Abstract
Background and objectives: Detection of chromosomal abnormalities in myeloproliferative disorders is important for proper diagnosis of these disorders. This study has investigated the presence of JAK2 mutation (V617F) in Egyptian patients with myeloproliferative disorders referred to National Cancer institute, Cairo University. Methods: The study involved 110 cases of Philadelphia negative Myeloproliferative diseases (MPDs), 70 cases with Polycythemia Vera (PV), 24 cases with Essential Thrombocytosis (ET) and 16 cases with Idiopathic Myelofibrosis (IMF) and 20 cases as a control group which represented as; (10 cases with secondary erythrocytosis, 1 case with reactive thrombocytosis, 4 cases as normal control and 5 as Philadelphia positive Chronic Myeloid Leukemia cases), they were collected from National Cancer Institute (NCI) over 3 years. We used ARMS technique for mutation detection. Results: The frequency of the V617F JAK2 mutation was highest in patients with PV where 56 out of 70 cases (80%) carried the mutation, followed by ET with 6 of 24 (25) and IMF with 2 of 16 (12.5%) . None of the cases with secondary Erythrocytosis, reactive thrombocytosis, the normal controls or Philadelphia positive CML cases carried the mutation. Conclusions: Our results are concordant with international published results for detection of this mutation. It is unequivocal now that V617F is met in many MPDs especially PRV. Finding this mutation in those patients is thought to have a big impact on the diagnosis and treatment of these disorders.
Keywords: JAK2 mutation, myeloproliferative disorders, polycythemia vera, essential thrombocytosis, idiopathic myelofibrosis (IMF), ARMS, Egypt
Introduction
Myeloproliferative disorders (MPDs) are a group of disorders characterized by clonal proliferation of one or more types of cells of myeloid series [1]. In these disorders, bone marrow shows increased numbers of progenitor cells of myeloid lineages while peripheral blood shows increased number of immature and mature cells accompanying with chance for potential transformation into AML [2,3].
Traditionally, MPDs were used to be classified into the “classic” and “atypical” categories. Classic category includes chronic myelogenous leukemia, polycythemia Vera (PV), essential thrombocythemia (ET) and chronic idiopathic myelofibrosis (CIM). The WHO has introduced a classification for MPDs in 2008. According to this classification, MPDs include chronic myeloid leukemia (CML), polycythemia Vera (PV), essential thrombocythemia (ET) and idiopathic myelofibrosis (IMF). In addition to some uncommon types as chronic neutrophilic leukemia (CNL), hypereosinophilic syndrome (HES) and chronic eosinophilic leukemia (CEL) [4].
This classification is based mainly on the predominant myeloid cell lineage expanded in the blood. PV is characterized by excessive production of erythrocytes, increased red cell mass as well as splenomegaly due to extramedullary hematopoiesis [5]. High platelet count is necessary in ET together with susceptibility for thrombosis and hemorrhage [6]. Finally Idiopathic myelofibrosis (IMF) shows bone marrow fibrosis, variable count of cells of myeloid series and hepatosplenomegaly [3].
According to WHO 2008 classification, the hallmark of chronic myelogenous leukemia (CML) is the presence of a t (9; 22) BCR-ABL1 fusion genes in cells of myeloid series which is present in about 90% of patients [7]. This mutation is detected by standard cytogenetic analysis in 95% and can be detected by more sophisticated methods (as FISH and RT-PCR) in the remaining 5% of cases [8]. Lack of specific diagnostic molecular markers for other typical MPDS represented a major problem over years. So, diagnosis depended on clinical and pathological features together with exclusion of other specific genetic abnormalities such as BCR-ABL1 fusion [9].
The Janus kinase/signal transducers and activators of transcription (JAK)/(STAT) pathway perform an essential role in the initiation of signal transduction. The presence of JAK2 is essential for normal hematopoiesis. This fact has been confirmed by defects in erythropoiesis which appeared in JAK2 deficient mice [10]. JAK2 is composed of two main domains, the first is an enzymatically active kinase domain (JAK homology 1 [JH1]) and a catalytically inactive pseudokinase domain (JH2). The JH2 domain plays an inhibitory role that generally inhibits the kinase activity of JAK2 [11].
In 2005, Janus kinase JAK2 mutation was discovered in some myeloproliferative disorders. The discovery of this mutation was represented a great progress in the understanding of BCR-ABL negative myeloproliferative disorders [12]. The most common mutation of JAK2 is a substitution of valine with phenylalanine at position 617 in the JH2 domain [13], which affects the function of the inhibitory pseudokinase JH2 domain causing an increased activity of progenitor cells of myeloid origin and excessive production of mature cells of myeloid origin [12,14]. Less commonly are mutations in exon 12, beside JAK2 mutation has been suspected to have a role in the development of AML in a single case report [15].
The presence of JAK2V617F mutation in hematopoietic cells causes hypersensitivity to cytokines and factor independent growth [15]. The presence of the erythropoietin, thrombopoietin or granulocyte-colony stimulating factor receptor/s is essential for this mutation to work, leading to functional hyperactivity and increased sensitivity for hematopoietic growth factor such as interleukin 3 (IL-3), stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and insulin-like growth factor-1 (IGF-1) [11,16-18].
