Abstract
Background: Alpha-fetoprotein (AFP) levels are routinely used for diagnosis and monitoring of hepatic diseases, but it has a limited value. Golgi protein 73 (GP73) has been suggested as a new marker for hepatic diseases. Objective: To explore the clinical value of serum GP73 in different diseases associated with hepatitis B virus (HBV) infection. Method: Between January 2010 and August 2014, serum samples from 88 patients with chronic hepatitis B (CHB), 78 patients with HBV-related liver cirrhosis (LC), and 194 patients with HBV-related primary hepatic cancer (PHC) were collected. Serum samples from 30 healthy volunteers were used as controls. ELISA and microparticle enzyme immunoassay were used to measure serum GP73 and AFP levels. Receiver operating characteristic (ROC) curves were used to analyze the diagnostic value of serum GP73 and AFP for PHC. Results: For the diagnosis of PHC, GP73 showed a sensitivity of 65.5% and specificity of 66.3%, while AFP levels showed sensitivity of 64.4% and specificity of 76.5%. Serial testing (both tests are positive) could increase the specificity (sensitivity of 45.9% and specificity of 85.5%) while parallel testing (any single positive test result) could increase the sensitivity (sensitivity of 84.0% and specificity of 57.2%). Serum GP73 and AFP levels were significantly different between Child-Pugh grades (P<0.001 for GP73 and P=0.044 for AFP). Significant differences in serum GP73 and AFP were found between TNM stages (all P<0.001). Conclusion: Serum GP73 had limited diagnostic value for HBV-related PHC. The combined use of serum GP73 and AFP levels improved the diagnostic efficacy.
Keywords: Hepatitis B virus, primary hepatic carcinoma, Golgi protein 73, alpha fetoprotein
Introduction
Early diagnosis of hepatic carcinoma is a clinical challenge and has been the focus of many research efforts. Alpha-fetoprotein (AFP) has been used for decades, but its sensitivity and specificity are only of 40-65% and 76-96%, respectively [1-3]. Other tumor markers like gamma-glutamyltransferase (GGT) and alkaline phosphatase (ALP) are sometimes used, but they are only auxiliary diagnostic indices that cannot satisfy the clinical needs [3].
Golgi protein 73 (GP73) is a new promising biomarker for the diagnosis of primary hepatic carcinoma (PHC). GP73 is a 73-kDa transmembrane glycoprotein first discovered by Kladney et al [4]. In normal adults, the hepatic concentration of GP73 is very low since GP73 is mainly expressed by the biliary epithelial cells but not by liver cells [5]. However, in patients with hepatic diseases, the hepatic expression of GP73 is significantly upregulated [6,7]. Therefore, GP73 might provide a solution for the early diagnosis of hepatic diseases. A study by Marrero et al. [8] has shown that GP73 had a 62% sensitivity for the early diagnosis of hepatic carcinoma compared with 25% for AFP. Several reports indicated that GP73 levels have an excellent clinical value for the diagnosis of PHC [8,9] and that the combined use of GP73 and AFP could even improve the detection rate of PHC [10]. On the other hand, Ozkan et al. [11] suggested that GP73 has a low value for the diagnosis and prognosis of early PHC while AFP was better. However, Ozkan et al. [11] included patients with hepatitis C virus (HCV)-associated PHC and their results are still controversial [12,13]. In addition, the incidence of HCV-associated PHC is higher in Europe and America than in Asia [14], while hepatitis B virus (HBV)-associated PHC is more frequent in Asia than in Europe and America [15]. Therefore, there is no conclusion on the diagnostic value of GP73, in particular for the early diagnosis of HBV-associated PHC. Furthermore, most of these previous studies used semi-quantitative methods for the measurement of GP73.
In 2009, a Chinese team invented a double-antibody sandwich ELISA to detect the serum GP73 levels [6]. However, their study revealed that serum GP73 levels were significantly different between patients with and without hepatic diseases, while increases of serum GP73 levels in patients with PHC were not significantly different from those of patients with other hepatic diseases.
