Abstract
The promoter region of telomerase reverse transcriptase (TERTp) and isocitrate dehydrogenase (IDH) have been regarded as biomarkers with distinct clinical and phenotypic features. Investigated the possible correlations between tumor location and genetic alterations would enhance our understanding of gliomagenesis and heterogeneity of glioma. We examined mutations of TERTp and IDH by direct sequencing and fluorescence in-situ hybridization in a cohort of 225 grades II and III diffuse gliomas. Correlation analysis between molecular markers and tumor locations was performed by Chi-square tests/Fisher’s exact test and multivariate logistic regression analysis. We found gliomas in frontal lobe showed higher frequency of TERTp mutation (P=0.0337) and simultaneously mutations of IDH and TERTp (IDH mut-TERTpmut) (P=0.0281) than frequency of biomarkers mutation of tumors in no-Frontal lobes, while lower frequency of TERTp mutation (P<0.0001) and simultaneously wild type of IDH and TERTp (IDH wt-TERTpwt) (P<0.0001) in midline than no-midline lobes. Logistic regression analysis indicated that locations of tumors associated with TERTp mutation (OR=0.540, 95% CI 0.324-0.900, P=0.018) and status of combinations of IDH and TERTp (IDH mut-TERTp mut vs. IDH wt-TERTp wt OR=0.162, 95% CI 0.075-0.350, P<0.001). In conclusion, grades II and III gliomas harboring TERTp mutation were located preferentially in the frontal lobe and rarely in midline. Association of IDH-TERTp status and tumor location suggests their potential values in molecular classification of grades II and III gliomas.
Keywords: Gliomas, heterogeneity, IDH mutation, TERT promoter mutation, tumor location
Introduction
Gliomas are the most common primary brain malignancies. They were classified by World Health Organization (WHO) into four grades according to the morphological resemblance of the neoplastic cells to normal glial tissues [1,2]. While glioblastomas (grade IV) had the most dismal prognosis [3-5], grades II and III gliomas showed a relatively favorable but highly variable survival [2]. Although the great improve had been made on the diagnosis and treatment of grades II and III gliomas in the past decades, patients with grades II and III gliomas had an inevitable recurrence and mortal result.
It had been increasingly valued by researchers that gliomas with diverse genetic abnormalities might arise from distinct cell types of origin and might be an important cause of tumor heterogeneity [6,7]. The association between genetic signature and tumor location is important for understanding the spatial origin of tumorigenesis, sub-classifying this kind of aggressive cancers and developing more intensive treatment measures to prolong the life of patients with grades II and III gliomas.
In the recent years, isocitrate dehydrogenase (IDH) mutation has been linked with special location distribution in diffuse gliomas. Approximately 70% to 80% of grades II and III diffuse gliomas and secondary glioblastomas, excluding ependymoma and pilocytic astrocytomas, harbor mutations at codon 132 (R132) of the isocitrate dehydrogenase 1 gene (IDH1) or at codon 172 (R172) of the isocitrate dehydrogenase 2 gene (IDH2) [5,8-11]. Many studies reported the preferential localization of glioblastomas [12-14] and lower-grade diffuse astrocytic gliomas [5,6,9,12,15-19] with IDH mutation in frontal lobe, suggesting this kind of tumors may arise from distinct cell types of origin.
Concurrently, it is well known that the promoter region of telomerase reverse transcriptase (TERTp) is a driver event in cancer development [19]. Shortening of telomere repeats cap at the ends of eukaryotic chromosomes with each cell division trigger cell death or senescence eventually [20]. TERTp encodes catalytic subunits of telomerase which maintain telomere length, and delay cellular senescence [20,21]. Tumors with TERTp mutation present distinct clinical and phenotypic features [19]. Analysis of the spatial distribution of TERTp mutation in grade II and III gliomas and combined analysis with the spatial distribution of IDH mutation may enhance the understanding of gliomagenesis. It would be a new perspective for neurosurgeons to consider grade II and III gliomas because of the association between genetic alterations and distinct prognosis of gliomas [22-25]. To our knowledge, there is no report about the regional distribution of TERTp mutation in WHO grade II and III gliomas. In this study, we examined the TERTp and IDH mutation of 225 WHO grade II and III gliomas, confirmed the brain lobes their located and analysis the associations of TERTp mutation, IDH mutation and tumor location.
