Abstract
Background: MicroRNA-20a (miRNA-20a or miR-20a) plays a key role in tumorigenesis and progression. But the prognostic value of miR-20a in cutaneous squamous cell carcinoma (CSCC) remains unclear. The aim of this study was to identify the association of miR-20a and the prognosis of CSCC patients. Methods: The miR-20a expression was detected using quantitative real-time polymerase chain reaction (qRT-PCR) in 152 CSCC tissues and matched adjacent normal tissues. Kaplan-Meier and Cox regression analysis were utilized to determine the association of miR-20a with overall survival as well as the prognosis of CSCC patients. Results: The expression of miR-20a was lower in CSCC tissues compared with adjacent normal tissues (P=0.000). Moreover, the expression of miR-20a was closely correlated with TNM stage (P=0.013). Kaplan-Meier analysis showed that patients with low miR-20a expression had significantly poorer overall survival than those with high miR-20a expression (P<0.05). Multivariate analysis revealed that miR-20a expression (P=0.001, HR=3.262, 95% CI: 1.635-6.520) could influence the prognosis and might be an independent prognostic predictor in CSCC. Conclusions: Our results indicated that low miR-20a expression was associated with tumor stage of CSCC and suggested that miR-20a expression would be a novel biomarker for predicting clinical outcomes in CSCC patients. The inhibition of miR-20a might even become a new therapeutic method for the treatment of CSCC.
Keywords: MicroRNA-20a, cutaneous squamous cell carcinoma, prognosis
Introduction
Cutaneous squamous cell carcinoma (CSCC) is the second most common non-melanoma skin cancer and a malignant tumor of keratinocytes which tends to metastasis and leads to mortality [1,2]. The most important risk factor for the occurrence of this cancer is UV exposure [3]. Its morbidity is greater in males than females, and increases with age [4]. Moreover, patients with CSCC also have an elevated the mortality rate of lung cancer, colorectal cancer, rectum cancer, prostate cancer and breast cancer [5]. Metastasis is the main reason that causes cancer-related death in CSCC which can lead to the 5-year survival rate less than 30% [6]. Therefore, the prognosis is unfavorable after metastasis in CSCC and it is necessary to study the molecular mechanisms underlying metastasis in CSCC as well as look for a new effective prognostic marker to improve clinical outcome.
MicroRNAs (miRNAs or miRs) are a large group of non-coding, single-stranded RNA with approximately 22 nucleotides in length [7]. These small molecules have been found to regulate endogenous gene expression through translational repression and messenger RNA cleavage [8]. Recent studies showed that miRNAs play key roles in the biology of various human cancers, including cell differentiation, proliferation, apoptosis, invasion and angiogenesis [9,10]. It also has been confirmed to be important diagnostic or prognostic markers in various cancers. MiR-20a (microRNA-20a) belongs to the miR-17-92 cluster, which includes six microRNAs: miR-17-5p, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a-16 [11]. It plays different roles in many tumors including prostate cancer, anaplastic thyroid cancer, and gastric cancer [12-14]. Besides, miR-20a was also considered to be decreased and correlated with the recurrence and prognosis of hepatocellular carcinomas [15]. However, although miR-20a had been reported to be an inhibitor for the metastasis and proliferation of CSCC [16], its prognostic implication in CSCC had never been explored.
The purpose of this study was to investigate the expression of miR-20a and explored whether it was related to the prognosis of CSCC.
Methods and materials
Patients and tissue samples
A total of 152 patients with CSCC were collected at the department of plastic and reconstructive surgery, First Affiliated Hospital of Bengbu Medical College. These CSCC cases included 102 men (67.1%) and 50 women (32.9%), with a median age of 53.9 years. No previous local or systemic treatment had been conducted on these patients before the operation or biopsy. All protocols were approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College and all participators had signed written informed consent in advance.
