Abstract
Background: The sialyl-Tn (sTn) antigen is a mucin-associated carbohydrate antigen expressed by numerous human carcinomas, and is also claimed to be a prognostic factor in colorectal cancer. But the associations between sTn and colorectal cancer remain elusive and controversial. Here, we investigated the expression profile of sTn antigen in a series of human colorectal tissue samples including normal colon, colorectal adenomas, and colorectal carcinomas (CRCs), with an aim to analyzing whether sTn plays a role in the progression and development of Chinese patients with CRCs. Methods: Immunohistochemical staining of sTn antigen was performed in formalin-fixed, paraffin-embedded colonic sections from 4 healthy controls, 44 patients with colorectal adenomas, and 186 patients with primary CRCs. Results: No sTn antigen was detected in normal colonic tissues. There were 41 of 44 patients with colorectal adenomas (93.2%), and 141 of 186 patients with CRCs (75.8%) found to express sTn antigen. The patterns of sTn localization were different in adenomas and carcinomas of colonic tissues. Colorectal adenomas showed predominant supranuclear distribution of sTn antigen, while carcinomas revealed apical membrane, mucin droplet and diffuse cytoplasmic localization. Notably, sTn was significantly associated with the degree of differentiation (P = 0.006) and perineural invasion (P = 0.041) of the tumors, but was independent of age, gender, tumor location, depth of penetration, status of lymph nodes, lymphovascular invasion and TNM stage. Conclusions: These results indicate that sTn may play a role in initiating colorectal carcinogenesis and promoting tumor progression. Determination of sTn expression and localization may assist in evaluating malignant status of colorectal lesions.
Keywords: sTn antigen, colorectal adenoma, colorectal carcinoma, expression pattern
Introduction
Colorectal cancer (CRC) is the third most common type of cancer and the fourth leading cause of cancer-related death worldwide [1]. In China, the estimate of new cases diagnosed with CRC in 2011 was 310,244, with an estimated 149,722 deaths [2]. It was reported that the tumorigenesis and malignant transformation of human CRC are closely associated with aberrant glycosylation of proteins and lipids, resulting in exposure of the immature truncated O-glycan structures, which may serve as tumor-associated markers [3]. Among them, sialyl-Tn (sTn) antigen has been a research focus for decades. STn (Neu5Ac 2-6GalNAc -O-Ser/Thr), also referred to as CD175s, is formed by the sialylation of Tn antigen (GalNAc-O-Ser/Thr), which represents the common core O-glycan carbohydrate structure [4]. The biosynthesis of sTn is performed by a specific sialyltransferase called ST6GalNAc I, which blocks further elongation of O-linked carbohydrate chains.
Discovered as a tumor marker, sTn antigen is expressed by more than 80% of human carcinomas including CRC. The reported frequency of sTn expression in CRC is approximately between 75-95% [5]. Moreover, sTn antigen has been correlated with a poor prognosis and low overall survival for patients with CRC, but the reported data are controversial [6-8]. For example, Nakagoe et al. reported that sTn expression was useful for predicting survival in CRC patients, whereas Lundin et al. concluded that sTn did not provide any prognostic information regarding CRC patients. As is known, the adenoma-carcinoma sequence is the one of the most important fundamental features in CRC tumorigenesis [9]. However, only a few studies have examined the expression of sTn antigen in the premalignant lesions of CRC, such as colorectal adenomas and dysplasia. In addition, the reports vary a lot as to the frequency of sTn antigen expression and its association with clinicopathological characteristics in colorectal cancer. The differences may be due to different countries of the cases studied. In this study, we aimed to examine the expression profiles of sTn antigen in a series of human colonic tissue samples including normal mucosa, adenomas, and carcinomas, and also investigate the associations between sTn and clinicopathological features in Chinese patients with CRCs.
Materials and methods
Tissue specimens
The tissue specimens were obtained by endoscopic polypectomy and surgical resection from 44 patients with colorectal adenomas and 186 patients with colorectal adenocarcinomas. These patients were treated at Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China, from January 2007 to December 2009. The 4 normal colorectal mucosae were taken at autopsy from individuals without colorectal malignancies. Using hematoxylin and eosin (HE) staining method, colorectal adenomas were classified as mild, moderate and severe dysplasia according to the criteria of Konishi and Morson [10]. Likewise, colorectal carcinomas were histologically classified as well, moderately, poorly differentiated and mucinous adenocarcinomas, using the WHO criteria [11]. The tumor stage was assessed according to the TNM classification of CRC [12]. None of the patients had received preoperative radiotherapy and/or chemotherapy.
