Abstract
There have been several studies on gallbladder carcinogenesis, and mutations of the KRAS, TP53, and CDKN2A genes have been reported in gallbladder carcinoma. The DBC1 gene (deleted in breast cancer 1) was initially cloned from region 8p21, which was homozygously deleted in breast cancer. DBC1 has been implicated in cancer cell proliferation and death. The functional role of DBC1 in normal cells and the role of DBC1 loss in cancer are not entirely clear. And DBC1 expression and its clinical implications in gallbladder carcinoma have yet to be thoroughly elucidated. Therefore, we evaluated DBC1 expression in 104 gallbladder carcinoma tissues in relation to survival and other prognostic factors via immunohistochemical analysis. DBC1 expression was divided into two categories: high DBC1 expression was observed in 32/104 cases (30.8%) and low expression in 72/104 cases (69.2%). High DBC1 expression correlated significantly with favorable clinicopathologic variables. Furthermore, in survival analysis, the high-DBC1 expression group showed a better survival rate compared to the low-DBC1 expression group. In conclusion, high DBC1 expression is associated with several favorable clinicopathologic factors in gallbladder carcinoma. These findings suggest that loss of DBC1 expression plays a role in tumorigenesis and tumor progression in gallbladder carcinoma.
Keywords: DBC1 (CCAR2), gallbladder carcinoma
Introduction
Gallbladder carcinoma is a relatively uncommon neoplasm that shows considerable geographic variation in incidence [1]. Mortality rates are highest among American Indian women from the Southwest United States and among Chilean and Japanese women [2]. Gallbladder carcinoma has a propensity to directly invade the liver, and also frequently metastasizes to the liver and pericholedochal lymph nodes [3].
Several studies of gallbladder carcinogenesis have been performed, and mutations of the KRAS, TP53, and CDKN2A genes have been previously reported in gallbladder carcinoma [4]. Gallbladder carcinoma develops through accumulation of multiple genetic alterations involving oncogenes, tumor suppressor genes, and DNA repair genes [5]. Factors that have been evaluated as possible predictors of prognosis in gallbladder carcinoma include stage, surgical margins, grading, DNA content, KRAS, HER2 oncogene, and angiogenesis [6-10].
The DBC1 gene (deleted in breast cancer 1) was initially cloned from region 8p21, which was homozygously deleted in breast cancer. DBC1 messenger RNA is lost in several breast, lung, and colon cancer cell lines [11]. Loss of DBC1 results in the inhibition of cell death and possibly promotes tumorigenesis [12]. However, according to previous studies, the role of DBC1 in tumorigenesis is more puzzling. DBC1 is deleted in several types of cancer and has been suggested to suppress tumor development [11,13].
DBC1 expression and its clinical implications in gallbladder carcinoma have not been investigated. Therefore, we compared the expression levels of DBC1 in normal and cancer tissue. We evaluated the different DBC1 expression levels in gallbladder carcinoma tissue in relation to survival and other prognostic factors via immunohistochemical analysis.
Materials and methods
Patients and tissue samples
Tissue samples from 104 cases of gallbladder carcinoma were utilized in the present study. All tumors were surgically resected at Kyung Hee University Medical Center between 1982 and 2009. Surgical treatment for the 104 patients included the following: cholecystectomy with lymph node dissection and concomitant hepatic segmentectomy in 61 cases, cholecystectomy with concomitant hepatic segmentectomy in 25, laparoscopic cholecystectomy with lymph node dissection in 7 cases, and laparoscopic cholecystectomy alone in 11 cases. No preoperative chemotherapy or radiotherapy was performed. The age of the patients ranged from 27 to 85 years (median age: 61.9 years). The mean patient follow-up duration was 46.5 months (range: 2-247 months). Among the total of 104 patients, 48 (46.2%) patients died of disease and 41 (39.4%) patients remained alive at the study start date. Fifteen (14.4%) patients were lost during the follow-up period. Of the 104 patients, 16 (15.4%) had disease recurrence during the follow-up period. The mean disease-free interval was 17.8 months. For each case, three investigators (K.Y. Won, Y.W. Kim, and J.H. Lee) reviewed all of the original hematoxylin and eosin-stained sections. Clinicopathologic variables were evaluated, including age, gender, histologic grade, tumor size, primary tumor (pT), nodal (pN), and distant metastasis (M), TNM stage group, lymphatic invasion, vascular invasion, nerve invasion, status of the resection margin, and local recurrence. The TNM stage was classified in accordance with the 7th edition of the AJCC cancer staging protocols.