The tumor tissues and adjacent tissues were obtained with surgical removal and frozen in liquid nitrogen immediately. Then the tissues were stored at -80°C until use. Clinicopathological characteristics for these patients, including age, gender, location, tumor size, tumor grade, pT classification, pN classification and stage were detailed in a database. Tumor differentiation was determined based on the World Health Organization tumor classification criteria. TNM (tumor, nodes, and metastasis) stage of cutaneous carcinoma was defined according to the Union for International Cancer Control. A 5-years’ follow-up was conducted according to a telephone or questionnaire. The overall survival referred to the period of time from the date of diagnosis until death from any cause.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted and purified from all the 152 CSCC tissues and matched adjacent normal specimens using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). Only those total RNA samples with an OD A260/A280 ratio close to a value of 2.0, which indicates that the RNA was pure, were subsequently analyzed. The cDNAs were synthesized using gene-specific primers according to the TaqMan MicroRNA assays protocol (Applied Biosystems, Foster City, CA, USA). Then the PCR reaction was performed in the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, California, USA). U6 was taken as the internal control. Each sample was examined in triplicate, and the raw data were presented as the relative quantification of miR-20a expression evaluated by the comparative cycle threshold (CT) method using SDS 2.2.2 software (Applied Biosystems), normalized with respect to U6. The mean normalized miR-20a expression ± the standard deviation (SD) was calculated from triplicate analyses.
Statistical analysis
All statistical analysis was performed using SPSS version 18.0. Comparisons of miR-20a expression levels between CSCC tissues and adjacent normal tissues were estimated using T-test. The correlation between miR-20a expression and clinicopathological characteristics of patients with CSCC was evaluated by Χ2-test. Association of miR-20a expression with overall survival was estimated by Kaplan-Meier analysis, and the resulting curves were compared using the log-rank test. The multivariate analysis was used to evaluate the prognostic factors to patient’s survival via Cox regression analysis. P<0.05 was considered to be statistically significant.
Results
MiR-20a expression was significantly lower in CSCC tissues compared with adjacent normal tissues
With the purpose of revealing the expression and significance of miR-20a in CSCC, we first detected the expression of miR-20a in 152 cases of CSCC and adjacent normal tissues by qRT-PCR. The expression of miR-20a was significantly down-regulated in CSCC tissues compared with adjacent normal skin tissues (P=0.000; Figure 1). This result might reveal that miR-20a was a tumor suppressor in CSCC.
Figure 1.
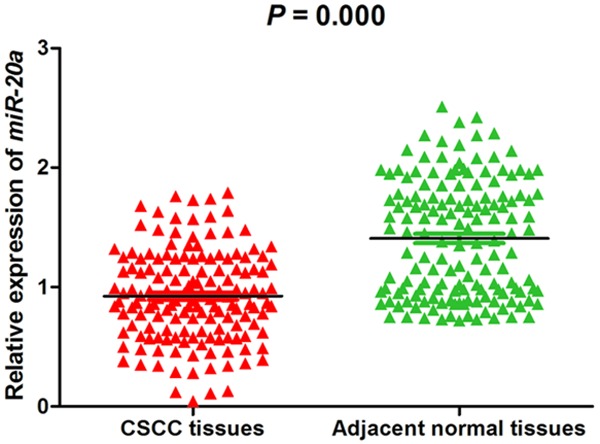
Expression level of miR-20a in the specimens of CSCC patients QRT-PCR Demonstrated that the expression level of miR-20a was lower in tumor tissues than in adjacent normal tissues (P<0.05).
Relationship between the expression of miR-20a and clinicopathological characteristics
The relationships between miR-20a expression and clinicopathological characteristics were analyzed by chi-square test. As shown in Table 1, the expression of miR-20a in CSCC was significantly associated with tumor stage (P=0.013). However, there were no correlation with other clinical features, such as age, gender, tumor size, grade of differentiation, pT classification, pN classification (P>0.05).
Table 1.