Patients and methods
Patients and tissue samples
A total of 225 grades II and III gliomas with formalin-fixed paraffin-embedded tissues available and imaging studies (MRI) at the time of the diagnosis or in the preoperative period available were selected from the Department of Neurosurgery, Huashan hospital (Shanghai, China) between 2001 and 2011 and from the Department of Anatomical and Cellular Pathology, Prince of Wales Hospital (Hong Kong) between 1990 and 2012 [26,27]. To confirm the tumors location perfectly, detailed radiological reports, operative reports and profiles of postoperative MRI were studied also. According to the 2007 WHO classification [2], there were 96 diffuse astrocytomas (WHO grade II; AII), 20 oligodendrogliomas (WHO grade II; OII), 47 oligoastrocytomas (WHO grade II; OAII), 54 anaplastic astrocytomas (WHO grade III; AAIII), 5 anaplastic oligodendrogliomas (WHO grade III; AOIII), 3 anaplastic oligoastrocytomas (WHO grade III; AOAIII). The cohort overlapped partly with previous studies [26,27]. This study was approved by the Ethics Committee of Shanghai Huashan Hospital and the New Territories East Cluster-Chinese University of Hong Kong Ethics Committee.
Tumor location
To consider the tumor locations, imaging profiles (MRI) and the clinical files were retrospectively reviewed by two neurosurgeons that had no idea of the molecular status of the patient. If there were disagree with the tumor categorization between them, a senior neurosurgeon would have the right to judge the tumor involvement. To simplify the analysis, the tumors were primarily assigned into three kinds of locations: frontal, midline and others [28]. Frontal gliomas included only the tumors located entirely in frontal lobe. Midline location included corpus callosum, thalamencephalon, periventricular location, brainstem and thoracic spinal cord [7], where tumors entirely located in. Others lobes included insular lobe (including insular, frontotemporal-insular, temporal-insular and frontal-insular lobe), temporal lobe (including temporal, frontotemporal, tempoparietal, temporal-occipital lobe), parietal lobe (including parietal, frontoparietal, parietal-occipital lobe) and Occipital lobe & Cerebellum (including occipital lobe and cerebellum).
Mutational analysis of TERTp and IDH
Among the 225 grade II and III gliomas that had been detected IDH mutation, 213 grade II and III gliomas were examined for mutations of TERTp. Detection of IDH1 and IDH2 alterations of the mutational hotspot codons R132 and R172 and mutation analysis of TERTp were performed as previously described respectively [26,27,29]. Briefly, crude cell lysate extracted from dewaxed sections from representative tumor area with tumor content greater than 70% was used for subsequent polymerase chain reaction (PCR) analysis. Primers sequences (the forward primer IDH1F: 5’-CGGTCTTCAGAGAAGCCATT-3’ and reverse primer IDH1-R: 5’-CACATTATTGCCAACATGAC-3’; forward primer IDH2-F: 5’-AGCCCATCATCTGCAAAAAC-3’ and reverse primer IDH2-R 5’-CTAGGCGAGGAGCTCCAGT-3’) were used to amplify fragments of PCR, while a 163 bp fragment spanning the two mutational hotspots (C228T and C250T) in promoter region of TERTp were amplified with TERT-F (5’-GTCCTGCCCCTTCACCTT-3’) and TERT-R (5’-CAGCGCTGCCTGAAACTC-3’). Sequencing was performed using Big Dye Terminator Cycle Sequencing kit v1.1. The products were resolved in Genetic Analyzer 3130xl and analyzed by Sequencing Analysis software.
Statistical analysis
Fisher’s exact test (or Chi-square tests when n>10) were performed to assess the genotype distribution of IDH and TERTp mutation in different tumor locations. Main effects multivariate logistic regression analysis was used to identify the factors associated with status of biomarker of this cohort of grade II and III gliomas. Gender (values: 1= female, 0= male), age (values: 1= group of patients ≤40 years old, 2≥40 years old), pathology (values: 1= GradeII, 2= Grade III) and locations of tumors (values: 1= Frontal, 2= Others lobe, 3= Midline) were selected as independent variables for the analysis of each biomarker status. Value of β, odds ratio (ORs), 95% confidence interval (95% CIs) and p-values of factors with status of each biomarkers status were calculated respectively. All statistical tests were two-sided, and the threshold for statistical significance was P<0.05. Analyses were conducted with SPSS for Windows version 20.0 (SPSS Inc, Chicago, IL, USA).