Immunohistochemistry
Immunohistochemistry was conducted on 4-µm tissue sections using the Bond Polymer Intense Detection system (Vision BioSystems, Victoria, Australia) according to the manufacturer’s instructions with minor modifications. In brief, 4-µm sections of formalin-fixed, paraffin-embedded tissue were deparaffinized using Bond Dewax Solution (Vision BioSystems), and an antigen retrieval procedure was conducted using Bond ER Solution (Vision BioSystems) for 30 minutes at 100°C. The endogenous peroxidase was quenched by incubating the tissues with hydrogen peroxide for 5 minutes. The sections were incubated for 15 minutes at ambient temperature with primary polyclonal antibodies for DBC1 (IHC-00135; Bethyl Laboratories, Montgomery, TX, USA) using a biotin-free polymeric horseradish peroxidase (HRP)-linker antibody conjugate system in a Bond-max automatic slide stainer (Vision BioSystems). The nuclei were counterstained with hematoxylin.
Evaluation of the immunohistochemical staining
The expression of DBC1, as determined by immunohistochemical staining, showed nuclear staining. DBC1 expression was analyzed using a semiquantitative scoring method. The score was calculated according to the intensity and proportion of the immunoreactivity. The intensity score was designated as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The proportion score was calculated as 0 (no staining), 1 (< 30% positivity of the tumor cells), 2 (30-60% positivity of the tumor cells) and 3 (≥ 60% positivity of the tumor cells). The total score was the sum of the intensity score and the proportion score. We regarded a total score of 0 to 4 as low expression and 5 to 6 as high expression. All slides were evaluated independently by two investigators (K.Y. Won and J.H. Lee) who were not aware of the identity of the patients or their clinical outcomes.
Statistical analysis
Pearson’s chi-squared test was employed to assess the association between DBC1 expression and several clinicopathologic variables. Univariate and multivariate survival analyses were used to investigate the prognostic value of DBC1 expression. Curves for overall survival were drawn according to the Kaplan-Meier method and differences were analyzed using the log rank test for univariate survival analysis. Multivariate survival analysis was performed on variables that achieved statistical significance in univariate survival analysis, using the Cox proportional hazards model (95% confidence interval) with a backward stepwise elimination method. Statistical analyses were performed using the SPSS software package (version 15.0; SPSS, Inc., Chicago, IL, USA). Overall survival was defined as survival from the date of surgery to the date of death due to cancer. A P-value of < 0.05 was regarded as statistically significant.
Results
DBC1 expression and its association with clinicopathologic variables
Adjacent normal gallbladder epithelial cells demonstrated diffuse moderate to strong nuclear DBC1 expression (Figure 1A, 1B). The DBC1 expression in areas of carcinoma was variable compared to the adjacent normal gallbladder mucosa. High DBC1 expression was observed in 32/104 cases (30.8%) (Figure 1C-F) and low expression in 72/104 cases (69.2%) (Figure 1G, 1H). DBC1 expression was significantly correlated with histologic grade (P = 0.022), lymph node metastasis (P = 0.040), TNM stage (P = 0.040), lymphatic invasion (P = 0.015), and surgical margin involvement (P = 0.031) (Table 1).
Figure 1.