Immunohistochemistry staining of sTn antigen
In brief, human colorectal tissues were fixed with formalin, processed and embedded in paraffin wax, and cut into 5 μm-thick sections by microtome. Immunohistochemistry was performed using the PV-9000 2-step plus ®Poly-HRP Anti-Mouse/Rabbit IgG Detection System (GBI, Bothell, WA, USA), according to the manufacturer’s instructions. Each section was dewaxed in xylene and rehydrated by a graded ethanol solution followed by antigen retrieval by heating the tissue in boiling EDTA buffer (diluted 1:50, pH 9.0) for 18 min. The sections were cooled, immersed in 0.3% H2O2 for 15 min to block the endogenous peroxidase activity and rinsed in PBS (PH 7.2-7.4) for 5 min. The samples were blocked with 10% goat serum at room temperature for 15 min and incubated with primary antibodies against sTn (B72.3, diluted 1:100, Abcam, Cambridge, UK) at 4°C overnight. After three washes with PBS, the sections were further processed using Polymer Helper + polyperoxidase-anti-mouse/rabbit IgG (ZSGB-BIO, Beijing, China) according to the instructions provided by the manufacturer. Reactivity was detected using DAB reagent sets (ZSGB-BIO, Beijing, China) for 5 min, and the cells were counterstained with hematoxylin. A negative control was made by replacing the primary antibody with immunoglobulin G.
Evaluation of sTn staining
Immunostaining of sTn antigen was independently assessed by two pathologists. Semiquantitative levels of sTn expression were obtained based on staining intensity and distribution [13]. Staining intensity (I) was graded as 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). The percentage (0-100%) of the extent of reactivity (R) scored as follows: 0 (no positive cells); 1 (positive cells rates < 5%), 2 (positive cells rates ≥ 5%), and 3 (positive cells rates ≥ 50%). Histochemistry score = I × R. The scores less than 4 were classified as the low expression and the rest as the high expression. The mean optical density was analyzed by Image-Pro Plus 6.0 software.
Statistical analysis
The correlations between the expression of sTn antigen and different groups of adenomas and clinicopathological parameters of CRCs were analyzed by the Fisher exact test and χ2 test. For all statistical analyses, SPSS 17.0 software (SPSS, Chicago, USA) was used, and P < 0.05 was considered as significant.
Results
There was no sTn antigen detected in normal colorectal mucosa (Figure 1A). In contrast, 41 of 44 Chinese patients with colorectal adenomas (93.2%), and 141 of 186 Chinese patients with CRCs (75.8%) were found to express sTn antigen. There was a significant difference in sTn antigen expression between adenomas and CRCs (P = 0.011). These observations suggest that sTn overexpression occurs earlier during the process of carcinogenesis. Moreover, in colorectal adenomas, there was a gradual increase in the frequencies and intensities of sTn expression from mild to moderate to severe dysplasia, but no statistical association was obtained between sTn and the dysplasia degree (P = 0.789) (Table 1). Notably, there were different patterns of sTn antigen localization in adenomas and carcinomas of colorectal tissues, respectively. As for colorectal adenomas, sTn antigen was found to locate predominantly in the supranuclear region of cells and accumulate at the luminal cell surface regardless of their degree of dysplasia (Figure 1B-D). In colorectal adenocarcinomas, sTn antigen was observed in the apical cell membranes, mucin droplet and the cytoplasm of the cancer cells (Figure 1E-H). Furthermore, we analyzed whether there was an association between sTn antigen expression and the clinicopathological characteristics of colorectal carcinomas. The results showed that sTn antigen was strongly correlated to poor histological differentiation (P = 0.006) and perineural invasion (P = 0.041) of the tumors, whereas it was not related to age, gender, tumor location, depth of penetration, status of lymph nodes, lymphovascular invasion and TNM stage (Table 2).
Figure 1.
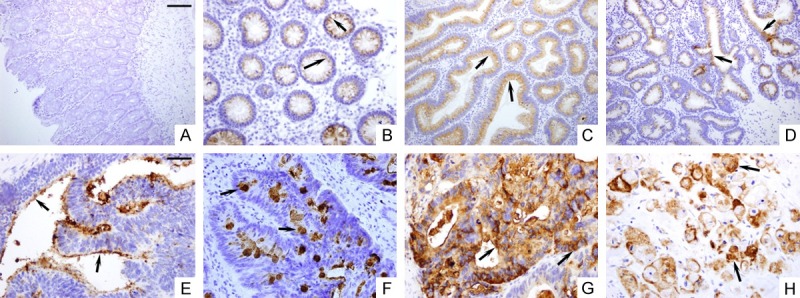
Immunohistochemical staining of normal, premalignant and malignant colorectal tissue sections with monoclonal antibody B72.3 to the sTn antigen. (A) Normal colorectal tissues had no sTn staining. Colorectal adenomas with mild dysplasia (B), moderate dysplasia (C) and severe dysplasia (D) showed consistently supranuclear distribution of sTn antigen. Colorectal carcinomas showed apical membrane (E), mucin droplet (F) and diffuse cytoplasmic distribution (G) of sTn antigen. (H) Mucinous adenocarcinoma showed diffuse cytoplasmic staining. Scale Bars (A-D): 100 um; (E-H): 50 um.