Results
Cohort characteristics and molecular data
IDH mutation was found in 149 of 225 (66.22%) cases examined, including one IDH1-R132S mutation, one IDH2-R172M mutation, three IDH2-R172K mutation and 144 IDH1-R132H mutations. IDH mutations were found in 65% (91/140) male patients and 68.24% (58/85) female patients. There were 117 patients younger than or equal to 40 years and 108 patients older than 40 years, with 72.12% (75/108) and 63.25% (74/117) harboring IDH mutations, respectively. Among the cohort, 68.75% (66/96) of AII, 90% (18/20) of OII and 82.98% (39/47) of OAII harbored IDH mutations. Grade III gliomas, including AAIII, AOIII and AOAIII showed IDH mutations in 41.94% (26/62) of cases (Table 1 and Table S1).
Table 1.
Clinical and molecular data of 225 WHO grade II and III gliomas
| Variables | All patients number (%) | IDH mutation positive/all (%) | TERTp mutation positive/all (%) |
|---|---|---|---|
| Age | |||
| Median | 40 | 39 | 44 |
| Mean (±SD) | 40.8±12.2 | 40.9±9.6 | 43.7±11.5 |
| Range | 3-79 | 23-79 | 13-70 |
| Sex | |||
| Male | 140 (62.22) | 91/140 (65) | 35/131 (26.72) |
| Female | 85 (37.78) | 58/85 (68.24) | 26/82 (31.71) |
| Pathology | |||
| II | 163 (72.44) | 123/163 (75.46) | 45/158 (28.48) |
| III | 62 (27.56) | 26/62 (41.93) | 16/55 (35.56) |
| Location | |||
| Frontal | 83 (36.89) | 67/83 (80.72) | 30/81 (37.04) |
| Insular | 13 (5.78) | 10/13 (76.92) | 4/13 (30.77) |
| Temporal | 61 (27.11) | 41/61 (67.21) | 15/53 (28.3) |
| Parietal | 35 (15.56) | 25/35 (71.43) | 9/33 (27.27) |
| O & C | 6 (2.67) | 1/6 (16.67) | 0/6 (0) |
| C & C | 6 (2.67) | 3/6 (50) | 0/6 (0) |
| Midline | 21 (9.33) | 2/21 (9.52) | 2/21 (9.52) |
Abbreviations: O & C, Occipital & Cerebellum; C & C, Corpus callosum & Cingulate gyrus. Frontal including tumors located entirely in frontal lobe; Midline including tumors located entirely in corpus callosum, thalamencephalon, periventricular location, brainstem and thoracic spinal cord; Insular including tumors involved in insular, frontotemporal-insular, temporal-insular and frontal-insular lobe; Temporal including tumors involved in temporal, frontotemporal, tempoparietal, temporal-occipital lobe; Parietal including tumors involved in parietal, frontoparietal, parietal-occipital lobe; O & C including tumors located entirely in occipital lobe and cerebellum; C & C including tumors located entirely in corpus callosum and cingulate gyrus.
Mutation in TERTp was found in 61 of 213 (28.64%) grade II and III gliomas examined. The mutations were found in 26.72% (35/131) of male patients and 31.7% (26/82) of female patients. There were110 patients younger than or equal to 40 years and 103 patients older than 40 years, with 19.42% (20/103) and 36.36% (40/110) showing TERTp mutation, respectively. Among the cohort, 14.89% (14/94) of AII, 73.68% (14/19) of OII, 37.78% (17/45) of OAII, 22.45% (11/49) of AAIII, 100% (3/3) of AOIII and 66.67% (2/3) of AOAIII harbored TERTp mutation respectively (Table 1).
Correlation between biomarker status and tumor location
To discover the tendency of the distributions of biomarker status on brain lobes, we calculated the approximate mutation rate of each biomarker across brain lobes. Figure 1A showed IDH mutation was identified in 67 of 83 (80.72%) frontal tumors, 10 of 13 (76.92%) insular tumors, 41 of 61 (67.21%) temporal tumors, 25 of 35 (71.43%) parietal tumors, 3 of 6 (50%) corpus callosum & cingulate gyrus tumors, 1 of 6 (16.67%) occipital & cerebellar tumors and 2 of 21 (9.52%) midline tumors. We found from Figure 1A that there was a degressive tendency of mutation rate of IDH from Frontal to Midline. Chi-square test or Fisher’s exact test had been used to find out that the rate of IDH mutation in the cohort of grade II and grade III gliomas were higher in the frontal lobe than non-frontal region (P=0.0004, Chi-square test) and lower in the midline than non-midline location (P<0.0001, Fisher’s exact test) (Figure 1B). Results of binary logistic regress confirmed this discover. As shown in Table 2, Locations of tumors after adjustment for gender, age and pathology (β=-1.456, OR=0.233, 95% CI 0.132-0.413, P<0.001) was found to be independently associated with status of IDH1/2. With the values of β is negative, the rate of IDH mutation has a degressive tendency from Frontal to midline.