Therefore, the issue of early diagnosis of PHC is still unresolved. Consequently, the present study aimed to investigate the diagnostic value of GP73 for PHC in patients with chronic HBV infection.
Patients and methods
Study design
This was a cross-sectional study that was carried out between January 2010 and August 2014 at the Ningbo No. 2 Hospital (Zhejiang, China). This study was approved by the Ethical Committee of the Ningbo No. 2 Hospital. All patients signed the informed consent.
Patients
Patients had to have received a confirmed diagnosis of chronic hepatitis B (CHB), HBV-related liver cirrhosis (LC), or HBV-related PHC. The diagnoses of CHB and related complications were made according to the current guidelines for HBV infection management [16]. Exclusion criteria were: 1) coinfection with hepatitis A, C, D or E; 2) infection with cytomegalovirus, human herpes virus or human immunodeficiency virus; 3) autoimmune hepatitis, fatty liver or alcoholic liver disease; 4) drug-induced hepatitis; or 5) missing data.
PHC was diagnosed according to internationally recognized standards [17]. The lesion had to be confirmed by pathological examination. The specific clinical diagnostic criteria were: 1) AFP >400 ng/mL after exclusion of active hepatic diseases, pregnancy, embryonic germline tumors, and metastatic liver cancer in the presence of palpable hepatic mass or imaging-confirmed space-occupying lesions with characteristics of hepatic carcinoma; or 2) AFP <400 ng/mL in the presence of a space-occupying lesion with characteristics of hepatic carcinoma confirmed by two imaging results (B-type ultrasound, CT, MRI, etc.) or two positive markers of hepatic carcinoma (AFP heterogeneity, or abnormal prothrombin, GGT isoenzyme II, and α-L-fucosidase) and one imaging-confirmed space-occupying lesions with characteristics of hepatic carcinoma, or clinical manifestation of hepatic carcinoma and affirmative extrahepatic metastatic lesions (including visible bloody ascites or cancer cells found in the ascites), with exclusion of metastatic cancer of liver. TNM staging and Child-Pugh scoring were applied to all patients with PHC [18,19].
Laboratory testing
Morning fasting venous blood (5 mL) was collected from all subjects. Serum was immediately isolated and stored at -80°C. An Abbott AxSYM Immunoassay System (Abbott Laboratories, Abbott Park, IL, USA) with original kits was used to perform the microparticle enzyme immunoassay for the measurement of AFP. An Olympus AU 2700 automatic biochemical analyzer with original kits (Olympus, Tokyo, Japan) was used to measure serum total protein (TP), albumin (ALB), aspartate transaminase (AST), alanine aminotransferase (ALT), AST/ALT, ALP, GGT and total bilirubin (TB).
Measurement of GP73
The quantitative GP73 enzyme-linked immune sorbent assay (ELISA) 96T detection kit (Hotgen Biotech, Beijing, China, lot No.: 20140502) and the Anthos PHOMO microplate reader (Anthos Labtec Instruments, Austria) were used. This kit uses the double-antibody sandwich ELISA method to quantify the GP73 concentrations from serum or plasma samples. The microplate is pre-coated with the monoclonal antibody against GP73, which binds the GP73 of the samples; the complex is then detected using HRP-conjugated GP73-polyclonal antibody and 3,3’,5,5’-tetramethylbenzidine as HRP substrate. The optical density (OD) value is measured by the microplate reader and a standard curve is created to calculate the GP73 concentration in the samples.
Statistical analysis
All data were analyzed using SPSS 16.0 (IBM, Armonk, NY, USA). The distribution of serum GP73, AFP, ALT, AST, AST/ALT, ALP, GGT, TP, ALB, and PT were skewed. Therefore, they are expressed as medians (quartiles) and were analyzed using the Mann Whitney U test (for pairwise comparisons), and the Kruskal-Wallis H test and Nemenyi test (for comparisons of multiple groups). Age and BMI were normally distributed. They are expressed as means ± standard deviation and were analyzed using the Student’s t test. Categorical data are expressed as frequencies. The receiver operating characteristic (ROC) curve was plotted and the area under the curve (AUC) was used to assess the diagnostic values of serum GP73 and AFP for PHC. The Z test for normality was used for the comparison of AUC. Two-sided P-values <0.05 indicated statistical significance.