The aim of work is to study the frequency of JAK2V617F mutation in Egyptian patients with myeloproliferative disorders were referred to National Cancer institute, Cairo University.
Patients and methods
Patients
Study subjects were recruited from outpatient clinic patients coming to National Cancer Institute, Cairo University. The study group was composed of 110 cases of Philadelphia negative myeloproliferative diseases (MPDs), 70 cases with polycythemia Vera (PV), 24 cases with essential thrombocytosis (ET), 16 cases with idiopathic myelofibrosis (IMF) and 20 control group divided as follow; 10 cases with 2ry erythrocytosis, 1 case with 2ry thrombocytosis, 4 cases as normal control and 5 cases as Philadelphia positive chronic myeloid leukemia (CML), collected from National Cancer Institute (NCI), Cairo University within 3 years.
General examination
All cases were subjected to: Complete clinical examination, complete blood counts and differential counts with microscopic examination of the peripheral smear, bone marrow aspirate, bone marrow biopsy and leukocyte alkaline phosphatase (LAP) score. Polymerase chain reaction (PCR) or fluorescent in-situ hybridization (FISH) runs on peripheral blood to exclude BCR-ABL gene rearrangement and red blood cell mass study to differentiate true from spurious polycythemia).
JAK2V617F detection
DNA extraction using salting out method, DNA was extracted by standard procedures after isolation of total leukocytes from peripheral blood following red cell lysis. Patients and controls were genotyped according to Jones et al., 2005 [2], (DNA tetra-primer ARMS assay) which was proven to be the most sensitive method for detection of the desired mutation. The sensitivity of ARMS technique was found to be 1% to 2%. Two primer pairs used to specifically amplify the normal and mutant sequences plus a positive control band in a single reaction. PCR primers were: forward outer (FO), 5_-TCCTCAGAACGTTGATGGCAG-3_; reverse outer (RO), 5_-ATTGCTTTCCTTTTTCACAAGAT-3_; forward wild-type-specific (Fwt), 5_-GCATTTGGTTTTAAATTATGGAGTATaTG-3_; reverse-mutant-specific (Rmt), 5_-GTTTTACTTACTCTCGTCTCCACAaAA-3_. Amplifications were performed for 30 cycles with Hot Start Taq polymerase (Qiagen, Crawley, United Kingdom), an annealing temperature of 60°C, 25-100 ng genomic DNA, and standard amplification conditions, except that the final concentrations of the outer primers and the mutant/wild-type-specific inner primers were 1 µM and 0.5 µM, respectively. Products were resolved on 2% agarose gels and visualized after staining with ethidium bromide. Primers FO and RO flank JAK2 exon 12 and should generate a control 463-bp band in all cases. Primers Fwt and RO generate a 229-bp wild-type (2343G)-specific product and primers FO and Rmt generate a 279-bp mutant (2343T)-specific product.
Statistical analysis
Statistical Package of GraphPad Prism 5 program was used for analysis of data. Data was summarized as mean ± SE. Comparison between groups was carried out using one way analysis of variance (ANOVA). If P values were significant, Tukey-Kramer multiple comparison test was used. The level of significance was accepted at P < 0.05.
Results
The frequency of the V617F JAK2 mutation was highest in polycythemia vera (PV) group, in which 56 of 70 cases (80%) cases carried the mutation, followed by essential thrombocytosis (ET) with 6 of 24 (25%) and idiopathic myelofibrosis (IMF) with 2 of 16 (12.5%) (Figure 1). None of the cases with secondary erythrocytosis or thrombocytosis or the normal controls, or Philadelphia positive CML carried the mutation. Those results are compatible with international published results for detection of this mutation.
Figure 1.
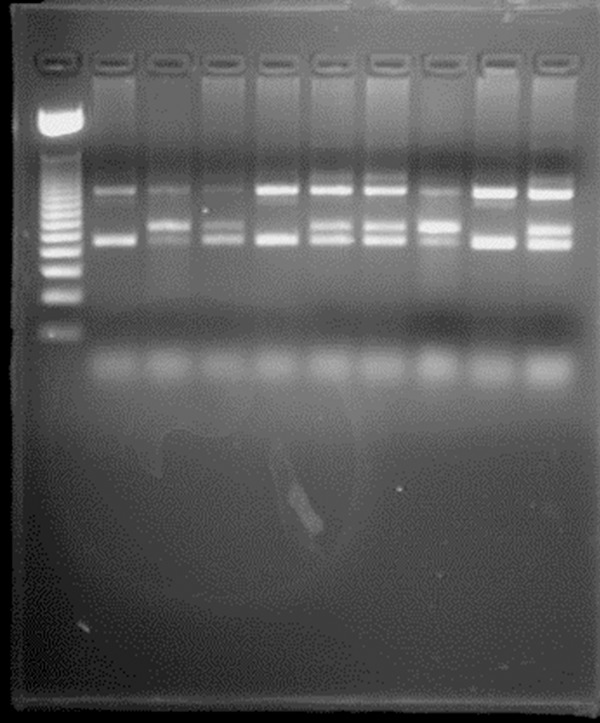
PCR amplification of Jack 2 cases and control.
