Abstract
It was previously reported that intrarenal renin angiotensin system (RAS) plays a pivotal role in the onset and progression of diabetic nephropathy (DN). Urinary angiotensinogen (UAGT) was employed as a special index of the intrarenal RAS status and enhanced significantly at a very early stage of chronic kidney disease and type 1 diabetes. On the basis of these findings, the present study was performed to test the hypothesis that UAGT levels are increase even before the development of DN in type 2 diabetic patients without hypertension. 102 patients with type 2 diabetes mellitus (T2DM) and 18 healthy volunteers were studied cross-sectionally. Clinical data were collected and morning spot urine samples were obtained from all participants. UAGT levels were detected by an enzyme-linked immunosorbent assay (ELISA). As a result, UAGT to creatinine ratio (UAGT/Cr) was significantly enhanced in T2DM patients before the appearance of urinary albumin (UALB) and further increased to a greater degree in albuminuric patients. UAGT/Cr levels were positively correlated with Log (UALB to creatinine ratio) and diastolic blood pressure, but negatively correlated with estimated glomerular filtration rate. These data indicate that elevated UAGT levels precede the onset of albuminuria in normotensive T2DM patients. UAGT might potentially serve as an early marker to determine intrarenal RAS activity and predict progressive kidney disease in T2DM patients without hypertension.
Keywords: Urinary angiotensinogen, albuminuria, normotensive type 2 diabetic patients
Introduction
Diabetic nephropathy (DN) is a major cause of end stage renal disease and associated with increased risk of cardiovascular disease and all-cause mortality [1,2]. Microalbuminuria is the most commonly used early marker of DN in clinical settings. However, the presence of albuminuria always indicates a more advanced stage of DN and implies a poor prognosis [2]. Therefore, it would be of great importance to identify a more sensitive marker to predict the progression of DN.
It has been reported that all of the components necessary to generate angiotensin II (Ang II) are present along the nephron, suggesting a functional renin angiotensin system (RAS) exist in the kidney [3]. Emerging evidence has demonstrated that intrarenal RAS plays a pivotal role in the onset and progression of renal disease including DN [4,5]. RAS inhibitors such as angiotensin receptor blockers (ARB) and angiotensin converting enzyme inhibitors (ACEI) have been widely used to decrease proteinuria and retard progression of renal insufficiency in patients with DN.
Angiotensinogen (AGT) is the only known substance of renin. Circulating AGT is mainly produced by the liver and could not filter through the glomerular basement membrane due to its high molecular weight. Therefore, urinary AGT (UAGT) is mainly derived from renal proximal tubular cells [3]. UAGT excretion was highly associated with kidney Ang II content but not plasma Ang II level in Ang II-infused rats as well as chronic kidney disease (CKD) patients, suggesting that UAGT could serve as a reliable index of intrarenal Ang II activity [6,7]. More importantly, UAGT increased at an earlier time point than urinary albumin (UALB) in experimental type 1 diabetic rats [8]. These data prompt us to further investigate the role of UAGT in type 2 diabetic patients. Here, we tested the hypothesis that UAGT levels are enhanced prior to the onset of albuminuria and correlated with clinical parameters in type 2 diabetic patients without hypertension, and UAGT might be a potential early marker for diabetic nephropathy.
Methods
Study design and sample collections
The study protocol was approved by the Peking University Biomedical Ethics Committee (Beijing, China). A total of 122 patients with type 2 diabetes mellitus (T2DM) hospitalized in Peking university third hospital from September 2013 to November 2013 were recruited. All patients were normotensive and had never received ACEI or ARB. Patients with primary glomerulonephritis, those with renal artery stenosis, and those with eGFR below 30 ml/min or undergoing dialysis were excluded. Consequently, 102 patients and 18 healthy volunteers were eligible for the study. To further evaluate the different distribution of UAGT levels in patients with or without albuminuria, diabetic patients were divided into three groups according to the levels of UALB to creatinine ratio (UALB/Cr): T2DM with normoalbuminuria (UALB/Cr < 20 mg/g), T2DM with microalbuminuria (20 mg/g < UALB/Cr < 300 mg/g), and T2DM with macroalbuminuria (UALB/Cr > 300 mg/g). Morning spot urine samples were obtained from all participants. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded on the same day.
Measurements
Urinary concentrations of AGT were measured with human AGT ELISA kits (IBL, Japan) following the manufacturer’s instructions. Briefly, urine samples were centrifuged at 3000 rpm for 10 min at 4°C. 100 μl of urine sample (1:8 dilution with ELISA buffer) was added to each well of the plates and incubated at 37°C for 1 h. Then the plates were washed seven times and incubated with horseradish peroxides-labeled monoclonal antibody against human angiotensinogen at 37°C for 30 min. After washing, 100 μl of TMB solution was added to each well and incubated in dark at room temperature for 30 min. Finally, the reaction was stopped with sulfuric acid and the absorbance values were measured at 450 nm.
