Abstract
Background: Non-alcoholic fatty hepatitis (NASH) is highly prevalent, mitochondria damage is the main pathophysiological characteristic of NASH. However, treatment for mitochondria damage is rarely reported. Methods: NASH model was established in rats, the protective effects of curcumin were evaluated by histological observation; structure and function assessments of mitochondria; and apoptotic genes expression. Results: NASH rats treated with curcumin displayed relatively slight liver damage when compared with NASH livers. The average mitochondrial length and width of NASH (12.0 ± 3.2 and 5.1 ± 1.1 micrometers) were significantly longer than that of normal (6.2 ± 2.1 and 2.1 ± 1.5 micrometers) and NASH treated with curcumin (7.4 ± 1.2 and 3.2 ± 1.5 micrometers) rats. The average malondialdehyde (MDA) and 4-hydroxy nonyl alcohol (HNE) levels in liver homogenates of NASH rats (4.23 ± 0.22 and 19.23 ± 2.3 nmol/Ml) were significantly higher than these in normal (1.32 ± 0.12 and 3.52 ± 0.43 nmol/mL) and NASH treated with curcumin (1.74 ± 0.11 and 4.66 ± 0.99 nmol/mL) rats. The expression levels of CytC, Casp3 and Casp8 of the NASH livers were significantly higher than normal and NASH treated with curcumin rats livers. Conclusion: Our data demonstrated that curcumin prevents the NASH by mitochondria protection and apoptosis reduction and provided a possible novel treatment for NASH.
Keywords: Curcumin, non-alcoholic fatty hepatitis, mitochondria damage, apoptosis
Introduction
Non-alcoholic fatty hepatitis (NASH), is a chronic liver inflammatory disease developed from nonalcoholic fatty liver disease (NAFLD), manifested pathologically as hepatic steatosis; hepatic cells ballooning; diffuse hepatic lobule mild inflammation; and/or collagen deposition around the central vein of liver and hepatic sinusoidal [1]. Although the pathogenesis of NASH is not very clear, the role of oxygen stress in the pathogenesis of NASH has been recognized by many scholars [2].
Oxidative stress is a state of dynamic imbalance between peroxide and antioxidant, which lead by molecular oxygen free radicals or reactive oxygen species (ROS) and its metabolites increasing or antioxidant mechanism weakening [3]. The short acting and local effects of ROS include lipid peroxidation with membrane phospholipids and membrane receptors related to multivalent unsaturated fatty acids, promoting fatty acid aggregation and trigger intracellular lipid peroxidation [4]. The by-products 4-hydroxy nonyl alcohol (HNE) and malondialdehyde (MDA) will be produced during above processes, the half-lives of HNE and MDA are much longer than that of ROS, and they will spread from the production site to intracellular and extracellular sites, thereby, amplify oxygen stress [5]. Compared with patients with simple steatosis, more than 90% of NASH patients displayed increasing levels of HNE and MDA [6]. The lipid peroxidation products can also, induce the synthesis and expression of transforming growth factor beta (TGF-beta) and monocyte chemoattractant protein-1 (MCP-1); promote neutrophil chemotaxis and tumor necrosis factor-alpha (TNF-alpha) generation; stimulate hepatic stellate cell to secrete collagen fiber [7]. In addition, the lipid peroxidation products may also induce cell apoptosis via the c-Jun N-terminal kinase (JNK) and transcription factor activated protein-1 (AP-1) signaling [7]. ROS induced TNF-alpha and lipid peroxides can also damage the mitochondrial respiratory chain and DNA, which leads to more ROS generation, forming a vicious cycle of oxidative stress [7].
In mammals, ROS are mainly produced by mitochondria [8]. Mass ROS can lead to the disorders of the membrane potential, penneability transition pore (PTP) and energy metabolism of mitochondria [9]. In recent years, studies show that NASH is accompanied by the liver mitochondria structure abnormal, including huge mitochondria, mitochondrial swelling, membrane crest grain decreased and mitochondrial matrix crystallization inclusion [10]. Study found that there is no significant difference of respiratory control rate (RCR) and phosphorus/oxygen (P/O) ratio between high-fat diet rats and normal diet control rats in short-term observation, however, the PCR and P/O ratio decreased significantly in rats with high fat diet after 8 to 12 weeks [11]. These evidences showed that the disorders of the respiratory function of mitochondria in the pathological process of NASH. Thus, protection on mitochondrial structure and function damage is a possible treatment for NASH. Curcumin is a phenolic compound found in the dietary spice turmeric, derived from the rhizome of Curcuma longa [12]. Curcumin can play a variety of mechanisms to play a role in antioxidant, these including elimination of hydrogen peroxide, hydroxyl radical, peroxynitrite, etc [13]. Curcumin can also enhance the body’s antioxidant capacity, 100 mg/kg and 200 mg/kg curcumin could remove the ROS induced by insecticide and increase the glutathione content in the Wistar rat and hence play a protective effect over lindane-induced oxidative damage in rat liver [14]. Although curcumin antioxidation effect has been confirmed, whether curcumin possesses any protective effect on NASH remains unclear. Our primary study showed that curcumin can prevent and amelio rate NASH via lipid reduction, insulin resistance and anti-inflammatory improvement [15]. However, the role of curcumin in the structure and function changes of mitochondria had not evaluated. In this study, the protective effects of curcumin were evaluated by histological observation; structure and function assessments of mitochondria; and apoptotic genes expression.