Results
Enrollment
Figure 1 presents the patients’ flowchart. A total of 373 patients with HBV infections were enrolled. One patient with HCV infection, four with fatty liver, three with alcoholic liver, one with autoimmune hepatitis, one drug-induced hepatitis, and three with missing data were excluded. Therefore, 360 patients were enrolled. Among these 360 patients, 88 had CHB (72 men and 16 women, mean age of 38.7±11.6 years old; 68, 19, and 1 were Child-Pugh grade A, B, and C, respectively), 78 had HBV-related LC (53 men and 23 women, mean age of 55.1±10.9 years old; 30, 27, and 21 were Child-Pugh grade A, B and, C, respectively), and 194 had HBV-related PHC (162 men and 32 women, mean age of 55.9±11.0 years old; 89, 70, and 35 were Child-Pugh grade A, B, and C, respectively; 34, 47, 91, and 22 were stage I, II, III, and IV, respectively; early hepatic carcinoma included TNM stages I and II). Table 1 presents the characteristics of the patients.
Figure 1.

Patients’ flowchart.
Table 1.
Characteristics of the patients
| HBV and HBV-LC | PHC | P | |
|---|---|---|---|
|
|
|||
| N=166 | N=194 | ||
| Sex (male, %) | 125, 75.3% | 162, 83.5% | 0.054 |
| Age | 46.3±13.9 | 55.9±11.0 | <0.001 |
| BMI (kg/m2) | 23.3±2.7 | 21.9±2.6 | <0.001 |
| Comorbidities (n, %) | 15, 9.0% | 43, 22.2% | <0.001 |
| Diabetes (n) | 7 | 12 | |
| Hypertension (n) | 7 | 30 | |
| Coronary heart disease (n) | 1 | 1 | |
| Child-Pugh | 0.045 | ||
| A (n, %) | 98, 59.0% | 89, 45.9% | |
| B (n, %) | 46, 27.7% | 70, 36.1% | |
| C (n, %) | 22, 13.3% | 35, 18.0% | |
| HBeAg positive (n, %) | 83, 50.0% | 51, 26.3% | <0.001 |
| HBV DNA >2 log (n, %) | 120, 72.3% | 116, 59.8% | 0.013 |
| Prothrombin time (s) | 14.45 (13.20-16.88) | 13.05 (11.70-15.08) | <0.001 |
| Total bilirubin (mmol/L) | 26.75 (15.83-48.70) | 21.55 (14.90-44.03) | 0.195 |
| TP | 66.60 (61.45-72.33) | 66.55 (60.90-71.93) | 0.471 |
| ALB | 35.60 (29.50-40.48) | 32.40 (28.45-36.70) | 0.001 |
| ALT | 47.50 (27.00-135.00) | 41.00 (27.00-63.75) | 0.055 |
| AST | 59.50 (36.25-103.00) | 58.00 (40.25-100.75) | 0.477 |
| AST/ALT | 1.08 (0.68-1.56) | 1.48 (1.11-1.99) | <0.001 |
| ALP | 102.50 (80.25-142.00) | 117.00 (86.00-197.50) | <0.001 |
| GGT | 73.50 (36.25-132.00) | 105.50 (54.00-194.75) | <0.001 |
| AFP | 9.73 (4.34-42.38) | 112.43 (13.82-1916.85) | <0.001 |
| GP73 | 123.25 (87.22-172.75) | 179.33 (111.52-249.36) | <0.001 |
HBV: hepatitis B virus; LC: liver cirrhosis; PHC: primary hepatic cancer; BMI: body mass index; TP: total protein; ALB: albumin; ALT: alanine transaminase; AST: aspartate transaminase; ALP: alkaline phosphatase; GGT: gamma-glutamyl transpeptidase; AFP: alpha-fetoprotein; GP73: Golgi protein 73.