Serum creatinine, Fasting blood glucose (FBG) and hemoglobin A1c (HbA1C) were measured by standard methods in our clinical laboratory in Peking university third hospital. The eGFR was calculated using the Modification of Diet in Renal Disease Formula: eGFR = 175 × [serum creatinine (mg/dl)]-1.154 × age-0.203 × 0.741 (× 0.742 if female). Urinary concentrations of albumin and creatinine were measured by an automated machine with reagent kits. UAGT and Urinary albumin excretion were adjusted with same sample creatinine and expressed as UAGT/Cr or UALB/Cr.
Statistical analysis
Data are presented as the means ± standard deviation and analyzed using SPSS 15.0 for Windows. Student’s t-tests and one-way ANOVA were used to compare group means. Pearson correlation analyses were used for parametric data. Multivariate regression analysis was performed to identify the association of UAGT/Cr and clinical parameters. Statistical significance was considered as P < 0.05.
Results
Patient characteristics and laboratory data
The demographics and clinical data of the participants are summarized in Table 1.
Table 1.
Characteristics of 102 patients with T2DM and 18 healthy controls
| Variables | Control group (n = 18) | Patients with T2DM (n = 102) | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Normoalbuminuria group (n = 32) | Microalbuminuria group (n = 48) | Macroalbuminuria group (n = 22) | |||
| Age (years) | 57.89 ± 12.65 | 62.38 ± 12.21 | 66.75 ± 13.21* | 53.73 ± 17.16#,Δ | 0.002 |
| Sex (M/F) | 9/9 | 20/12 | 36/12 | 12/10 | |
| SBP (mmHg) | 123.44 ± 6.17 | 124.63 ± 12.11 | 128.46 ± 12.05 | 128.73 ± 9.94 | 0.204 |
| DBP (mmHg) | 67.28 ± 4.38 | 69.50 ± 8.97 | 74.88 ± 8.37* | 78.00 ± 8.92*,# | < 0.001 |
| FBG (mmol/L) | 5.04 ± 0.58 | 8.06 ± 2.22* | 7.56 ± 2.61* | 7.00 ± 2.41* | < 0.001 |
| HbA1C (%) | 5.37 ± 0.38 | 8.59 ± 2.25* | 8.40 ± 1.46* | 7.85 ± 2.14* | < 0.001 |
| UALB/Cr (mg/g) | 4.71 ± 3.34 | 4.92 ± 3.36 | 43.51 ± 18.31*,# | 7436.18 ± 4427.59*,#,Δ | < 0.001 |
| eGFR (ml/min/1.73 m2) | 106.74 ± 18.38 | 89.11 ± 20.40 | 72.75 ± 23.41*,# | 48.71 ± 32.11*,#,Δ | < 0.001 |
P < 0.05 vs. healthy controls.
P < 0.05 vs. normoalbuminuria group.
P < 0.05 vs. microalbuminuria group.
UAGT levels in diabetic patients and healthy volunteers
Figure 1 exhibits UAGT/Cr levels in patients with T2DM and healthy controls. UAGT/Cr levels were significantly higher in diabetic patients with normoalbuminuria compared with healthy subjects (42.91 ± 41.72 μg/g vs. 7.35 ± 5.18 μg/g, P < 0.001), indicating that intrarenal RAS in diabetic patients is activated early in the premicroalbuminuric phase. As expected, UAGT/Cr levels were even higher in albuminuric T2DM patients when compared with their normoalbuminuric counterparts.
Figure 1.
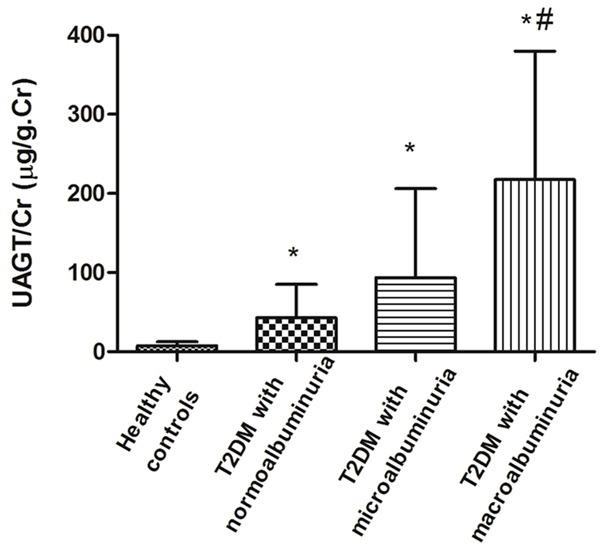
UAGT/Cr levels in diabetic patients and healthy controls. UAGT/Cr levels were significantly higher in T2DM patients with normoalbuminuria when compared to control group (42.91 ± 41.72 μg/g vs. 7.35 ± 5.18 μg/g), which further increased to 93.50 ± 111.53 μg/g in microalbuminuric patients and 217.64 ± 158.16 μg/g in macroalbuminuric patients. *P < 0.05 vs. healthy controls, #P < 0.05 vs. normoalbuminuria group.
