 | 0.543 |
| Special | 5 | 2 | 3 | |
| Tumor location | ||||
| Tibia or Femur | 43 | 22 | 21 | 0.456 |
| Other | 24 | 10 | 14 | |
Relationships of vitronectin expression with clinical outcome in osteosarcoma
To evaluate the influence of percentage of vitronectin-positive expression on patient prognosis, corresponding PFS and OS were calculated. Out of 67 patients, the median PFS and OS were 25 months [95% confidence interval (CI) 20-33] and 31 months (95% CI 27-39), respectively. Univariate survival analysis (Table 2) showed stronger vitronectin expression was correlated with shorter PFS (P = 0.002, log-rank test, Figure 2A) and OS (P = 0.001, log-rank test, Figure 2B). In addition, similar results were achieved for tumor size (Figure 2C, 2D) and neo-adjuvant chemotherapy (Figure 2E, 2F). However, we observed no significant difference between gender, age, histologic subtype, tumor location and PFS or OS. Multivariate survival analysis (Table 3) revealed strong vitronectin expression could serve as a significant prognostic predictor of shorter PFS (HR 2.788, P = 0.003), independent of tumor size and neo-adjuvant chemotherapy; similar results were identified for OS (HR 2.827, P = 0.003). In the same line, big tumor size and non-neoadjuvant chemoradiotherapy were also proved to be independent indicators of PFS and OS.
Table 2.
Univariate analysis of variables associated with PFS and OS by log-rank test
| Variables | Case number | PFS (months) | OS (months) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Median | 95% CI | P value | Median | 95% CI | P value | ||
| Vitronectin | |||||||
| Low | 32 | 44 | 31-57 | 0.002 | 52 | 46-58 | 0.001 |
| High | 35 | 19 | 8-30 | 27 | 14-40 | ||
| Gender | |||||||
| Male | 38 | 22 | 13-31 | 0.316 | 28 | 16-40 | 0.283 |
| Female | 29 | 43 | 19-67 | 48 | 31-65 | ||
| Age (years) | |||||||
| < 18 | 24 | 43 | 21-72 | 0.606 | 51 | 24-85 | 0.555 |
| ≥ 18 | 43 | 25 | 16-34 | 36 | 22-50 | ||
| Tumor size | |||||||
| < 5 | 16 | 59 | 29-88 | 0.043 | 52 | 36-68 | 0.044 |
| ≥ 5 | 51 | 22 | 16-28 | 30 | 21-39 | ||
| Histologic subtype | |||||||
| Conventional | 62 | 28 | 9-47 | 0.806 | 39 | 27-51 | 0.739 |
| Special | 5 | 43 | 14-73 | 51 | 13-89 | ||
| Tumor location | |||||||
| Tibia or Femur | 43 | 24 | 14-33 | 0.264 | 36 | 15-57 | 0.343 |
| Other | 24 | 56 | 27-85 | 45 | 25-65 | ||
| Neoadjuvant | |||||||
| Yes | 41 | 43 | 12-74 | 0.022 | 56 | 35-77 | 0.027 |
| No | 26 | 21 | 17-25 | 36 | 26-34 | ||
Figure 2.

Kaplan-Meier curves showing a correlation of percentage of vitronectin positive tumor cells with PFS (A) and OS (B), tumor size with PFS (C) and OS (D) and neo-adjuvant chemoradiotherapy with PFS (E) and OS (F) in the entire cohort. The osteosarcoma patients with vitronectin-strong expression demonstrated significantly poorer OS and DFS rates than those with vitronectin-weak expression, so do osteosarcoma patients with tumor size > 5 cm or without neoadjuvant chemotherapy.
Table 3.
Multivariate analysis of variables associated with OS and PFS
| Variables | OS | PFS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Vitronectin | 2.827 | 1.438-5.554 | 0.003 | 2.788 | 1.420-5.474 | 0.003 |
| Neoaujuvant | 0.478 | 0.251-0.911 | 0.025 | 0.446 | 0.234-0.851 | 0.014 |
| Tumor size | 3.037 | 1.244-7.412 | 0.015 | 3.357 | 1.355-8.317 | 0.009 |
Discussion
Osteosarcoma is the most common primary malignant bone tumor [20], and has a high capacity for distant metastasis, predominantly seen in lung tissues. With the application of comprehensive treatment including preoperative chemotherapy, operation and postoperative chemotherapy, 5-year survival rate of patients has remarkably enhanced to 50%-60% [21,22] while metastasis or recurrence of the disease still remains as high as 30 to 40% and the disease is fatal to the majority of the patients [23]. Thus, to explore the tumor metastasis-associated biomarkers of osteosarcoma is of great significance and urgent need.