Figure 1.
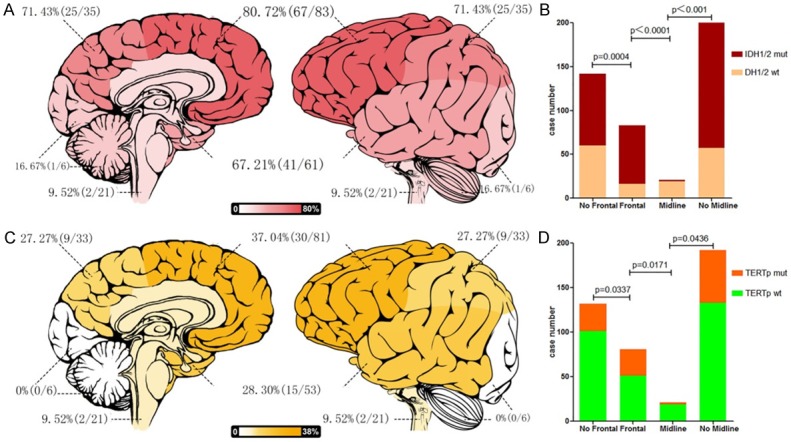
Correlations between locations distribution and molecular status of WHO grade II and III gliomas. The rate of IDH mutation (A) and TERT promoter mutation (C) of WHO grade II and III gliomas decreased gradually from frontal lobe to Midline. WHO grade II and III gliomas with IDH mutation (B) and TERT promoter mutation (D) is more preferentially located in Frontal and repulsively in midline. Footnotes: Frontal including tumors located entirely in frontal lobe; Midline including tumors located entirely in corpus callosum, thalamencephalon, periventricular location, brainstem and thoracic spinal cord; Insular lobe including tumors involved in insular, frontotemporal-insular, temporal-insular and frontal-insular lobe; Temporal lobe including tumors involved in temporal, frontotemporal, tempoparietal, temporal-occipital lobe; Parietal lobe including tumors involved in parietal, frontoparietal, parietal-occipital lobe; Occipital lobe & Cerebellum including tumors located entirely in occipital lobe and cerebellum.
Table 2.
ORs, 95% CIs and p-values of classes of gliomas location according to status of IDH
| Variables | β | P | OR | 95% CI |
|---|---|---|---|---|
| Gender | -0.286 | 0.396 | 0.751 | 0.388-1.455 |
| Age | -0.387 | 0.237 | 0.679 | 0.358-1.289 |
| Pathology | -1.483 | 0.000 | 0.227 | 0.114-0.453 |
| Location | -1.456 | 0.000 | 0.233 | 0.132-0.413 |
Abbreviations: CI, confidence interval; OR, odds ratio.
TERTp mutation was found in 37.04% (30/81) of frontal tumors, 30.77% (4/13) of insular tumors, 28.30% (15/53) temporal tumors, 9 of 33 (27.27%) parietal tumors, 0 of 6 (0%) corpus callosum & cingulate gyrus tumors, 0 of 6 (0%) occipital & cerebellar tumors and 2 of 21 (9.52%) midline tumors (Figure 1C). We found also that there was a degressive tendency of mutation rate of TERTp from Frontal to Midline. The rat of TERTp mutation in the cohort of grades II and grade III gliomas were higher in the frontal lobe than non-frontal region (P=0.0337, Chi-square tests) and lower in the midline than non-midline location (P=0.0438, Fisher’s exact test) (Figure 1D). Results of binary logistic regress confirmed this discover too. Locations of tumors after adjustment for gender, age and pathology (β=-0.616, OR=0.540, 95% CI 0.324-0.900, P=0.018) was found to be independently associated with status of TERTp. With the values of β is negative, the rate of TERTp mutation has a degressive tendency from frontal lobe to midline also (Table 3).
Table 3.