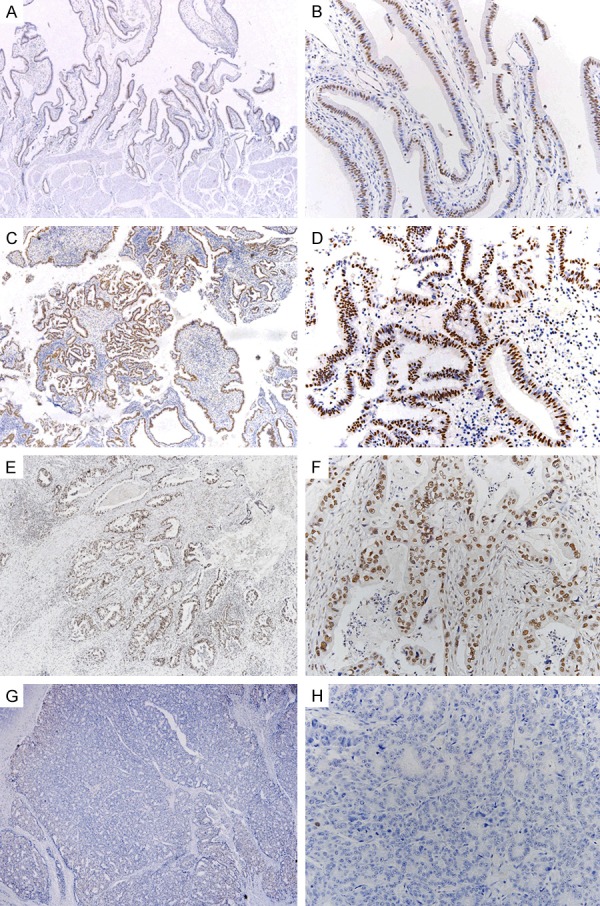
A, B. DBC1 expression in normal gallbladder epithelium. Adjacent normal gallbladder epithelial cells show diffuse strong nuclear DBC1 expression. C-H. Variable DBC1 expression in several gallbladder carcinoma cells. C. Well differentiated carcinoma cells show diffuse strong nuclear DBC1 expression; (original magnification, × 40); D. Magnified view of C (original magnification, × 200); E. Moderately differentiated carcinoma cells show diffuse strong nuclear DBC1 expression; F. Magnified view of E; G. Poorly differentiated carcinoma cells show low nuclear DBC1 expression in a few cells; H. Magnified view of G.
Table 1.
Correlation between DBC1 expression and clinicopathological variables in 104 Gallbladder carcinomas
| Variables | N | DBC1 expression | P value | |
|---|---|---|---|---|
|
|
||||
| Low | High | |||
| Gender | ||||
| female | 53 | 36 (67.8) | 17 (32.1) | .468 |
| male | 51 | 36 (70.6) | 15 (29.4) | |
| Age | ||||
| < 62 | 44 | 33 (75.0) | 11 (25.0) | .191 |
| > 62 | 60 | 39 (65.0) | 21 (35.0) | |
| Histologic grade | ||||
| Well/Mod | 89 | 58 (65.2) | 31 (34.8) | .022* |
| Poor | 15 | 14 (93.3) | 1 (6.7) | |
| Size | ||||
| < 2.5 cm | 51 | 33 (64.7) | 18 (35.3) | .294 |
| > 2.5 cm | 43 | 31 (72.1) | 12 (27.9) | |
| Primary tumor (T) | ||||
| T1-2 | 69 | 44 (63.8) | 25 (36.2) | .069 |
| T3-4 | 35 | 28 (80.0) | 7 (20.0) | |
| Lymph node metastasis | ||||
| Present | 22 | 19 (86.4) | 3 (13.6) | .040* |
| Absent | 82 | 53 (64.6) | 29 (35.4) | |
| Distant metastasis | ||||
| Present | 13 | 8 (61.5) | 5 (38.5) | .364 |
| Absent | 91 | 64 (70.3) | 27 (29.7) | |
| TNM stage | ||||
| I-II | 50 | 30 (60.0) | 20 (40.0) | .040* |
| III-IV | 54 | 42 (77.8) | 12 (22.2) | |
| Lymphatic invasion | ||||
| Present | 54 | 43 (79.6) | 11 (20.4) | .015* |
| Absent | 50 | 29 (58.0) | 21 (42.0) | |
| Vascular invasion | ||||
| Present | 15 | 9 (60.0) | 6 (40.0) | .29 |
| Absent | 89 | 63 (70.8) | 26 (29.2) | |
| Nerve invasion | ||||
| Present | 24 | 19 (79.2) | 5 (20.8) | .172 |
| Absent | 80 | 53 (66.3) | 27 (33.8) | |
| Surgical margin involvement | ||||
| Present | 9 | 9 (100) | 0 | .031* |
| Absent | 95 | 63 (66.3) | 32 (33.7) | |
| Local recurrence | ||||
| Present | 16 | 10 (62.5) | 6 (37.5) | .359 |
| Absent | 88 | 62 (70.5) | 26 (29.5) | |
NOTE. Values are n (%).