Relationship between clinicopathological characteristics and miR-20a expression in patients with CSCC
| Parameters | Cases (n) | miR-20a expression level | Χ2 | P-value | |
|---|---|---|---|---|---|
|
| |||||
| Low (n=98) | High (n=54) | ||||
| Age (yeas) | |||||
| ≤55 | 80 | 53 | 27 | 0.233 | 0.630 |
| >55 | 72 | 45 | 27 | ||
| Gender | |||||
| Male | 102 | 72 | 50 | 0.712 | 0.399 |
| Female | 50 | 26 | 24 | ||
| Location | |||||
| Neck | 32 | 18 | 14 | 1.083 | 0.406 |
| Face | 48 | 30 | 18 | ||
| Head | 72 | 50 | 22 | ||
| Tumor size (cm) | |||||
| ≤5 | 67 | 40 | 27 | 1.191 | 0.275 |
| >5 | 85 | 58 | 27 | ||
| Grade of differentiation | |||||
| Well to moderate | 141 | 89 | 52 | 1.557 | 0.212 |
| Poor | 11 | 9 | 2 | ||
| pT classification | |||||
| T1-T2 | 62 | 41 | 21 | 0.125 | 0.723 |
| T3-T4 | 90 | 57 | 33 | ||
| pN classification | |||||
| N0 | 115 | 72 | 43 | 0.717 | 0.397 |
| N1 | 37 | 26 | 11 | ||
| TNM Stage | |||||
| I-II | 108 | 63 | 45 | 6.141 | 0.013 |
| III | 46 | 35 | 9 | ||
Decrease expression of miR-20a in CSCC was associated with poor prognosis
To further explore the clinical relevance of miR-20a, overall survival curves were plotted according to miR-20a expression level by the Kaplan-Meier analysis. As shown in Figure 2, patients with low miR-20a expression had a significantly shorter overall survival than those with high miR-20a expression which was tested by the log-rank test (P=0.001, Figure 2). To get insight into the survival prediction potential of miR-20a, we performed multivariate analysis with Cox regression analysis. The results showed that miR-20a expression (HR=3.262, 95% CI=1.632-6.520, P=0.001) was an independent prognostic factor for patients with CSCC (Table 2).
Figure 2.
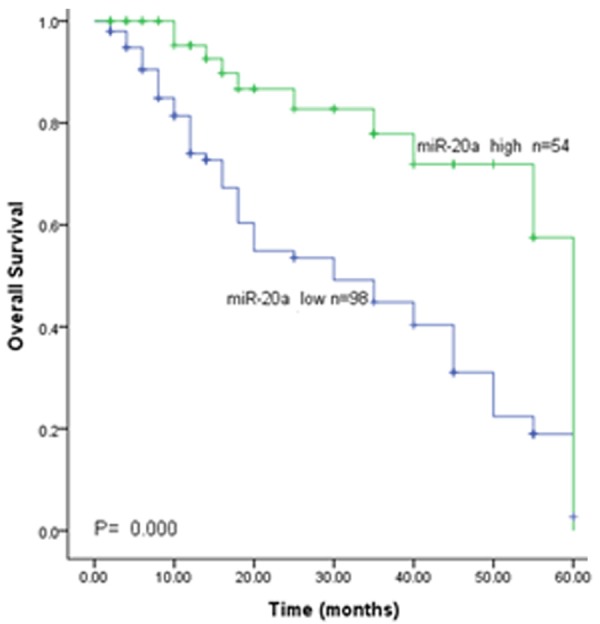
Kaplan-Meier analysis of with the miR-20a expression indicated that patients with low miR-20a expression lived shorter than those with high miR-20a expression.
Table 2.
Multivariate analysis of different prognostic factors in the patients with CSCC
| Parameters | HR | 95% CIs | P-value |
|---|---|---|---|
| High MiR-20a expression | 3.262 | 1.632-6.520 | 0.001 |
| Low MiR-20a expression | |||
| Tumor stage | 1.398 | 0.785-2.490 | 0.255 |
HR: hazard ratio; CI: confidence interval.
Discussion
MicroRNAs play an important role in tumor development and occurrence according to act as a tumor suppressor or oncogene. This potential effect of miRNAs is expected to make it to be molecular markers in the diagnosis and prognosis of cancers so that provide new therapy strategies.