ORs, 95% CIs and p-values of classes of gliomas location according to status of TERTp
| Variables | β | P | OR | 95% CI |
|---|---|---|---|---|
| Gender | 0.092 | 0.774 | 1.097 | 0.584-2.061 |
| Age | 0.539 | 0.089 | 1.715 | 0.921-3.192 |
| Pathology | 0.025 | 0.946 | 1.025 | 0.504-2.085 |
| Location | -0.616 | 0.018 | 0.540 | 0.324-0.900 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Distribution of grade II and III gliomas with combined status of IDH and TERTp
As the results shown above, IDH mutation and TERTp mutation in grades II and III gliomas shared similar spatial distributions across brain lobes. Therefore, we analyzed the regional distribution of the tumors according to combined status of IDH and TERTp (IDH-TERTp). Results of Chi-square test or Fisher’s exact test identified that simultaneously mutations of IDH and TERTp (IDH mut-TERTp mut) subgroup was preferentially located in frontal lobe (P=0.0281) and simultaneously wild type of IDH and TERTp (IDH wt-TERTp wt) subgroup was preferentially located in midline regions (P<0.0001). We identified the rate of IDH mut-TERTp wt were lower in midline tumors (P=0.0004), but did not identify any other association between IDH mut-TERTpwt subgroup and IDH wt-TERTp mut subgroup with location (Table 4). To confirm the result, we used a main effects multivariate logistic regression analysis to identify if the IDH-TERTp status correlated with tumor locations. For dependent variables, the IDH mut-TERTp mut subgroup was set as 1, while subgroup of IDH mut-TERTp wt and IDH wt-TERTp mut were set as 2, IDH wt-TERTp wt subgroup was set as 3 (Table S1). As shown in Table 6, Locations of tumors after adjustment for gender, age and pathology (IDH mut-TERTp mut vs. IDH wt-TERTp wt β=-1.819, OR=0.162, 95% CI 0.075-0.350, P<0.001; IDH mut-TERTp wt & IDH wt-TERTp mut vs. IDH wt-TERTp wt β=-1.411, OR=0.244, 95% CI 0.131-0.453, P<0.001) was found to be independently associated with status of IDH-TERTp. The result of the regression analysis shown there was a depressive of rate of IDH-TERTp mutation from Frontal to Midline (Table 5).
Table 4.
Regional distribution of the tumors according to combined status of IDH and TERTp
| Number | Frontal P/N | no Frontal P/N | P (F vs. NF) | Midline P/N | no Midline P/N | P (M vs. NM) | |
|---|---|---|---|---|---|---|---|
| IDH wt-TERTp wt | 57 | 10/71 | 47/85 | 0.0002 | 17/4 | 40/152 | <0.0001 |
| IDH wt-TERTp mut | 13 | 5/76 | 8/124 | 1.0000 | 2/19 | 7/185 | 0.6309 |
| IDH mut-TERTp wt | 95 | 41/40 | 54/78 | 0.2015 | 2/19 | 93/99 | 0.0004 |
| IDH mut-TERTp mut | 48 | 25/56 | 23/109 | 0.0281 | 0/21 | 48/144 | 0.0050 |
Abbreviations: P/N, numbers of gliomas with positive/negative biomarker mutation; P (F vs. NF), P values between Frontal and no-Frontal; P (M vs. NM), P values between Midline and no-Midline.
Table 6.
Correlations between locations distribution and molecular status of subgroups of WHO grade II and III gliomas
| Number | Frontal P/N | no Frontal P/N | P (F vs. NF) | Midline P/N | no Midline P/N | P (M vs. NM) | |
|---|---|---|---|---|---|---|---|
| II | |||||||
| TERTp | 158 | 23/38 | 22/75 | 0.0468 | 0/11 | 45/102 | 0.0346 |
| IDH1/2 | 163 | 56/6 | 67/34 | 0.0006 | 0/11 | 123/29 | <0.0001 |
| III | |||||||
| TERTp | 55 | 7/13 | 9/26 | 0.5434 | 2/8 | 14/31 | 0.7055 |
| IDH1/2 | 62 | 11/10 | 15/26 | 0.2329 | 2/8 | 24/28 | 0.3316 |
| Man | |||||||
| TERTp | 131 | 15/29 | 20/67 | 0.1750 | 2/15 | 33/81 | 0.2376 |
| IDH1/2 | 140 | 40/5 | 52/43 | <0.0001 | 2/15 | 90/33 | <0.0001 |
| Female | |||||||
| TERTP | 82 | 15/22 | 11/34 | 0.1191 | 0/4 | 26/52 | 0.3058 |
| IDH1/2 | 85 | 27/11 | 31/16 | 0.6159 | 0/4 | 58/23 | 0.0087 |
| >40 | |||||||
| TERTp | 103 | 19/25 | 17/42 | 0.130 | 2/7 | 34/60 | 0.4894 |
| IDH1/2 | 108 | 35/10 | 32/31 | 0.0051 | 1/8 | 66/33 | 0.0017 |
| ≤40 | |||||||
| TERTp | 110 | 11/26 | 14/59 | 0.2122 | 0/12 | 25/73 | 0.0646 |
| IDH1/2 | 117 | 32/6 | 50/29 | 0.0301 | 1/10 | 81/25 | <0.0001 |
Abbreviations: P/N, numbers of gliomas with positive/negative biomarker mutation; P (F vs. NF), P values between Frontal and no-Frontal; P (M vs. NM), P values between Midline and no-Midline.