ROC curve analysis of the diagnostic efficiency of GP73 and AFP
Results from the ROC curve analysis for the whole population (normal volunteers and those with HBV-related diseases) indicated a slightly poorer diagnostic efficiency for PHC with serum GP73 levels compared with serum AFP levels (Z=2.177, P=0.029) (Figure 2).
Figure 2.
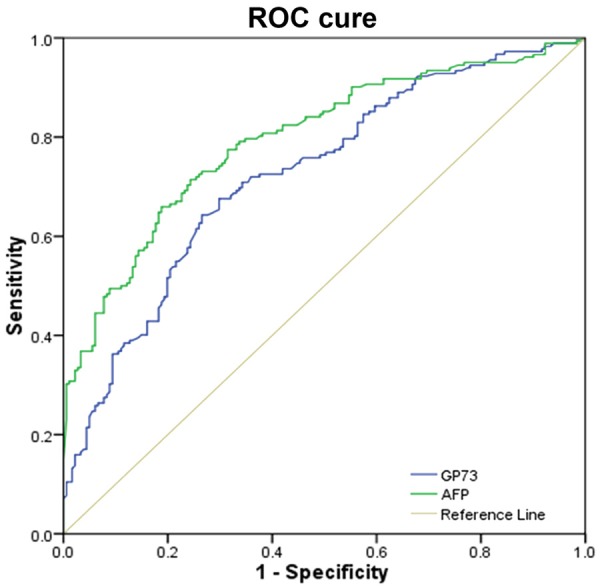
ROC curve of the efficiency of serum GP73 and AFP in diagnosing PHC from the whole study population.
Diagnostic value of serum GP73 and AFP levels for PHC
Based on the ROC curve analysis, the cutoff values for AFP [43.26 ng/mL, AUC (95% CI) 0.776 (0.731-0.816), P<0.001] and GP73 [145 ng/mL, AUC (95% CI) 0.713 (0.666-0.758), P<0.001] showed that some PHC patients with normal AFP levels had elevated GP73 level, indicating that these two indices were to some extend complementary. Therefore, we further investigated the combination of the two markers, and found that serial testing (both rests are positive) could increase the specificity while parallel testing (any single positive test result) could increase the sensitivity (Table 2).
Table 2.
Diagnostic values of GP73 and AFP for PHC in patients with CHB
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| AFP | 64.4% (125/194) | 76.5% (127/166) | 76.2% (125/164) | 64.8% (127/196) |
| GP73 | 65.5% (127/194) | 66.3% (110/166) | 69.4% (127/183) | 62.2% (110/177) |
| AFP+GP73 | 45.9% (89/194) | 85.5% (142/166) | 78.2% (89/113) | 57.5% (142/247) |
| AFP or GP73 | 84.0% (163/194) | 57.2% (95/166) | 69.7% (163/234) | 75.4% (95/126) |
PPV: positive predictive value; NPV: negative predictive value; AFP: alpha-fetoprotein; GP73: Golgi protein 73.
Comparison of serum GP73 levels in PHC with various disease degrees (Child-Pugh A, B, and C)
Serum GP73 and AFP levels were significantly different between Child-Pugh grades (P<0.001 for GP73 and P=0.044 for AFP). Serum GP73 levels were significantly increased with the Child-Pugh grade (all P<0.05) while the difference in AFP levels was only significant between Child-Pugh grades A and C (P<0.05) (Table 3).
Table 3.