Materials and methods
Animals and diets
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal study was approved by the Committee on the Ethics of Animal Experiments of The Sixth People’s Hospital Affiliated to Shanghai Jiaotong University.
As we reported previously [15], male Sprague-Dawley rats with an average body weight of 190-210 g were housed six per cage under conditions of room temperature with free access to food and water. All the rats were fed a standard laboratory diet for a week. The rats were then randomly divided into three groups of twelve rats each: normal group, high fat diet group and high fat diet treated with curcumin (6 weeks later) group with twelve each. Rats in the normal group were fed a standard diet, while those in the other two groups received a high-fat diet composed of 18.0% protein, 45.0% fat, and 37.0% carbohydrates. Two rats from each group were euthanized at the end of the 6th week to detect pathological changes. Then, rats in the treatment group were gavaged with 50 mg/kg of curcumin (Sigma, St. Louis, MO) suspended in 0.5% carboxymethyl cellulose (CMC) daily for 6 weeks. The normal group and NASH group received an equal volume of 0.5% CMC (Sigma, St. Louis, MO) as controls.
Histological analysis
As we reported previously [15], Liver pathology was assessed using hematoxylin and eosin (H&E) and Oil Red O staining by board-certified pathologists in our hospital according following criterions [16]: steatosis was graded S0-S3 based on the percentage of hepatocytes in the biopsy involved, S0 corresponded to none; S1 was < 33%; S2 was 33-66%; S3 was > 66%; intra-acinar (lobular) inflammation was graded L0-L3 based on inflammatory foci per 10× with 20× ocular, L0 corresponded to none; L1 was 1-2/10×; L2 was up to 4/10×; L3 was > 4/10×; and portal tract inflammation was graded as none, mild, moderate, and severe (P0-P3).
Electron microscopy
To observe the mitochondria structure of rat liver, the rat livers were isolated. After rinsing in 0.9% cooled saline for three times, the liver tissue were cut into 250-500 nm slices. Then, the liver sections were fixed immediately by adding primary fixative (2% paraformaldehyde and 2.5% glutaraldehyde in a 0.1 M sodium cacodylate, pH 7.4, buffer) at 4°C, followed by 1 hour of incubation on ice. After washing three times in an ice-cold 0.1 M sodium cacodylate buffer containing 3 μM calcium chloride for 3 min, the primary fixed liver tissues were then incubated with 1% osmium tetroxide, 0.8% potassium ferrocyanide, and 3 μM calcium chloride in 0.1 M sodium cacodylate for 60 min on ice. After washing with distilled water three times for 3 min, the fixed liver tissues were stained and stabilized in 2% uranyl acetate for 30 min on ice. Liver tissues were dehydrated in an ice-cold ethanol series of 20%, 50%, 70% and 90% successively, on ice for 3 min each. The liver tissues were then dehydrated at room temperature three times for 3 min each in 100% ethanol. Then, the liver tissues were infiltrated in a mixture of 50% ethanol and 50% Durcupan ACM resin (Sigma, St. Louis, MO) for 60 min with agitation at room temperature, followed by 100% Durcupan ACM twice for 1 hour with agitation, after which the samples were placed in an oven to polymerize at 60 to 80° for at least 48 hours.
The mitochondria structure was observed following the methods reported by Sun et al [16,17]. Briefly, single-tilt series three-dimensional reconstructions were obtained from semi-thick samples in a tilt series every 2° from -60° to +60° on a JOEL 4000EX electron microscope (JOEL, Peabody, MA) operated at 400 kV. The IMOD software suite (an open-source, cross-platform suite of modeling, display and image processing programs used for 3D reconstruction and modeling of microscopy images) was used to process the images in each tilt series (http://bio3d.colorado.edu/). X-Voxtrace software enabled volume segmentation of tomographic data using manual tracing followed by rendering using Synu (National Center for Microscopy and Imaging Resources, University of California, San Diego, CA). Tomograms were converted to Tiff stacks for measurements of crista junction sizes using Image J (http://rsb.info.nih.gov/ij/).