Statistically significant by the chi-square test.
Results of the disease-free survival and overall survival analysis
Univariate analysis for overall survival demonstrated that DBC1 expression (P = 0.0278) (Figure 2), histologic grade (P = 0.0009), primary tumor (T) (P < 0.00001), TNM stage (P < 0.00001), lymphatic invasion (P < 0.00001), vascular invasion (P < 0.00001), neural invasion (P = 0.0041), and local recurrence (P = 0.0095) were identified as significant prognostic factors for patients with gallbladder carcinoma (Table 2). Univariate analysis for disease-free survival demonstrated that primary tumor (T) (P = 0.0144), lymphatic invasion (P = 0.0057), and neural invasion (P = 0.0215) were significant prognostic factors (Table 2). Multivariate analysis for overall survival revealed that DBC1 expression, primary tumor (T), vascular invasion, and local recurrence were independent predictors of survival in gallbladder carcinoma (Table 3).
Figure 2.
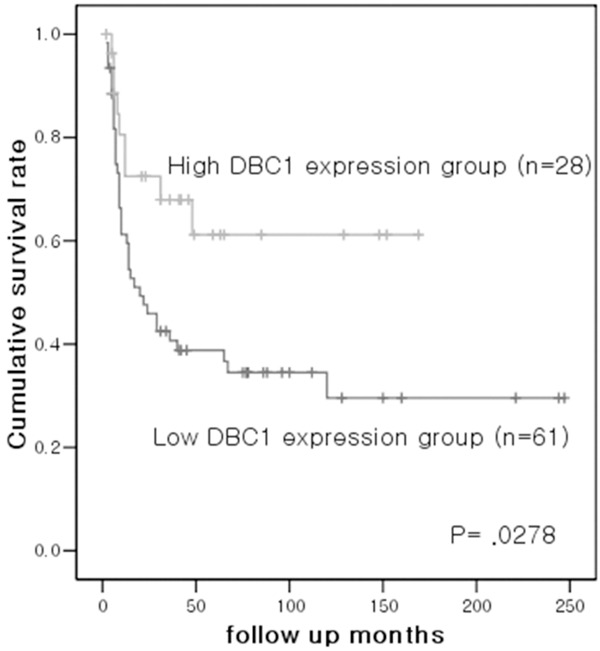
The high-DBC1 expression group shows a favorable survival rate compared to the low-DBC1 expression group in gallbladder carcinoma patients.
Table 2.