Table 5.
ORs, 95% CIs and p-values of classes of gliomas location according to combined status of IDH and TERTp
| Variables | β | P | OR | 95% CI |
|---|---|---|---|---|
| IDH wt-TERTp wt | 1 | |||
| IDH mut-TERTp mut | ||||
| Gender | -0.043 | 0.923 | 0.958 | 0.399-2.299 |
| Age | 0.190 | 0.665 | 1.209 | 0.513-2.852 |
| Pathology | -1.395 | 0.009 | 0.248 | 0.087-0.709 |
| Location | -1.819 | 0.000 | 0.162 | 0.075-0.350 |
| IDH mut-TERTp wt & IDH wt-TERTp mut | ||||
| Gender | -0.135 | 0.720 | 0.874 | 0.417-1.830 |
| Age | -0.024 | 0.948 | 0.976 | 0.479-1.991 |
| Pathology | -0.774 | 0.049 | 0.461 | 0.213-0.998 |
| Location | -1.411 | 0.000 | 0.244 | 0.131-0.453 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Biomarker distribution in subgroups by age, gender and histology
We further evaluated the effects of age, gender and histology on the spatial distribution of molecular markers as shown in Table 6. In both age subgroups of patients above 40 years and patients at or under 40 years, IDH mutation frequency was significantly higher in frontal tumors and lower in midline tumors. For gender, the frequencies of IDH mutation were higher in frontal tumors and lower in midline tumors of male patients. Among the female patients, there was no statistical difference in IDH mutation rate between different tumor locations. There were not any associations between TERTp mutation and tumor locations in subgroups of gliomas classified by age and gender. Evaluating the cohort according to histological grade, biomarker-location associations were mainly identified in grade II tumors, with frontal tumors demonstrating higher rate of IDH mutation and TERTp mutation, and midline tumors showing lower rate of IDH mutation and TERTp mutation. Results of regression analysis shown pathology independently associated with IDH1/2 mutation (β=-1.483, OR=0.227, 95% CI 0.114-0.453, P<0.001) (Table 2) and status of IDH-TERTp (IDH mut-TERTp mut vs. IDH wt-TERTp wt β=-1.395, OR=0.248, 95% CI 0.087-0.709, P=0.009; IDH mut-TERTp wt & IDH wt-TERTp mut vs. IDH wt-TERTp wt β=-0.774, OR=0.049, 95% CI 0.213-0.998, P=0.049) (Table 5).
Discussion
In this study, we found that WHO grade II and III gliomas located in frontal lobe were preferentially associated with IDH, TERTp and IDH mut-TERTp mut, while gliomas in midline were preferentially associated with IDH, TERTp and IDH wt-TERTp wt, evenly after adjustment for gender, age and pathology. It is in concordance with previous data [6,15,18] that frontal lobe was a preferential location for gliomas harboring IDH mutation as compared to other cerebral regions. Chen et al. recently reported that the glutaminergic neurotransmitter specialization of human neocortex, especially frontal lobe, created a metabolic niche favorable for the development of IDH1 mutant tumors [30]. Their findings may explain the biological mechanisms for the preferential distribution of IDH mutated grades II and III gliomas in frontal lobes. Midline location in this study included corpus callosum, thalamencephalon, periventricular location, brainstem and thoracic spinal cord. The menchanism of the special distribution of gliomas in midline without IDH mutation remained undiscovered. In the subset analysis, association between IDH mutation and frontal localization was observed in male patients but not female patients. Given that the case numbers in the individual gender subsets of the cohort were small, further study evaluating the effect of gender on the regional distribution of IDH mutation in a larger cohort should be conducted.