ROS measures
To study the ROS level in normal, NASH and NASH treated with curcumin rat livers, overnight food-deprived rats were anesthetized with ketamine (0.2 mL/100 g) at the end of the 12th week. Body weight was measured. The liver was removed carefully and washed with 0.9% cooled saline. The livers were then homogenized, the homogenates were centrifuged at 1500 RPM for 10 minutes, the supernatants were collected and used for HNE and MDA measures. The liver contents of MDA was analyzed by MAD assay kits (BlueGene, Shanghai, China) in accordance with the manufacturer’s instructions as we reported previously [15]. The HNE was measured using the approach established by Spies-Martin et al [19].
Gene expression analysis
To study the apoptotic gene expression profile in rat groups, real-time quantification reverse transcript PCR (qRT-PCR) analysis was used. Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed to cDNA using the Two-Step RT-PCR Clone Kit (Biovisualab, Shanghai, China). Quantitative PCR was performed using a 7500 Real-time PCR System (Applied Biosystems, Foster City, CA) and the GREAT Real-Time SYBR PCR Kit (Biovisualab, Shanghai, China). The relative mRNA expression level was calculated using the 2-ΔΔCT method with the CT values normalized using β-actin as an internal control [18].
The genes and their corresponding primers were: CytC (forward: 5’CACAGATGCCAACAAGAACAAAGG3’; reverse: 5’AGGGATGTACTTTTTGGGATTTTCC3’); Casp3 (forward: 5’TGTATGCTTACTCTACCGCACCCG3’; reverse: 5’GCGCAAAGTGACTGGATGAACC3’); Casp8 (forward: 5’ACGTCTGGGCAACGAAGAACTG3’; reverse: 5’GCACCAGGACATCATTGATGGAC3’); Casp9 (forward: 5’GCGACATGATCGAGGATATTCAGC3’; reverse: 5’TGCCTCCCTCGAGTCTCAAGATC3’).
Statistical analysis
All statistics were analyzed using SPSS17.0 (SPSS Inc., Chicago, IL). All results are shown as the mean of three or more replicates. Data are presented as mean ± standard deviation. The data of three groups were analyzed by one-way ANOVA followed by Kruskal-Wallis test. P values < 0.05 were considered significant.
Results
Histological observation showed that curcumin could protect liver
As expected, rats feed with high fat diet developed into NASH after 6 weeks, as showed in Figure 1, NASH livers displayed hepatic steatosis; hepatic cells ballooning; diffuse hepatic lobule mild inflammation; and collagen deposition around the central vein. While, high fat diet rats treated with curcumin displayed relatively slight liver damage when compared with NASH livers. Oil Red O staining further showed steatosis in NASH liver and curcumin treatment could reduce the fat deposit in liver.
Figure 1.
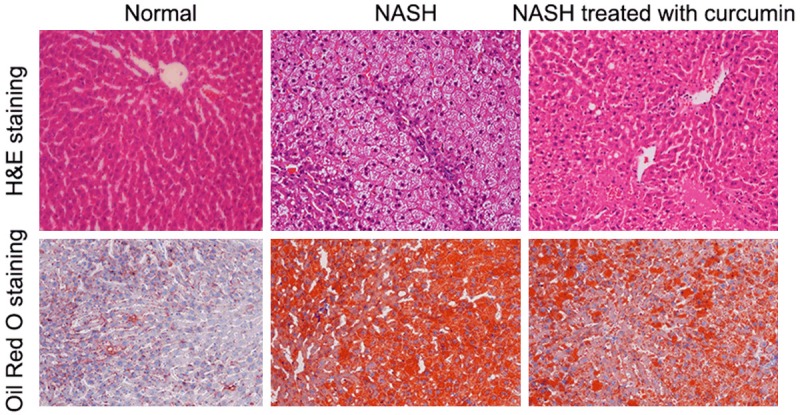
Histological observation. Upper half, H&E staining ×200; lower half, Oil Red O staining ×200.