Univariate analysis of clinicopathological variables for overall survival rate in 104 Gallbladder carcinomas
| Variables | Disease free survival (P value) | Overall survival (P value) |
|---|---|---|
| Sex | .2969 | 0.4694 |
| Age (< 62 vs. ≥ 62) | .8780 | 0.6064 |
| Histologic grade (well to mod vs. poor) | .1872 | 0.0009* |
| Tumor size (< 2.5 cm vs. ≥ 2.5 cm) | .6736 | .2381 |
| Primary tumor (T) (T1, 2 vs. T3, 4) | .0144* | < 0.00001* |
| Lymph node metastasis | .2879 | .2519 |
| Distant metastasis | N.A | .0675 |
| TNM stage (I, II vs. III, IV) | .2330 | < 0.00001* |
| Lymphatic invasion | .0057* | < 0.00001* |
| Vascular invasion | .4091 | < 0.00001* |
| Neural invasion | .0215* | 0.0041* |
| Local recurrence | N.A | 0.0095* |
| DBC1 expression (high vs. low) | .8394 | .0278* |
Statistically significant.
N.A: not applicable.
Table 3.
Multivariate analysis of clinicopathological variables for overall survival rate in 104 Gallbladder carcinomas
| Variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Histologic grade (well to mod vs. poor) | 1.326 (0.588-2.993) | .497 |
| Primary tumor (T) (T1, 2 vs. T3, 4) | 2.882 (1.030-8.067) | .044* |
| TNM stage (I, II vs. III, IV) | 1.361 (0.508-3.644) | .540 |
| Lymphatic invasion | 1.918 (0.867-4.240) | .108 |
| Vascular invasion | 2.950 (1.273-6.833) | .012* |
| Neural invasion | 0.904 (0.413-1.979) | .800 |
| Local recurrence | 2.797 (1.204-6.499) | .017* |
| DBC1 expression (high vs. low) | 0.429 (0.194-0.950) | .037* |
Statistically significant.
Abbreviations: HR, Hazard ratio; CI, confidence interval.
Discussion
In this study, we evaluated the characteristics of DBC1 expression in gallbladder carcinoma. We observed that adjacent normal gallbladder epithelial cells demonstrate diffuse moderate to strong nuclear DBC1 expression. Comparing to normal gallbladder epithelial cells, carcinoma cells showed lower DBC1 expression. The relative loss of DBC1 expression in gallbladder carcinoma is an interesting finding. We also found that the group with high DBC1 expression was more likely to have favorable clinicopathologic variables, including a better histologic grade, negative lymph node metastasis, low TNM stage, negative lymphatic invasion, and a negative surgical resection margin. Furthermore, in survival analysis, the high-DBC1 expression group had a better survival rate compared to the low-DBC1 expression group. These findings suggest that loss of DBC1 expression in gallbladder carcinoma might play a role in tumorigenesis and tumor progression.
The function of DBC1 in human cancer has been controversial. It has been suggested to promote or suppress cancer cell growth in different studies. Recently, Qin et al. reported that DBC1 suppressed cell proliferation and tumorigenesis in wild type p53 background in vitro and in vivo [14]. And Noguchi et al. indicated that DBC1 expression assessed by immunohistochemical stain was associated with tumor regression and a favorable prognosis [15]. And Kang et al. showed that DBC1 expression in gastric adenocarcinoma was correlated with good prognostic factors including lower histologic grade, intestinal type of Lauren classification, lower pathologic T stage, lower lymph node stage, and absence of lymphatic invasion [16]. These results are similar to ours in that DBC1 expression was correlated with good prognostic factors.
However, there have been conflicting reports on the overexpression of DBC1 and poor prognosis in several cancers including breast [17-19], esophageal [20], gastric [21], colorectal [22], soft tissue sarcoma [23], clear cell renal cell carcinoma [24], and diffuse large B cell lymphoma [25]. Lee et al. reported that patients with DBC1-expressing breast carcinoma showed more frequent distant metastatic relapse and significantly lower overall survival and relapse-free survival if they had received endocrine therapy. Their reports have shown that DBC1 and estrogen receptor (ER) collaborate to suppress apoptosis and promote hormone-independent breast cancer cell growth [17].