Comparison of GP73 and AFP levels according to Child-Pugh stages and TNM staging
| Child-Pugh grade | A (n=88) | B (n=71) | C (n=35) | P | |
|
| |||||
| GP73 (ng/mL) | 139.8 (97.50-191.50) | 192.5 (138.10-246.00) | 260 (211.00-323.50) | <0.001 | |
| AFP (ng/mL) | 79.55 (9.33-513.01) | 140.23 (29.60-1269.00) | 907.58 (24.28-14670.00) | 0.044 | |
|
| |||||
| TMN stage | I (n=34) | II (n=47) | III (n=91) | IV (n=22) | P |
|
| |||||
| GP73 (ng/mL) | 158 (84.00-245.02) | 146 (97.00-191.20) | 192 (138.67-254.00) | 215.24 (159.50-282.03) | 0.001 |
| AFP (ng/mL) | 46.09 (8.38-154.40) | 31.06 (7.00-228.28) | 260.5 (32.40-1000.00) | 454.55 (44.65-6431.00) | <0.001 |
Comparison of serum GP73 levels in patients with different TNM stages
Significant differences in serum GP73 and AFP were found between the TNM stages (all P<0.001). Serum GP73 and AFP levels were increased with TNM stages, with significantly higher levels of serum GP73 and AFP found in patients with stages III and IV compared with stages I and II (all P<0.05) (Table 3).
Discussion
The aim of the present study was to explore the clinical value of serum GP73 in different diseases associated with HBV infection. Results showed that for the diagnosis of PHC, GP73 showed a sensitivity of 65.5% and specificity of 66.3%, while AFP levels showed sensitivity of 64.4% and specificity of 76.5%. Serial testing (both rests are positive) could increase the specificity (sensitivity of 45.9% and specificity of 85.5%) while parallel testing (any single positive test result) could increase the sensitivity (sensitivity of 84.0% and specificity of 57.2%). Serum GP73 and AFP levels were significantly different between Child-Pugh grades. Significant differences in serum GP73 and AFP were found between TNM stages.
The results of the present study suggest that serum GP73 had a limited value for the diagnosis of HBV-related PHC and therefore could not replace AFP for the diagnosis of PHC, which is consistent with some previous studies [2,20]. However, some other studies reported that GP73 had a better diagnostic value than AFP [8,12,21,22]. These differences might be due to the populations being studied including the causes of liver cancer, ethnicity, level of income, etc.
Previous studies revealed that the combined use of serum GP73 and AFP could increase the diagnostic efficiency for PHC [20]. Therefore, we analyzed the complementarity between the two tests, and found that they were actually complementary to one another since some patients with PHC had normal AFP levels but elevated GP73 levels. Therefore, we further investigated the combined diagnosis targeting the differentiation from PHC from HBV-related LC, and found that serial testing (the two tests are positive) could increase the specificity while parallel testing (only one positive test) could increase the sensitivity. As a result, it might be possible to use the two tests to diagnose PHC.
Our results showed that serum GP73 increased with disease severity of CHB, LC, and PHC, indicating that serum GP73, to some extent, reflect liver damage. Indeed, Marrero et al. [8] showed that GP73 were higher in patients with early PHC compared with cirrhosis without cancer. Hu et al. [22] showed that GP73 had a good capability to differentiate cirrhosis from stage I and II PHC. Wang et al. [21] showed that GP73 were lower in patients with cholangiocarcinoma compared with patients with HBV-induced PHC.
In addition, the present study revealed that serum GP73 and AFP levels were increased with TNM stages, and significantly higher levels of serum GP73 and AFP were found in patients with stage III and IV cancer compared with stages 1 and 2. These results are consistent with previous studies [23,24], but it is still controversial [6,11].
The present study is not without limitations. Indeed, the sample size was relatively small. In addition, only two serum markers were examined. A more comprehensive panel of markers could reveal even better combinations for the diagnosis of PHC. Indeed, a previous study revealed that serum GP73 combined with serum desgamma carboxy prothrombin (DCP) provided a good diagnostic yield [25]. Additional studies are necessary to address these issues, and large-scale diagnostic studies are necessary to determine the best cut points.
Conclusion
Serum GP73 had limited diagnostic value for HBV-related PHC. The combined use of serum GP73 and AFP levels improved the diagnostic efficacy.
Acknowledgements
The study was supported by Chinese foundation for hepatitis prevention and control-Tian Qing liver disease research fund subject (TQGB20120081).
Disclosure of conflict of interest
None.