Curcumin could protect mitochondria structure in NASH rats
To know if curcumin could prevent mitochondria structure abnormal, the mitochondria structure of normal, NASH and NASH treated with curcuminrats livers were studied by electron microscope observation. At least two mitochondrias of each liver sample were randomly selected to quantify the mitochondria structure. As showed in Table 1, the average length of the mitochondria of the normal, NASH and NASH treated with curcumin livers were 6.2 ± 2.1, 12.0 ± 3.2 and 7.4 ± 1.2 micrometers, respectively. The average width of the mitochondria of the normal, NASH and NASH treated with curcumin rats were 2.1 ± 1.5, 5.1 ± 1.1 and 3.2 ± 1.5 micrometers, respectively. The average mitochondrial length and width of NASH was significantly longer than that of normal and NASH treated with curcumin livers. The average mitochondrial length and width of NASH treated with curcumin rats were similar to normal rats. In conclusion, curcumin could protect mitochondria structure in NASH livers.
Table 1.
The structure changes of mitochondria
| Measurements | Normal | NASH | NASH treated with curcumin |
|---|---|---|---|
| Length (micrometers) | 6.2 ± 2.1 | 12.0 ± 3.2* | 7.4 ± 1.2** |
| Width (micrometers) | 2.1 ± 1.5 | 5.1 ± 1.1* | 3.2 ± 1.5** |
Data are presented as the means ± standard deviation.
P < 0.05 when compared with normal rats;
P < 0.05 when compared with NASH rats.
Curcumin could prevent the oxidative stress in liver
Since above observation showed that curcumin could protect mitochondria structure in NASH rats, we then check if curcumin could ease the oxidative stress in liver. As showed in Table 2, the average MDA of the normal, NASH and NASH treated with curcumin rats were 1.32 ± 0.12, 4.23 ± 0.22 and 1.74 ± 0.11 nmol/mL, respectively, the MDA in NASH liver is significantly higher than that in the livers of normal and NASH treated with curcumin rats. The average HNF of the normal, NASH and NASH treated with curcumin rats were 3.52 ± 0.43, 19.23 ± 2.3 and 4.66 ± 0.99 nmol/mL, respectively, the HNF in NASH liver is also significantly higher than that in the livers of normal and NASH treated with curcumin rats. In conclusion, NASH liver showed higher level of ROS, curcumin could ease the oxidative stress significantly.
Table 2.
Contents of HNF and MDA in livers of each group
| ROS | Normal | NASH | NASH treated with curcumin |
|---|---|---|---|
| MDA (nmol/mL) | 1.32 ± 0.12 | 4.23 ± 0.22* | 1.74 ± 0.11** |
| HNF (nmol/mL) | 3.52 ± 0.43 | 19.23 ± 2.3* | 4.66 ± 0.99** |
Data are presented as the means ± standard deviation. ROS, reactive oxygen species; HNE, 4-hydroxy-2-nonenal; MDA, malondialdehyde.
P < 0.05 when compared with normal rats;
P < 0.05 when compared with NASH rats.
Curcumin could prevent apoptotic genes expression
To evaluate if the curcumin could prevent apoptotic genes expression, the transcription level of CytC, Casp3, 8 and 9 were quantified by real time qRT-PCR. The mRNA level of normal rats liver tissues were treated as 1, the relative CytC, Casp3 and Casp8 of the NASH livers were 3.44, 7.83 and 5.72, respectively, which were significantly higher than normal and NASH treated with curcumin rats livers (Table 3). The relative Casp9 mRNA levels were similar between normal, NASH and NASH treated with curcumin rats livers (Table 3). In conclusion, the apoptotic genes in NASH livers were activated, while, curcumin could quell the apopotic genes activation significantly.
Table 3.
Expression characteristics of apoptotic genes
| Genes | Normal | NASH | NASH treated with curcumin |
|---|---|---|---|
| CytC | 1.00 (0.77~1.23) | 3.44 (2.99~5.77)* | 1.67 (0.89~1.89)** |
| Casp3 | 1.00 (0.81~1.41) | 7.83 (4.67~9.11)* | 2.10 (1.09~3.11)** |
| Casp8 | 1.00 (0.73~1.31) | 5.72 (4.12~8.77)* | 1.77 (1.01~3.33)** |
| Casp9 | 1.00 (0.85~1.29) | 1.51 (0.98~2.27) | 1.11 (0.99~2.12) |
The genes expression levels were calculated by the 2-ΔΔCT method. Data were presented as 2-ΔΔCT and range.
indicated significance (P < 0.05) when compared with normal rats;
indicated significance (P < 0.05) when compared with NASH rats.
CytC, Cytochrome C, Somatic; Casp, caspase.