The tumor suppressive functions of DBC1 have been reported in several processes. First, DBC1 enhances the acetylation of apoptotic targets such as p53 and FOXO through inhibition of NAD+-dependent SIRT1 (silent mating-type information regulation 2 homologue 1) deacetylase by directly binding to the catalytic domain of SIRT1 [12]. Therefore, DBC1 triggers the death of cancer cells following genotoxic and oxidative stress. Second, caspase-dependent processing and activation of the proapoptotic activity of DBC1 may function in tumor suppression [26]. Third, DBC1 activates retinoic acid receptor alpha (RARα). Therefore, it possibly contributes to the induction of tumor suppressor genes through RARα and inhibition of cancer cell growth [27].
Taken together, these conflicting results on DBC1 expression in various cancers raise the possibility that DBC1 might act as both a tumor suppressor and a tumor inducer. Thus, further studies of DBC1 expression in various cancers are needed to identify its mechanism of action in carcinogenesis.
In conclusion, high DBC1 expression is associated with several favorable clinicopathologic factors in gallbladder carcinoma. These findings suggest that loss of DBC1 expression may play a role in tumorigenesis and progression of gallbladder carcinoma. DBC1 also has the possibility to act as a tumor suppressor in gallbladder carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Wistuba II, Albores-Saavedra J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 1999;6:237–44. doi: 10.1007/s005340050113. [DOI] [PubMed] [Google Scholar]
- 2.Tavani A, Negri E, La Vecchia C. [Biliary tract tumors] . Ann Ist Super Sanita. 1996;32:615–9. [PubMed] [Google Scholar]
- 3.Shirai Y, Tsukada K, Ohtani T, Watanabe H, Hatakeyama K. Hepatic metastases from carcinoma of the gallbladder. Cancer. 1995;75:2063–8. doi: 10.1002/1097-0142(19950415)75:8<2063::aid-cncr2820750806>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Hanada K, Itoh M, Fujii K, Tsuchida A, Ooishi H, Kajiyama G. K-ras and p53 mutations in stage I gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Cancer. 1996;77:452–8. doi: 10.1002/(SICI)1097-0142(19960201)77:3<452::AID-CNCR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218–29. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 6.Nevin JE, Moran TJ, Kay S, King R. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer. 1976;37:141–8. doi: 10.1002/1097-0142(197601)37:1<141::aid-cncr2820370121>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Roa I, Araya JC, Shiraishi T, Yatani R, Wistuba I, Villaseca M, De Aretxabala X. DNA content in gallbladder carcinoma: a flow cytometric study of 96 cases. Histopathology. 1993;23:459–64. doi: 10.1111/j.1365-2559.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 8.Malats N, Porta M, Pinol JL, Corominas JM, Rifa J, Real FX. Ki-ras mutations as a prognostic factor in extrahepatic bile system cancer. PANK-ras I Project Investigators. J. Clin. Oncol. 1995;13:1679–86. doi: 10.1200/JCO.1995.13.7.1679. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Takano Y, Kakita A, Okudaira M. An immunohistochemical and molecular biological study of c-erbB-2 amplification and prognostic relevance in gallbladder cancer. Pathol Res Pract. 1993;189:283–92. doi: 10.1016/S0344-0338(11)80511-X. [DOI] [PubMed] [Google Scholar]