Vitronectin plays a role in guiding cell adhesion, migration, cell cycle progression and cell differentiation. It is noteworthy that vitronectin is involved in facilitating accelerated disease progression of many cancers, such as human breast cancer [13], melanoma [14], hepatocellular carcinoma [15] and colorectal adenocarcinoma etc. [16]. In this paper, we demonstrated for the first time that high percentage of vitronectin expression in osteosarcoma is a predictor of poor survival, importantly, it is an independent outcome factor in patients with osteosarcoma, suggesting that vitronectin is associated with osteosarcoma progression and metastasis.
There may be several mechanisms for vitronectin to enhance the progression and metastasis of osteosarcoma. It had been demonstrated that vitronectin may induce cancer stem cell differentiation by downregulation of stem cell genes through αvβ3 integrin-mediated mechanisms [24] and seems to influence cell adhesion and motility as well as formation of cancer cell aggregates [25]. On the cellular phase, VN-binding integrins (both on tumor and endothelial cells) and uPAR system together play an essential role for tumor progression and neovascularization, and these cell surface molecules may offer feasible therapeutic alternatives for imaging and cancer treatment [10-12]. VN binding played a key role in tumor growth through accelerating tumor activity of uPAR in vivo, and host deficiency in VN greatly damaged tumor formation [26]. Recent studies show that vitronectin significantly promoted Vascular Endothelial Growth Factor-Mediated Angiogenesis [27]. And VEGF overexpression indicated a poor prognosis for patients with osteosarcoma had been verified by a meta-analysis [28]. Tumor angiogenesis is a prerequisite for solid tumor growth and contributes to the initial step of metastatic spreading, facilitating the dissemination of tumor cells into the blood circulation [29]. Meanwhile, as a main Receptor, αvβ3 integrin expression improved the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. And echistatin, a RGD-containing peptide antagonist of αvβ3, decreased osteosarcoma cell adhesion to vitronectin [30]. Thus, vitronectin blockade is an attractive candidate for therapy in humans with osteosarcoma to cut down metastasis from primary to lung tissue and potentially improve patient outcomes.
Vitronectin expression was also seen in vascular endothelial cells in our study, suggesting that the cross talk between vascular endothelial cells and sarcoma cells might be mediated by vitronectin signal molecular, which could facilitate tumor angiogenesis and even may be associated with invasion and metastasis. Furthermore, we found there were no associations between vitronectin expression and gender, age, tumor size, tumor location, implying that patient age, gender or performance status might not influence vitronectin expression level.
Whereas, some limitations are still found in our study. Firstly, as a retrospective study and being specifically designed, it is highly possible that some biases may occur when collecting patient information. Secondly, sample size of 67 cases is relatively small, which would require further large-scale studies to be done in order to offer more convincing evidence for the future clinical employment of VN inhibition.
In conclusion, the findings of our study revealed that higher vitronectin expression was noted in osteosarcoma tissues compared with osteochondroma tissues and its overexpression in patients with osteosarcoma had no correlation with clinicopathological characteristics including gender, age, tumor size, histologic subtype and tumor location. And we confirmed that the high percentage of vitronectin positive tumor cells in osteosarcoma was considered as a vital independent predictor for poorer prognosis, indicating that the overexpression of VN is a promising prognostic factor for osteosarcoma progression and metastasis. Hence, our study suggested that the inhibition of vitronectin may be a potential therapeutic target in osteosarcoma.
Acknowledgements
This study was funded by key project of Fujian Science and Technology Department (grant number 2011YZ0002-1).
Disclosure of conflict of interest
None.
References
- 1.Iwamoto Y, Tanaka K, Isu K, Kawai A, Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, Furuse K, Minamizaki T, Kawaguchi N, Yamawaki S. Multiinstitutional phase II study of neoadjuvant chemotherapy for osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop Sci. 2009;14:397–404. doi: 10.1007/s00776-009-1347-6. [DOI] [PubMed] [Google Scholar]
- 2.Melillo G, Semenza GL. Meeting report: exploiting the tumor microenvironment for therapeutics. Cancer Res. 2006;66:4558–60. doi: 10.1158/0008-5472.CAN-06-0069. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–16. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 4.DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ, Raz A, Matrisian LM, Sloane BF, Noel A, Hendrix MJ, Coussens L, Padarathsingh M. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol. 2004;164:1131–9. doi: 10.1016/S0002-9440(10)63200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 6.Izumi M, Yamada KM, Hayashi M. Vitronectin exists in two structurally and functionally distinct forms in human plasma. Biochim Biophys Acta. 1989;990:101–8. doi: 10.1016/s0304-4165(89)80019-4. [DOI] [PubMed] [Google Scholar]