Discussion
Accumulating evidence suggest that mitochondrial dysfunction plays a key role in the physiopathology of NASH [20,21]. The NASH relative mitochondrial dysfunction displayed in respiratory chain deficiency and textural anomaly [20,21]. Although a large number of researches have revealed the damage of mitochondria in NASH, rare studies have focused on the strategy to protect mitochondria from damage. In this study, we demonstrated that: 1) curcumin displayed protective effect on liver injury histologically; 2) curcumin could protect mitochondria structure in NASH livers, the mitochondria structure of NASH livers treated with curcumin are similar to the normal control livers, contrast with the former, NASH liver without treatment displayed larger mitochondria, mitochondrial swelling, membrane crest grain decreased and mitochondrial matrix crystallization inclusion; 3) curcumin could prevent the oxidative stress in liver, NASH liver showed higher level of MDA and HNF, curcumin could ease the oxidative stress significantly; 4) curcumin could quell the apopotic genes activation significantly.
In mammalians, mitochondria play a major role in fuel oxidation and ATP formation [20-22]. Mitochondria dysfunction will not only lead to the disorders of fuel oxidation and ATP formation but also might activate many pathophysiological pathways [15]. These pathophysiological pathways and molecules are nuclear factor-erythroid 2-related factor-2 (Nrf2) and pro-inflammatory cytokines [20-22].
Nrf2 could be activated by electrophilic agents and play a central role in mediating a cytoprotective response against a wide variety of stress and toxic insults [15]. In our previous report, we demonstrated that curcumin could attenuate liver injury in NASH animal model possibly due to an Nrf2 activation-mediated effect [15]. We also evaluated that the levels of TNF-alpha and IL-6 are significantly higher in NASH animal body, and curcumin treatment could reduce elevation of these inflammatory cytokines [15]. Taken our data together, our studies suggest that curcumin might be modified into a drug target NASH in the feature.
In this report, we omitted the morphological and functional liver parameters and the data for serum lipids and insulin resistance. In our previous report, we showed that; alanine aminotransferase and aspartate aminotransferase were elevated significantly in NASH rats; serum triglyceride, total cholesterol, free fatty acids and homeostasis model assessment of insulin resistance were also significantly higher in NASH rats than that of normal control rats. In this study, we mainly focused on the structure and function assessments of mitochondria and apoptotic genes expression.
The NASH is developed from nonalcoholic fatty liver disease (NAFLD), the latter is the most common liver disease worldwide with prevalence estimates ranging from 25% to 45% [23]. Up to 25% of individuals with NAFLD also have NASH [24]. Since NASH is risk factor for cirrhosis, many studies including our report had only focused on NASH but ignored the NAFLD, this should be one limitation of our study. We only fixed one dose (50 mg/kg of curcumin) and had not performed dose-effect experiment for curcumin, this should be another deficiency of this study.
Acknowledgements
The cross research foundation for medicine and engineering, Shanghai Jiao Tong University (YG2013MS49).
Disclosure of conflict of interest
None.
References
- 1.Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450–65. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 2.Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011;31:1432–48. doi: 10.1111/j.1478-3231.2011.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poljsak B, Šuput D, Milisav I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid Med Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escribá PV, González-Ros JM, Goñi FM, Kinnunen PK, Vigh L, Sánchez-Magraner L, Fernández AM, Busquets X, Horváth I, Barceló-Coblijn G. Membranes: a meeting point for lipids, proteins and therapies. J Cell Mol Med. 2008;12:829–875. doi: 10.1111/j.1582-4934.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayala A, Muñoz MF, Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, Budillon G, Cimino L, Di Carlo A, Di Marino MP, Morisco F, Picciotto F, Terracciano L, Vecchione R, Verde V, Del Vecchio Blanco C. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001;35:568–74. doi: 10.1016/s0168-8278(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 7.Pessayre D, Mansouri A, Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G193–199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- 8.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205–18. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P. Mitochondria-Ros Crosstalk in the Control of Cell Death and Aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serviddio G, Bellanti F, Vendemiale G, Altomare E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:233–44. doi: 10.1586/egh.11.11. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 13.Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. 2011;6:e26012. doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R, Sharma P. Hepato protective Effect of Curcumin on Lindane-induced Oxidative Stress in Male Wistar Rats. Toxicol Int. 2011;18:124–9. doi: 10.4103/0971-6580.84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Wang L, Lu Q, Da W. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci. 2014 doi: 10.1007/s11845-014-1226-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Sun MG, Williams J, Munoz-Pinedo C, Perkins GA, Brown JM, Ellisman MH, Green DR, Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat Cell Biol. 2007;9:1057–65. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- 18.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 19.Spies-Martin D, Sommerburg O, Langhans CD, Leichsenring M. Measurement of 4-hydroxynonenal in small volume blood plasma samples: modification of a gas chromatographic-mass spectrometric method for clinical settings. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:231–9. doi: 10.1016/s1570-0232(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 20.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–40. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria insteatohepatitis. Semin Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 23.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]