In CSCC, there were also many microRNAs that proved to be participated in such as miR-361-5p, miR-125b, miR-199a, miR-31, miR-365, and so on [6,17-20]. miR-20a has been found to have different roles in various tumors and acts as oncogene or tumor suppressor in previous studies. Indeed, miR-20a promoted gastric cancer [14], gallbladder carcinoma [21], and prostate cancer [22]. On the contrary, it could inhibit in hepatic cancer [15] and oral squamous cell cancer [23]. It was also verified to be enhanced in the circulation and the tissue as well as associated with a higher risk of tumor metastasis in squamous cancer of the cervix, squamous cell cancer of the esophagus and CSCC [16,24,25]. In the present study, we investigated the miR-20a expression by qRT-PCR in 152 cases of CSCC and adjacent normal tissues. Results showed that the expression level of miR-20a was decreased in CSCC compared with that in adjacent normal specimens. This might indicate that miR-20a was a tumor suppressor in CSCC.
Based on the relative expression analysis, we investigated the association of miR-20a with clinicopathological features. We found that miR-20a expression was closely associated with the TNM stage of CSCC, as results revealed that low level of miR-20a expression was more frequently to be detected in tumors with advanced TNM stage. These findings reveal that the abnormal expression of miR-20a might play important roles in the tumorigenesis and progression of CSCC patients.
Furthermore, we assessed the prognostic role of miR-20a in CSCC. Kaplan-Meier analysis and log-rank test showed that patients with low miR-20a expression had worse overall survival compared with those with high levels of miR-20a expression. The statistically significant association indicated that miR-20a could be related to the prognosis of CSCC. To further evaluate the prognostic value of miR-20a in CSCC, we performed a Cox regression analysis adjusted for gender, age and other parameters related to survival of CSCC. Results proved that decreased miR-20a expression could be an independent prognostic marker in CSCC.
In summary, our investigation provides the effective evidence for the first time that the down-regulation of miR-20a may serve as a novel molecular marker to predict the tumor progression and inferior prognosis of CSCC patients. However, the sample size is small and the current study has not elucidated the exact molecular mechanisms of miR-20a acting on CSCC which make this study has some limitation. Hence, some further verified experiments need to be conducted.
Disclosure of conflict of interest
None.
References
- 1.DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145–149. doi: 10.1053/j.seminoncol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Xia YH, Li M, Fu DD, Xu SL, Li ZG, Liu D, Tian ZW. Effects of PTTG down-regulation on proliferation and metastasis of the SCL-1 cutaneous squamous cell carcinoma cell line. Asian Pac J Cancer Prev. 2013;14:6245–6248. doi: 10.7314/apjcp.2013.14.11.6245. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt J, Seidler A, Diepgen TL, Bauer A. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291–307. doi: 10.1111/j.1365-2133.2010.10118.x. [DOI] [PubMed] [Google Scholar]
- 4.Rahimi S. Squamous cell carcinoma of skin: a brief review. Clin Ter. 2013;164:143–147. doi: 10.7417/CT.2013.1534. [DOI] [PubMed] [Google Scholar]
- 5.Johannesdottir SA, Lash TL, Jensen AO, Farkas DK, Olesen AB. Mortality in cancer patients with a history of cutaneous squamous cell carcinoma--a nationwide population-based cohort study. BMC Cancer. 2012;12:126. doi: 10.1186/1471-2407-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SH, Zhou JD, He QY, Yin ZQ, Cao K, Luo CQ. MiR-199a inhibits the ability of proliferation and migration by regulating CD44-Ezrin signaling in cutaneous squamous cell carcinoma cells. Int J Clin Exp Pathol. 2014;7:7131–7141. [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Zhang P, Yang J, Liu Z, Yang Z, Qin H. Candidate microRNA biomarkers in human colorectal cancer: systematic review profiling studies and experimental validation. Int J Cancer. 2012;130:2077–2087. doi: 10.1002/ijc.26232. [DOI] [PubMed] [Google Scholar]