- 7.Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 8.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539–44. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 9.Pola C, Formenti SC, Schneider RJ. Vitronectin-αvβ3 integrin engagement directs hypoxia-resistant mTOR activity and sustained protein synthesis linked to invasion by breast cancer cells. Cancer Res. 2013;73:4571–8. doi: 10.1158/0008-5472.CAN-13-0218. [DOI] [PubMed] [Google Scholar]
- 10.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rüegg C, Alghisi GC. Vascular integrins: therapeutic and imaging targets of tumor angiogenesis. Recent Results Cancer Res. 2010;180:83–101. doi: 10.1007/978-3-540-78281-0_6. [DOI] [PubMed] [Google Scholar]
- 12.Mekkawy AH, Morris DL, Pourgholami MH. Urokinase plasminogen activator system as a potential target for cancer therapy. Future Oncol. 2009;5:1487–99. doi: 10.2217/fon.09.108. [DOI] [PubMed] [Google Scholar]
- 13.Aaboe M, Offersen BV, Christensen A, Andreasen PA. Vitronectin in human breast carcinomas. Biochim Biophys Acta. 2003;1638:72–82. doi: 10.1016/s0925-4439(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 14.Tas F, Karabulut S, Bilgin E, Tastekin D, Duranyildiz D. Clinical significance of serum fibronectin and vitronectin levels in melanoma patients. Melanoma Res. 2014;24:475–9. doi: 10.1097/CMR.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 15.Zhu W, Li W, Yang G, Fu C, Jiang G, Hu Q. Vitronectin silencing inhibits hepatocellular carcinoma in vitro and in vivo. Future Oncol. 2015;11:251–8. doi: 10.2217/fon.14.202. [DOI] [PubMed] [Google Scholar]
- 16.Tomasini-Johansson BR, Sundberg C, Lindmark G, Gailit JO, Rubin K. Vitronectin in colorectal adenocarcinoma--synthesis by stromal cells in culture. Exp Cell Res. 1994;214:303–12. doi: 10.1006/excr.1994.1262. [DOI] [PubMed] [Google Scholar]
- 17.Kamiyama F, Maeda T, Yamane T, Li YH, Ogukubo O, Otsuka T, Ueyama H, Takahashi S, Ohkubo I, Matsui N. Inhibition of vitronectin-mediated haptotaxis and haptoinvasion of MG-63 cells by domain 5 (D5(H)) of human high-molecular-weight kininogen and identification of a minimal amino acid sequence. Biochem Biophys Res Commun. 2001;288:975–80. doi: 10.1006/bbrc.2001.5864. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Kobuke S, Haruyama N, Hoshino H, Kulkarni AB, Nishimura F. Adhesive and migratory effects of phosphophoryn are modulated by flanking peptides of the integrin binding motif. PLoS One. 2014;9:e112490. doi: 10.1371/journal.pone.0112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF, Kleinerman ES. Association of alphavbeta3 integrin expression with the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. Clin Exp Metastasis. 2004;21:747–53. doi: 10.1007/s10585-005-0599-6. [DOI] [PubMed] [Google Scholar]
- 20.Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137:89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Harting MT, Blakely ML. Management of osteosarcoma pulmonary metastases. Semin Pediatr Surg. 2006;15:25–9. doi: 10.1053/j.sempedsurg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Carrle D, Bielack S. Osteosarcoma lung metastases detection and principles of multimodal therapy. Cancer Treat Res. 2009;152:165–84. doi: 10.1007/978-1-4419-0284-9_8. [DOI] [PubMed] [Google Scholar]
- 23.Hu F, Wang W, Zhou HC, Shang XF. High expression of periostin is dramatically associated with metastatic potential and poor prognosis of patients with osteosarcoma. World J Surg Oncol. 2014;12:287. doi: 10.1186/1477-7819-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurt EM, Chan K, Serrat MA, Thomas SB, Veenstra TD, Farrar WL. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells. 2010;28:390–8. doi: 10.1002/stem.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellouche S, Fernandes J, Leroy-Dudal J, Gallet O, Dutoit S, Poulain L, Carreiras F. Initial formation of IGROV1 ovarian cancer multicellular aggregates involves vitronectin. Tumour Biol. 2010;31:129–39. doi: 10.1007/s13277-010-0017-9. [DOI] [PubMed] [Google Scholar]
- 26.Pirazzoli V, Ferraris GM, Sidenius N. Direct evidence of the importance of vitronectin and its interaction with the urokinase receptor in tumor growth. Blood. 2013;121:2316–23. doi: 10.1182/blood-2012-08-451187. [DOI] [PubMed] [Google Scholar]
- 27.Li R, Luo M, Ren M, Chen N, Xia J, Deng X, Zeng M, Yan K, Luo T, Wu J. Vitronectin regulation of vascular endothelial growth factor-mediated angiogenesis. J Vasc Res. 2014;51:110–7. doi: 10.1159/000360085. [DOI] [PubMed] [Google Scholar]
- 28.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, Zhang CQ. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–60. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 29.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 30.Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF, Kleinerman ES. Association of alphavbeta3 integrin expression with the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. Clin Exp Metastasis. 2004;21:747–53. doi: 10.1007/s10585-005-0599-6. [DOI] [PubMed] [Google Scholar]