We found that grades II and III gliomas in frontal lobe show high TERTp mutation rate, on the counterparts, low TERTp mutation rate in midline. To the best of our knowledge, this is the first study reported it. Dominik et al. had classified glioblastomas into six subgroups based on their global DNA methylation patterns [7]. They suggested there were a specific anatomically-defined subset of gliomas with H3F3A-K27M mutation almost exclusively arose from midline locations, which was consistent with results of several other researchers’ studies [31,32]. Our results of this report suggested that there might be an anatomically-defined subset of gliomas with biological character of IDH wt-TERTp wt in grades II and III diffuse gliomas located in midline. Although the biological mechanism of the special distribution of gliomas with TERTp mutation requires further elucidation, our results shed light on potential anatomical cellular origins of grades II and III gliomas and improved further research in this field.
As our previously study showed, TERTp mutation had been recognized as a dismal molecular marker in prognostic classification of diffuse gliomas [26]. Killela et al. had identified that gliomas exhibit IDH mut-TERTp mut had a best prognosis for exhibiting a median over survival (OS) of 125 months, while gliomas exhibit TERTp mutation alone with a poorest OS (11.5 months) in their cohort of gliomas [19]. In keeping with this study, Eckel-Passow et al. recently found that patients with IDH wt-TERTp wt and without co-deletion of 1p19q had poor OS than IDH mut-TERTp mut or IDH mutation alone, but better OS than TERTp mutation alone, after adjustment for age and grade [22]. Data of this study may imply neurosurgeons that grades II and III gliomas located in frontal lobe where is more accessible to surgery would be associated with different prognostic biomarkers. Moreover, our results suggest a gloomier prognosis of patients with grades II and III gliomas located in midline for being barely inaccessible to surgery and associated with poor prognostic biomarkers. So, there would be a more crucial need to design new therapeutic agents for grades II and III gliomas located in midline.
In conclusion, we have investigated the spatial distribution of grades II and III gliomas with specially status of IDH, TERTp and IDH-TERTp, suggested that there are some anatomically-defined subset of gliomas with special biomarkers, enhanced neurosurgeons’ understanding of grades II and III gliomas in specific area of brain, and would stimulated more researchers to study in this field.
Acknowledgements
This work was supported by grant 13JC1408000 from the Science and Technology Com-mission of Shanghai Municipality, the Research Special Fund for Public Welfare Industry of Health (No. 201402008), and the National key clinical specialist construction Programs of China (Oncology). We thank Dr. Xiao-Qin Wang for helpful instructions about statistics treatment.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F, Forrest WF, Pujara K, Carrillo JA, Pandita A, Ellingson BM, Bowers CW, Soriano RH, Schmidt NO, Mohan S, Yong WH, Seshagiri S, Modrusan Z, Jiang Z, Aldape KD, Mischel PS, Liau LM, Escovedo CJ, Chen W, Nghiemphu PL, James CD, Prados MD, Westphal M, Lamszus K, Cloughesy T, Phillips HS. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J. Clin. Oncol. 2011;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Fruhwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Durken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Liu XY, Gerges N, Korshunov A, Sabha N, Khuong-Quang DA, Fontebasso AM, Fleming A, Hadjadj D, Schwartzentruber J, Majewski J, Dong Z, Siegel P, Albrecht S, Croul S, Jones DT, Kool M, Tonjes M, Reifenberger G, Faury D, Zadeh G, Pfister S, Jabado N. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124:615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 9.Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 2011;28:177–183. doi: 10.1007/s10014-011-0029-1. [DOI] [PubMed] [Google Scholar]
- 10.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta R, Webb-Myers R, Flanagan S, Buckland ME. Isocitrate dehydrogenase mutations in diffuse gliomas: clinical and aetiological implications. J Clin Pathol. 2011;64:835–844. doi: 10.1136/jclinpath-2011-200227. [DOI] [PubMed] [Google Scholar]