Univariate correlation analyses
Figure 2 shows the univariate correlation analyses between UAGT/Cr and other clinical data in all subjects. UAGT/Cr levels were not correlated with age, gender, SBP, FBG, HbA1C. However, UAGT/Cr levels were significantly correlated positively with Log (UALB/Cr) (r = 0.534, P < 0.001) and DBP (r = 0.396, P < 0.001), and correlated negatively with eGFR (r = -0.288, P = 0.003).
Figure 2.
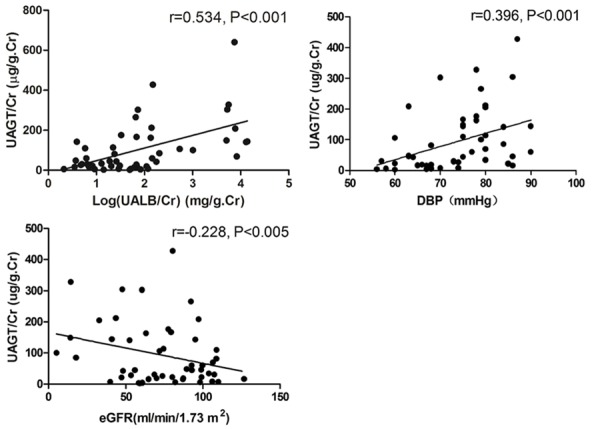
Univariate correlation analyses between UAGT/Cr and clinical data. UAGT/Cr levels were significantly correlated positively with Log (UALB/Cr) (r = 0.534, P < 0.001) and DBP (r = 0.396, P < 0.001), but correlated negatively with eGFR (r = -0.288, P = 0.003).
Multivariate regression analysis
To further identify the explanatory variables for UAGT/Cr, a backward elimination multivariate linear regression analysis was performed. The original model included UALB/Cr, DBP and eGFR. As a result, UAGT/Cr was independently predicted by UALB/Cr (β = 0.013, P < 0.001) and DBP (β = 3.319, P = 0.007) but not eGFR (β = -0.031, P = 0.946; Table 2).
Table 2.
Multivariate regression analysis for UAGT/Cr
| Correlation | Regression | |||
|---|---|---|---|---|
|
| ||||
| r | p | B | p | |
| DBP (mmHg) | 0.396 | 0.000** | 3.319 | 0.007* |
| eGFR (ml/min/1.73 m2) | -0.288 | 0.003* | -0.031 | 0.946 |
| UALB/Cr (mg/g. cr) | 0.521 | 0.000** | 0.013 | 0.000** |
Discussion
In the present study, we examined the UAGT levels in normotensive T2DM patients with or without albuminuria and three major findings were obtained. First, UAGT/Cr levels were significantly increased in diabetic patients with normoalbuminuria compared with healthy volunteers. Secondly, UAGT/Cr levels were even higher in albuminuric patients. Thirdly, UAGT/Cr levels were significantly correlated with UALB/Cr, DBP and eGFR. These data suggest that UAGT might be employed as an early index of intrarenal RAS status in T2DM patients without hypertension.
Although plasma AGT is mainly produced and secreted by the liver, the kidneys also synthesize AGT. Due to its high molecular weight, little plasma AGT is expected to filter through the glomerular basement membrane. Consequently, UAGT is regarded to reflect locally formed AGT that is produced by proximal tubular cells of the kidney [3]. Accumulated evidence has implicated that UAGT excretion is highly correlated with intrarenal angiotensin II (Ang II) activity and could serve as a reliable biomarker for monitoring intrarenal RAS status [6,7].
It is reported that plasma rennin and angiotensin converting enzyme activities are not enhanced in diabetic patients, indicating no changes in circulating RAS [9]. The focus of interest on RAS in diabetic patients has recently shifted towards the role of intrarenal RAS. It has been previously reported that intrarenal AGT mRNA and protein expression were significantly higher in patients with diabetes than in control subjects and paralleled renal dysfunction [10]. In addition, AGT and Ang II production was increased in cultured glomerular mesangial cells under high glucose conditions [11]. Consistent with these studies, our results indicate that the intrarenal RAS is activated in T2DM patients with or without albuminuria, as evidenced by a significant increase in UAGT/Cr levels. Importantly, UAGT/Cr levels are significantly higher in normoalbuminuric T2DM patients compared with healthy subjects while the UALB/Cr levels are comparable. In a previous study, Saito and colleagues showed that UAGT increased in patients with type 1 diabetes even before diagnosis of proteinuria or microalbuminuria. Taken together, it is suggested that in patients with diabetes, the increase in UAGT levels precedes the onset of albuminuria. UAGT might function as an early marker for diabetic nephropathy.