- 10.Giatromanolaki A, Sivridis E, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Koukourakis MI. Thymidine phosphorylase expression in gallbladder adenocarcinomas. Int J Surg Pathol. 2002;10:181–8. doi: 10.1177/106689690201000303. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi M, Meth JL, von Klitzing C, Wei W, Esposito D, Rodgers L, Walsh T, Welcsh P, King MC, Wigler MH. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc Natl Acad Sci U S A. 2002;99:13647–52. doi: 10.1073/pnas.212516099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–6. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Chen J, Lou Z. p30 DBC is a potential regulator of tumorigenesis. Cell Cycle. 2009;8:2932–5. doi: 10.4161/cc.8.18.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin B, Minter-Dykhouse K, Yu J, Zhang J, Liu T, Zhang H, Lee S, Kim J, Wang L, Lou Z. DBC1 functions as a tumor suppressor by regulating p53 stability. Cell Reports. 2015;10:1324–34. doi: 10.1016/j.celrep.2015.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguchi A, Kikuchi K, Zheng H, Takahashi H, Miyagi Y, Aoki I, Takano Y. SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer. Cancer Med. 2014;3:1553–61. doi: 10.1002/cam4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang Y, Jung WY, Lee H, Lee E, Kim A, Kim BH. Expression of SIRT1 and DBC1 in Gastric Adenocarcinoma. Korean J Pathol. 2012;46:523–31. doi: 10.4132/KoreanJPathol.2012.46.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Kim KR, Noh SJ, Park HS, Kwon KS, Park BH, Jung SH, Youn HJ, Lee BK, Chung MJ, Koh DH, Moon WS, Jang KY. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42:204–13. doi: 10.1016/j.humpath.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Hiraike H, Wada-Hiraike O, Nakagawa S, Saji S, Maeda D, Miyamoto Y, Sone K, Tanikawa M, Oda K, Nakagawa K, Yano T, Fukayama M, Taketani Y. Expression of DBC1 is associated with nuclear grade and HER2 expression in breast cancer. Exp Ther Med. 2011;2:1105–9. doi: 10.3892/etm.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung JY, Kim R, Kim JE, Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101:1738–44. doi: 10.1111/j.1349-7006.2010.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Kim JH, Yu EJ, Lee KW, Park CK. The overexpression of DBC1 in esophageal squamous cell carcinoma correlates with poor prognosis. Histol Histopathol. 2012;27:49–58. doi: 10.14670/HH-27.49. [DOI] [PubMed] [Google Scholar]
- 21.Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH, Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, Moon WS, Jang KY. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453–9. doi: 10.1158/1078-0432.CCR-08-3329. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Gu Y, Sha S, Kong X, Zhu H, Xu B, Li Y, Wu K. DBC1 is over-expressed and associated with poor prognosis in colorectal cancer. Int J Clin Oncol. 2014;19:106–12. doi: 10.1007/s10147-012-0506-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Yu TK, Kim KM, Park HS, Lee JH, Moon WS, Lee H, Chung MJ, Jang KY. Expression of SIRT1 and DBC1 is associated with poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e74738. doi: 10.1371/journal.pone.0074738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noh SJ, Kang MJ, Kim KM, Bae JS, Park HS, Moon WS, Chung MJ, Lee H, Lee DG, Jang KY. Acetylation status of P53 and the expression of DBC1, SIRT1, and androgen receptor are associated with survival in clear cell renal cell carcinoma patients. Pathology. 2013;45:574–80. doi: 10.1097/PAT.0b013e3283652c7a. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Bae JS, Noh SJ, Kim KM, Lee H, Moon WS, Chung MJ, Kang MJ, Lee DG, Jang KY. Expression of DBC1 and Androgen Receptor Predict Poor Prognosis in Diffuse Large B Cell Lymphoma. Transl Oncol. 2013;6:370–81. doi: 10.1593/tlo.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundararajan R, Chen G, Mukherjee C, White E. Caspase-dependent processing activates the proapoptotic activity of deleted in breast cancer-1 during tumor necrosis factor-alpha-mediated death signaling. Oncogene. 2005;24:4908–20. doi: 10.1038/sj.onc.1208681. [DOI] [PubMed] [Google Scholar]
- 27.Garapaty S, Xu CF, Trojer P, Mahajan MA, Neubert TA, Samuels HH. Identification and characterization of a novel nuclear protein complex involved in nuclear hormone receptor-mediated gene regulation. J Biol Chem. 2009;284:7542–52. doi: 10.1074/jbc.M805872200. [DOI] [PMC free article] [PubMed] [Google Scholar]