- 13.Ikota H, Nobusawa S, Tanaka Y, Yokoo H, Nakazato Y. High-throughput immunohistochemical profiling of primary brain tumors and non-neoplastic systemic organs with a specific antibody against the mutant isocitrate dehydrogenase 1 R132H protein. Brain Tumor Pathol. 2011;28:107–114. doi: 10.1007/s10014-010-0016-y. [DOI] [PubMed] [Google Scholar]
- 14.Jha P, Suri V, Sharma V, Singh G, Sharma MC, Pathak P, Chosdol K, Jha P, Suri A, Mahapatra AK, Kale SS, Sarkar C. IDH1 mutations in gliomas: first series from a tertiary care centre in India with comprehensive review of literature. Exp Mol Pathol. 2011;91:385–393. doi: 10.1016/j.yexmp.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Gorovets D, Kannan K, Shen R, Kastenhuber ER, Islamdoust N, Campos C, Pentsova E, Heguy A, Jhanwar SC, Mellinghoff IK, Chan TA, Huse JT. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18:2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 16.Qi S, Yu L, Li H, Ou Y, Qiu X, Ding Y, Han H, Zhang X. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014;7:1895–1902. doi: 10.3892/ol.2014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourne TD, Schiff D. Update on molecular findings, management and outcome in low-grade gliomas. Nat Rev Neurol. 2010;6:695–701. doi: 10.1038/nrneurol.2010.159. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang T, Li S, Fan X, Ma J, Wang L, Jiang T. Anatomical localization of isocitrate dehydrogenase 1 mutation: a voxel-based radiographic study of 146 low-grade gliomas. Eur J Neurol. 2015;22:348–354. doi: 10.1111/ene.12578. [DOI] [PubMed] [Google Scholar]
- 19.Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, Friedman H, Friedman AH, Keir ST, He J, He Y, McLendon RE, Herndon JE 2nd, Yan H, Bigner DD. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5:1515–1525. doi: 10.18632/oncotarget.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Annu Rev Pathol. 2013;8:49–78. doi: 10.1146/annurev-pathol-020712-164030. [DOI] [PubMed] [Google Scholar]
- 22.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O’Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, LanXiao W, Fei Y. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103:269–273. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 24.Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 25.Metellus P, Coulibaly B, Colin C, de Paula AM, Vasiljevic A, Taieb D, Barlier A, Boisselier B, Mokhtari K, Wang XW, Loundou A, Chapon F, Pineau S, Ouafik L, Chinot O, Figarella-Branger D. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120:719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 26.Chan AK, Yao Y, Zhang Z, Chung NY, Liu JS, Li KK, Shi Z, Chan DT, Poon WS, Zhou L, Ng HK. TERT promoter mutations contribute to subset prognostication of lower-grade gliomas. Mod Pathol. 2015;28:177–186. doi: 10.1038/modpathol.2014.94. [DOI] [PubMed] [Google Scholar]
- 27.Yao Y, Chan AK, Qin ZY, Chen LC, Zhang X, Pang JC, Li HM, Wang Y, Mao Y, Ng HK, Zhou LF. Mutation analysis of IDH1 in paired gliomas revealed IDH1 mutation was not associated with malignant progression but predicted longer survival. PLoS One. 2013;8:e67421. doi: 10.1371/journal.pone.0067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laigle-Donadey F, Martin-Duverneuil N, Lejeune J, Criniere E, Capelle L, Duffau H, Cornu P, Broet P, Kujas M, Mokhtari K, Carpentier A, Sanson M, Hoang-Xuan K, Thillet J, Delattre JY. Correlations between molecular profile and radiologic pattern in oligodendroglial tumors. Neurology. 2004;63:2360–2362. doi: 10.1212/01.wnl.0000148642.26985.68. [DOI] [PubMed] [Google Scholar]
- 29.Chan AK, Yao Y, Zhang Z, Chung NY, Liu JS, Li KK, Shi Z, Chan DT, Poon WS, Zhou L, Ng HK. TERT promoter mutations contribute to subset prognostication of lower-grade gliomas. Mod Pathol. 2015;28:177–86. doi: 10.1038/modpathol.2014.94. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Nishimura MC, Kharbanda S, Peale F, Deng Y, Daemen A, Forrest WF, Kwong M, Hedehus M, Hatzivassiliou G, Friedman LS, Phillips HS. Hominoid-specific enzyme GLUD2 promotes growth of IDH1R132H glioma. Proc Natl Acad Sci U S A. 2014;111:14217–14222. doi: 10.1073/pnas.1409653111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bechet D, Gielen GG, Korshunov A, Pfister SM, Rousso C, Faury D, Fiset PO, Benlimane N, Lewis PW, Lu C, David Allis C, Kieran MW, Ligon KL, Pietsch T, Ellezam B, Albrecht S, Jabado N. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol. 2014;128:733–741. doi: 10.1007/s00401-014-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