Table 1.
Immunohistochemistry analysis of sTn antigen expression in colorectal adenomas with different degree of dysplasia
| Grade | Number of patients | sTn expression | P value | |
|---|---|---|---|---|
|
| ||||
| High | low | |||
| Mild dysplasia | 20 | 18 | 2 | 0.789 |
| Moderate dysplasia | 14 | 13 | 1 | |
| Severe dysplasia | 10 | 10 | 0 | |
Table 2.
The relationship between sTn antigen expression and clinicopathological factors in colorectal carcinoma
| Variable | Number of patients | sTn expression | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Age (years) | ||||
| < 60 | 56 | 43 | 13 | 0.838 |
| ≥ 60 | 130 | 98 | 32 | |
| Gender | ||||
| Male | 112 | 85 | 27 | 0.973 |
| Female | 74 | 56 | 18 | |
| Location | ||||
| Colon | 91 | 72 | 19 | 0.302 |
| Rectum | 95 | 69 | 26 | |
| Differentiation | ||||
| Well + Moderate | 151 | 109 | 43 | 0.006 |
| Poor + Mucinous | 35 | 32 | 2 | |
| Invasive depth | ||||
| T1 + T2 | 36 | 23 | 13 | 0.063 |
| T3 + T4 | 150 | 118 | 32 | |
| Lymph nodes metastasis | ||||
| N0 | 90 | 63 | 27 | 0.073 |
| N1 + N2 | 96 | 78 | 18 | |
| Vessel embolus | ||||
| Present | 58 | 45 | 11 | 0.342 |
| Absent | 128 | 96 | 34 | |
| Perineural invasion | ||||
| Present | 36 | 32 | 4 | 0.041 |
| Absent | 150 | 109 | 41 | |
| TNM Stage | ||||
| I + II | 88 | 61 | 27 | 0.050 |
| III + IV | 98 | 80 | 18 | |
Discussion
Abnormal sialylation is a glycoconjugate change frequently observed in the malignant transformation of epithelial cells in a variety of tumors, including colorectal cancer. Indeed, this aberrant process can produce a number of different sialylated oligosaccharides, including sialyl- Lewis A, sialyl- Lewis X, sialyl-T, and sialyl-Tn (sTn). It has been reported that sTn is involved in a number of important biological properties of cancer cells, such as immunological functions, tumor progression and metastasis [14]. Thus, sTn exposure is a characteristic feature of colorectal epithelial cancer cells, and is associated with poor prognosis and overall low survival of CRC patients. However, the expression profile of sTn in the colorectal adenoma-carcinoma sequence remains controversial. Some studies observed a very high frequency of sTn antigen expression in colorectal adenomas [15], whereas others only detected weak staining of sTn in early premalignant lesions [16]. In addition, the associations between sTn expression and the clinicopathological features of CRC are also elusive. The observational variations were likely due to environmental or ethnic differences. To date, there is still lack of the profile of sTn expression in Chinese patients with colorectal lesions (adenomas, carcinomas).
In this study, we observed that in contrast to the absence of sTn expression in normal colon, there was a significantly prominent expression of sTn antigen in Chinese patients with adenomas (93.2%), as well as in patients with CRCs (75.8%). Of particular interest, patients with adenomas had a higher frequency of sTn expression than patients with CRCs. sTn is speculated to be carried by various glycoproteins such as mucins and its expression generally represents incomplete glycosylation of the mucins. It is known that abnormal glycosylation of the mucins interferes with normal cellular signaling, and may be an early step in neoplastic transformation of a normal epithelial cell. Thus, enhanced levels of sTn expression in adenomas seem to be associated with the malignant potential of cells, which are sometimes lost in the fully transformed, poorly differentiated state.