A particular concern is whether urinary AGT is a nonspecific consequence of proteinuria. AGT is a protein and UAGT levels were positively correlated with proteinuria in several previous studies as well as in ours. Therefore, it is possible that augmented UAGT in diabetes is a nonspecific result of proteinuria. However, Nakano et al. has reported that after intravenously infused with human AGT, there is only a very slight increase of urinary human AGT in rats with advanced kidney disease [9]. Also, UAGT/Cr levels were similar in patients with minimal change nephropathy and control subjects, even though patients with minimal change nephropathy had severe proteinuria [12]. On the other hand, we observed in this study that UAGT/Cr levels were significantly higher in normoalbuminuric T2DM patients compared with healthy volunteers. Collectively, these data indicate that UAGT is not simply a nonspecific consequence of proteinuria.
Another important finding of our study was that UAGT/Cr levels were significantly correlated positively with Log (UALB/Cr) and DBP, but negatively with eGFR. It has been suggested that UAGT concentration reflects the activity of intrarenal angiotensin II, which is a central mediator resulting in renal injury including albuminuria and renal insufficiency. Thus, it can be speculated with caution that urinary protein excretion and the decline of eGFR in T2DM patients could be a consequence of the intrarenal RAS activation. Previous evidence demonstrated that in patients with CKD, UAGT excretion was associated with the risk for deterioration of renal function, the severity of renal histopathology, as well as the therapeutic effect of RAS inhibitor [7,13,14]. These data together with our present study suggest that UAGT might potentially be a noninvasive tool to predict the severity and progression of diabetic nephropathy.
Our investigation has some limitations. Firstly, we did not measure plasma AGT levels. However, several clinical and experimental studies have revealed that plasma AGT levels were similar between patients with CKD and healthy subjects [14-16]. Secondly, because of its cross-sectional design and small sample size, we failed to identify the dynamic change of UAGT and its relationship with long term outcomes in patients with T2DM. Therefore, prospective studies with a larger number of patients and a longer observation period are warranted.
In conclusion, we have demonstrated that UAGT levels increased significantly even before the onset of microalbuminuria in T2DM patients, indicating activated intrarenal RAS in the early phase of diabetes. The observation suggests that UAGT might be a novel early marker for diabetic nephropathy.
Disclosure of conflict of interest
None.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Kobori H, Kamiyama M, Harrison-Bernard LM, Navar LG. Cardinal role of the intrarenal renin-angiotensin system in the pathogenesis of diabetic nephropathy. J Investig Med. 2013;61:256–264. doi: 10.231/JIM.0b013e31827c28bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Yukimura T, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 8.Kamiyama M, Zsombok A, Kobori H. Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. J Pharmacol Sci. 2012;119:314–323. doi: 10.1254/jphs.12076fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Pe- terdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamiyama M, Urushihara M, Morikawa T, Konishi Y, Imanishi M, Nishiyama A, Kobori H. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int J Mol Sci. 2013;14:23045–23062. doi: 10.3390/ijms141123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Singh AK, Alavi N, Leehey DJ. Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol. 2003;14:873–880. doi: 10.1097/01.asn.0000060804.40201.6e. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SM, Jang HR, Lee YJ, Lee JE, Huh WS, Kim DJ, Oh HY, Kim YG. Urinary angiotensinogen levels reflect the severity of renal histopathology in patients with chronic kidney disease. Clin Nephrol. 2011;76:117–123. doi: 10.5414/cn107045. [DOI] [PubMed] [Google Scholar]
- 14.Jang HR, Lee YJ, Kim SR, Kim SG, Jang EH, Lee JE, Huh W, Kim YG. Potential role of urinary angiotensinogen in predicting antiproteinuric effects of angiotensin receptor blocker in non-diabetic chronic kidney disease patients: a preliminary report. Postgrad Med J. 2012;88:210–216. doi: 10.1136/postgradmedj-2011-130441. [DOI] [PubMed] [Google Scholar]
- 15.Kocyigit I, Yilmaz MI, Unal A, Ozturk F, Eroglu E, Yazici C, Orscelik O, Sipahioglu MH, Tokgoz B, Oymak O. A link between the intrarenal renin angiotensin system and hypertension in autosomal dominant polycystic kidney disease. Am J Nephrol. 2013;38:218–225. doi: 10.1159/000354317. [DOI] [PubMed] [Google Scholar]
- 16.Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]