References
- 1.Jorge MS, Vasconcelos MG, Junior EF, Barreto LA, Rosa LR, de Lima LL. [Solvability of mental health care in the Family Health Strategy: social representation of professionals and users] . Rev Esc Enferm USP. 2014;48:1060–1068. doi: 10.1590/S0080-623420140000700014. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272–1282. doi: 10.1007/s00535-010-0278-5. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249:53–65. doi: 10.1016/S0378-1119(00)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol. 2012;5:874–881. [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Y, Chen W, Zhao Y, Chen L, Peng T. Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Ann Clin Biochem. 2009;46:38–43. doi: 10.1258/acb.2008.008088. [DOI] [PubMed] [Google Scholar]
- 7.Wright LM, Huster D, Lutsenko S, Wrba F, Ferenci P, Fimmel CJ. Hepatocyte GP73 expression in Wilson disease. J Hepatol. 2009;51:557–564. doi: 10.1016/j.jhep.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, Chan DW, Dowell BL. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, alpha-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011;49:711–718. doi: 10.1515/CCLM.2011.097. [DOI] [PubMed] [Google Scholar]
- 10.Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Mullhaupt B, Clavien PA, Bahra M, Neuhaus P, Wild P, Fritzsche F, Moch H, Jochum W, Kristiansen G. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49:1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- 11.Ozkan H, Erdal H, Tutkak H, Karaeren Z, Yakut M, Yuksel O, Koklu S. Diagnostic and prognostic validity of Golgi protein 73 in hepatocellular carcinoma. Digestion. 2011;83:83–88. doi: 10.1159/000320379. [DOI] [PubMed] [Google Scholar]
- 12.Cao FF, Yu S, Jiang ZY, Bao YX. Diagnostic accuracy of Golgi protein 73 in primary hepatic carcinoma using ELISA: a systematic review and meta-analysis. Clin Lab. 2014;60:587–597. doi: 10.7754/clin.lab.2013.130312. [DOI] [PubMed] [Google Scholar]
- 13.Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1:593–598. doi: 10.3892/mco.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveria Andrade LJ, D’Oliveira A, Melo RC, De Souza EC, Costa Silva CA, Parana R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1:33–37. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi: 10.1111/jgh.12220. [DOI] [PubMed] [Google Scholar]
- 16.Uribe LA, O’Brien CG, Wong RJ, Gish RR, Tsai N, Nguyen MH. Current treatment guidelines for chronic hepatitis B and their applications. J Clin Gastroenterol. 2014;48:773–783. doi: 10.1097/MCG.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 17.Hepatobiliary Cancers (version 2.2015) Fort Washington: National Comprehensive Cancer Network; 2015. NCCN Clinical Practice Guidelines in Oncology (NCCN Guildeines) [Google Scholar]
- 18.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 19.Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 20.Tian L, Wang Y, Xu D, Gui J, Jia X, Tong H, Wen X, Dong Z, Tian Y. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer. 2011;129:1923–1931. doi: 10.1002/ijc.25838. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Long RE, Comunale MA, Junaidi O, Marrero J, Di Bisceglie AM, Block TM, Mehta AS. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:1914–1921. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu JS, Wu DW, Liang S, Miao XY. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Med Oncol. 2010;27:339–345. doi: 10.1007/s12032-009-9215-y. [DOI] [PubMed] [Google Scholar]
- 23.Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Graff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR, Fimmel CJ. Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol. 2004;99:1087–1095. doi: 10.1111/j.1572-0241.2004.30572.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G, Lu X, Sang X, Zhao H, Zhong S, Huang J, Zhang H. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J Gastroenterol Hepatol. 2011;26:1207–1212. doi: 10.1111/j.1440-1746.2011.06733.x. [DOI] [PubMed] [Google Scholar]
- 25.Huo Q, Zheng Z, Liu J, Li C, Hu C. [Diagnosis value of combined detection of serum golgi protein 73, desgamma carboxy prothrombin and alpha-fetoprotein in primary hepatic carcinoma] . Zhonghua Yi Xue Za Zhi. 2015;95:757–760. [PubMed] [Google Scholar]