Characteristically, immunostaining localization showed that sTn antigen was consistently located in the supranuclear region in colorectal adenomas, while colorectal carcinomas revealed apical membrane, mucin droplet and diffusion cytoplasmic distribution. Previous studies reported that sTn overexpression was observed at the apical or luminal cell surface in several other epithelial benign lesions [17-19], but was mainly located in cytoplasm of tumor cells [20]. These different patterns in the cellular distribution of sTn antigen seem important, which may assist in evaluating the process of malignant transformation of epithelial cells. The changes of mucin biosynthetic pathway and intracellular transport of glycosyltransferase may explain the distinctive patterns of sTn distribution in premalignant and malignant tissues, respectively. Wang et al. [21] examined the subcellular localization and biosynthetic pathway of sTn in a series of colorectal tissues using high-resolution immunoelectron microscopy. In normal colorectal mucosa, sTn expression could not be visualized due to O-acetylation of sialic acid residues. In colorectal adenomas, sTn sialylation took place in the trans-Golgi apparatus, whereas in CRCs, sTn sialylation occurred in all the Golgi compartments and in the rough endoplasmic reticulum (RER). Egea et al. [22] demonstrated that the enzyme molecules normally resident in the Golgi apparatus could be distributed retrogradely to the RER during malignant transformation of colon epithelial cells.
To our knowledge, it is the first report as to the frequency of sTn expression in Chinese patients with CRCs, which is in accordance with other previous reports. Our data indicated that the presence of elevated sTn expression was significantly associated with poor histological differentiation and perineural invasion of the tumors, but did not correlated with age, gender, tumor location, depth of penetration, status of lymph nodes, lymphovascular invasion and TNM stage. Although there was no statistical significance between sTn expression and TNM stage, patients with stage III and IV had a higher trend of sTn expression (P = 0.05). Poorly differentiated adenocarcinomas and mucinous carcinomas with higher sTn expression were often associated with an adverse outcome. Similar finding was reported by Itzkowize et al. [20] who showed that sTn antigens was preferentially expressed by poorly differentiated adenocarcinomas and mucinous carcinomas. Likewise, perineural invasion was also known to be a useful marker for a more aggressive tumor phenotype and poor prognosis in CRCs [23]. These observations thus strengthen the clinical role of sTn antigen in promoting tumor progression and predicting clinical outcomes of colorectal cancer.
The mechanisms as to sTn expression in colorectal adenomas and carcinomas are yet to be clear. It is suggested that during tumorigenesis of colorectal epithelia, mucins undergo characteristic changes of glycosylation and distribution, resulting in the exposure of sTn antigen in the cells of adenoma and adenocarcinoma. Taken together, sTn antigen not only is a tumor marker but also plays an important role in the sequence of adenoma-carcinoma. Moreover, the different immunohistochemistry staining localization of sTn antigen may assist in distinguishing the process of malignant transformation from a diagnostic standpoint.
Acknowledgements
This work was supported by the National Science and Technology Support Program of China (2013BAI04B01) and Natural Science Foundation of China (NO. 81572825).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Zheng R, Zhang M, Zhang S, Sun X, Chen W. Incidence and mortality of colorectal cancer in China, 2011. Chin J Cancer Res. 2015;27:22–28. doi: 10.3978/j.issn.1000-9604.2015.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akamine S, Nakagoe T, Sawai T, Tsuji T, Tanaka K, Hidaka S, Shibasaki S, Nanashima A, Yamaguchi H, Nagayasu T, Yasutake T. Differences in prognosis of colorectal cancer patients based on the expression of sialyl Lewisa, sialyl Lewisx and sialyl Tn antigens in serum and tumor tissue. Anticancer Res. 2004;24:2541–2546. [PubMed] [Google Scholar]
- 4.Lundin M, Nordling S, Roberts PJ, Lundin J, Carpelan-Holmstrom M, von Boguslawsky K, Haglund C. Sialyl Tn is a frequently expressed antigen in colorectal cancer: No correlation with patient prognosis. Oncology. 1999;57:70–76. doi: 10.1159/000012003. [DOI] [PubMed] [Google Scholar]