- 11.Jung YJ, Kim JW, Park SJ, Min BY, Jang ES, Kim NY, Jeong SH, Shin CM, Lee SH, Park YS, Hwang JH, Kim N, Lee DH. c-Myc-mediated overexpression of miR-17-92 suppresses replication of hepatitis B virus in human hepatoma cells. J Med Virol. 2013;85:969–978. doi: 10.1002/jmv.23534. [DOI] [PubMed] [Google Scholar]
- 12.Qiang XF, Zhang ZW, Liu Q, Sun N, Pan LL, Shen J, Li T, Yun C, Li H, Shi LH. miR-20a promotes prostate cancer invasion and migration through targeting ABL2. J Cell Biochem. 2014;115:1269–1276. doi: 10.1002/jcb.24778. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Zhang L, Kebebew E. MiR-20a is upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS One. 2014;9:e96103. doi: 10.1371/journal.pone.0096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Zhang Z, Yu M, Li L, Du G, Xiao W, Yang H. Involvement of miR-20a in promoting gastric cancer progression by targeting early growth response 2 (EGR2) Int J Mol Sci. 2013;14:16226–16239. doi: 10.3390/ijms140816226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan MQ, Huang CB, Gu Y, Xiao Y, Sheng JX, Zhong L. Decrease expression of microRNA-20a promotes cancer cell proliferation and predicts poor survival of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:21. doi: 10.1186/1756-9966-32-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Liu R, Luo C, Zhou X, Xia K, Chen X, Zhou M, Zou Q, Cao P, Cao K. MiR-20a inhibits cutaneous squamous cell carcinoma metastasis and proliferation by directly targeting LIMK1. Cancer Biol Ther. 2014;15:1340–1349. doi: 10.4161/cbt.29821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanitz A, Imig J, Dziunycz PJ, Primorac A, Galgano A, Hofbauer GF, Gerber AP, Detmar M. The expression levels of microRNA-361-5p and its target VEGFA are inversely correlated in human cutaneous squamous cell carcinoma. PLoS One. 2012;7:e49568. doi: 10.1371/journal.pone.0049568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu N, Zhang L, Meisgen F, Harada M, Heilborn J, Homey B, Grander D, Stahle M, Sonkoly E, Pivarcsi A. MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. J Biol Chem. 2012;287:29899–29908. doi: 10.1074/jbc.M112.391243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A, Landen NX, Meisgen F, Lohcharoenkal W, Stahle M, Sonkoly E, Pivarcsi A. MicroRNA-31 is overexpressed in cutaneous squamous cell carcinoma and regulates cell motility and colony formation ability of tumor cells. PLoS One. 2014;9:e103206. doi: 10.1371/journal.pone.0103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Zhou L, Zheng L, Guo L, Wang Y, Liu H, Ou C, Ding Z. miR-365 promotes cutaneous squamous cell carcinoma (CSCC) through targeting nuclear factor I/B (NFIB) PLoS One. 2014;9:e100620. doi: 10.1371/journal.pone.0100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y, Liu C, Yang J, Liu G, Feng F, Tang J, Hu L, Li L, Jiang F, Chen C, Wang R, Yang Y, Jiang X, Wu M, Chen L, Wang H. MiR-20a triggers metastasis of gallbladder carcinoma. J Hepatol. 2013;59:518–527. doi: 10.1016/j.jhep.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Pan JH, Song B, Xiong EQ, Chen ZW, Zhou ZS, Su YP. Suppression of CX43 expression by miR-20a in the progression of human prostate cancer. Cancer Biol Ther. 2012;13:890–898. doi: 10.4161/cbt.20841. [DOI] [PubMed] [Google Scholar]
- 23.Chang CC, Yang YJ, Li YJ, Chen ST, Lin BR, Wu TS, Lin SK, Kuo MY, Tan CT. MicroRNA-17/20a functions to inhibit cell migration and can be used a prognostic marker in oral squamous cell carcinoma. Oral Oncol. 2013;49:923–931. doi: 10.1016/j.oraloncology.2013.03.430. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Yao D, Li Y, Chen H, He C, Ding N, Lu Y, Ou T, Zhao S, Li L, Long F. Serum microRNA expression levels can predict lymph node metastasis in patients with early-stage cervical squamous cell carcinoma. Int J Mol Med. 2013;32:557–567. doi: 10.3892/ijmm.2013.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Liao J, Yang M, Shi Y, Peng Y, Wang Y, Pan E, Guo W, Pu Y, Yin L. Circulating miR-155 expression in plasma: a potential biomarker for early diagnosis of esophageal cancer in humans. J Toxicol Environ Health A. 2012;75:1154–1162. doi: 10.1080/15287394.2012.699856. [DOI] [PubMed] [Google Scholar]