- 5.Julien S, Videira PA, Delannoy P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules. 2012;2:435–466. doi: 10.3390/biom2040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Imada T, Rino Y, Hatori S, Takahashi M, Amano T, Kondo J, Suda T. Sialyl Tn antigen expression is associated with the prognosis of patients with advanced colorectal cancer. Hepatogastroenterology. 1999;46:208–214. [PubMed] [Google Scholar]
- 8.Nakagoe T, Sawai T, Tuji T, Jibiki M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Matuo T, Tagawa Y. Prognostic value of expression of sialosyl-Tn antigen in colorectal carcinoma and transitional mucosa. Dig Dis Sci. 2002;47:322–330. doi: 10.1023/a:1013765904875. [DOI] [PubMed] [Google Scholar]
- 9.Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma-carcinoma sequence in large bowel. Lancet. 1978;1:245–247. doi: 10.1016/s0140-6736(78)90487-7. [DOI] [PubMed] [Google Scholar]
- 10.Konishi F, Morson BC. Pathology of colorectal adenomas: a colonoscopic survey. J Clin Pathol. 1982;35:830–841. doi: 10.1136/jcp.35.8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jass JR, Sobin LH, Watanabe H. The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162–2167. doi: 10.1002/1097-0142(19901115)66:10<2162::aid-cncr2820661020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Kumamoto K, Ishibashi K, Hatano S, Amano K, Kuwabara K, Ohsawa T, Okada N, Kumagai Y, Baba H, Tsuji Y, Haga N, Ishida H. Clinical outcome of Stage IV colorectal cancer on the basis of the seventh edition of the TNM classification. Gan To Kagaku Ryoho. 2012;39:2164–2166. [PubMed] [Google Scholar]
- 13.Song H, Li C, Li R, Geng J. Prognostic significance of AEG-1 expression in colorectal carcinoma. Int J Colorectal Dis. 2010;25:1201–1209. doi: 10.1007/s00384-010-1009-3. [DOI] [PubMed] [Google Scholar]
- 14.Pant KD, Jain A, McCracken JD, Thompson K. Immunohistochemical examination of anti-STn monoclonal antibodies LLU9B4, B72.3, and B35.2 for their potential use as tumor markers. Dig Dis Sci. 2008;53:2189–2194. doi: 10.1007/s10620-007-0137-2. [DOI] [PubMed] [Google Scholar]
- 15.Listrom MB, Little JV, McKinley M, Fenoglio-Preiser CM. Immunoreactivity of tumor-associated glycoprotein (TAG-72) in normal, hyperplastic, and neoplastic colon. Hum Pathol. 1989;20:994–1000. doi: 10.1016/0046-8177(89)90271-2. [DOI] [PubMed] [Google Scholar]
- 16.Wolf BC, D’Emilia JC, Salem RR, DeCoste D, Sears HF, Gottlieb LS, Steele GD Jr. Detection of the tumor-associated glycoprotein antigen (TAG-72) in premalignant lesions of the colon. J Natl Cancer Inst. 1989;81:1913–1917. doi: 10.1093/jnci/81.24.1913. [DOI] [PubMed] [Google Scholar]
- 17.Baldus SE, Zirbes TK, Monig SP, Engel S, Monaca E, Rafiqpoor K, Hanisch FG, Hanski C, Thiele J, Pichlmaier H, Dienes HP. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewis(a), Sialosyl-Lewis(x) and sialosyl-Tn. Tumour Biol. 1998;19:445–453. doi: 10.1159/000030036. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Yonezawa S, Nomoto M, Ueno K, Kim YS, Sato E. Expression of mucin antigens and Lewis X-related antigens in carcinomas and dysplasia of the pharynx and larynx. Pathol Int. 1996;46:646–655. doi: 10.1111/j.1440-1827.1996.tb03667.x. [DOI] [PubMed] [Google Scholar]
- 19.Hachiya T, Honda T, Kubo K, Sekiguchi M. Expression patterns of type II pneumocyte apical surface glycoconjugates in lung adenocarcinoma cells. Virchows Arch. 1999;434:63–69. doi: 10.1007/s004280050306. [DOI] [PubMed] [Google Scholar]
- 20.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 21.Wang F, Goto M, Kim YS, Higashi M, Imai K, Sato E, Yonezawa S. Altered GalNAc-alpha-2, 6-sialylation compartments for mucin-associated sialyl-Tn antigen in colorectal adenoma and adenocarcinoma. J Histochem Cytochem. 2001;49:1581–1592. doi: 10.1177/002215540104901212. [DOI] [PubMed] [Google Scholar]
- 22.Egea G, Franci C, Gambus G, Lesuffleur T, Zweibaum A, Real FX. cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells. J Cell Sci. 1993;105:819–830. doi: 10.1242/jcs.105.3.819. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Huang X, Sun J, Gao P, Song Y, Chen X, Zhao J, Wang Z. Prognostic Value of Perineural Invasion in Colorectal Cancer: a Meta-Analysis. J Gastrointest Surg. 2015;19:1113–1122. doi: 10.1007/s11605-015-2761-z. [DOI] [PubMed] [Google Scholar]