Discussion
The most important frequent mutation in the BCR-ABL1-negative MPDs is the detection of the JAK2V617F mutation, especially in polycythemia vera (PV) [12]. Mutations in exon 12 of JAK2 has been also detected in one third of V617F-negative PV cases. This mutation is also present in some cases with an apparently isolated erythrocytosis [3]. Detection of JAK2 V617F mutation in patients with MPDS has emboldened researchers to develop inhibitors that target JAK2 in those patients [19].
Our results are similar to the vast majority of other studies especially for PRV. However the frequency of V617F mutation was lower in cases of IMF and to a lesser extent in ET in our study than other researches. This may be explicated by the small number of cases of IMF in our study.
In comparison of our results, Horn et al., 2006 [20] detected V617F JAK2 mutation in 27 of 28 (96%) cases of polycythemia vera (PV), 17 of 23 (74%) cases of essential thrombocytosis (ET) and 28 of 45 (62%) cases of idiopathic myelofibrosis(IMF) using both allele specific multiplex polymerase chain reaction (PCR) and nested polymerase chain reaction (PCR). Also Kralovics et al., 2005 [21] detected V617F JAK2 mutation in 83 of 128 (65%)cases of polycythemia vera (PV), 21 of 93 (23%) cases of essential thrombocytosis (ET) and 13 of 23 (57%) of idiopathic myelofibrosis using microsatellite mapping of the 9 pLOH region that included the Janus Kinase 2 (JAK2) gene and DNA sequencing. Fantasia et al., 2014 [22] detected JAK2V617F mutation in 22/22 PV (100%), 29/38 ET (76.3%), and 5/9 PMF cases (55.5%) respectively, using a highly specific q-RT-PCR assay. Difference in the results is most probably due to differences in the used techniques that causing differences in the sensitivity of V617F detection in various laboratories [2].
In concomitant with our results vast majority of literatures detect V617F JAK 2 mutation in 65 to 97% of patients with polycythemia Vera (PV), 23 of 57% of those with essential thrombocytosis (ET) and 30 to 57% of idiopathic myelofibrosis. V617F JAK 2 mutation is absent in normal individuals, in patients with chronic myeloid leukemia (CML) or in patients with secondary erythrocytosis and thrombocytosis [2,13,23,24].
There is a growing evidence for the presence of JAK2 V617F mutation in disorders other than MPDs. In a study performed on large Chinese hospital population, JAK2 V617F mutation was detected in 37 samples from a total of 3935 cases. This result was surprising since blood tests of only one case of those positive samples was suggestive of PV. Such results are suggestive of the presence of the mutation in other disorders that do not fulfill the full criteria of MPDs [25].
Conclusion
JAK2 mutation detection is now integrated in the diagnosis of MPDs. In this study, we investigated the presence of JAK2 (V617F) mutation in Egyptian patients with MPDs and our results are comparable to published literature in the topic. Detection of exon 12 mutation in JAK2 (V617F) negative patients suspected with different MPDs is recommended, JAK2 mutation may be also present in disorders other than MPDs such as; different hypercoagulable states. Investigation for the presence of this mutation may be useful for detection of occult MPDs and early interference and treatment.
Disclosure of conflict of interest
None.
References
- 1.Levine R, Gilliland D. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones A. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 3.Jones A, Cross N. Inherited predisposition to myeloproliferative neoplasms. Ther Adv Hematol. 2013;4:237–253. doi: 10.1177/2040620713489144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, Them NC, Berg T, Elena C, Casetti IC, Milanesi C, Sant’antonio E, Bellini M, Fugazza E, Renna MC, Boveri E, Astori C, Pascutto C, Kralovics R, Cazzola M Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2013;123:1544–1551. doi: 10.1182/blood-2013-11-539098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaleskas V, Krause D, Lazarides K, Patel N, Hu Y, Li S, Van Etten RA. Molecular Pathogenesis and Therapy of Polycythemia Induced in Mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwemmers S, Will B, Waller C, Abdulkarim K, Johansson P, Andreasson B, Pahl HL. JAK2V617F-negative ET patients do not display constitutively active JAK/STAT signaling. Exp Hematol. 2007;35:1695–1703. doi: 10.1016/j.exphem.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shet A, Jahagirdar B, Verfaillie C. Chronic myelogenous leukemia: mechanisms underlying disease progression. Leukemia. 2002;16:1402–1411. doi: 10.1038/sj.leu.2402577. [DOI] [PubMed] [Google Scholar]
- 8.Cortes J, Talpaz M, Beran M, O’Brien S, Rios M, Stass S, Kantarjian HM. Philadelphia chromosome-negative chronic myelogenous leukemia with rearrangement of the breakpoint cluster region. Long term follow-up results. Cancer. 1995;75:464–470. doi: 10.1002/1097-0142(19950115)75:2<464::aid-cncr2820750209>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Murugesan G, Aboudola S, Szpurka H, Verbic M, Maciejewski J, Tubbs R, Hsi ED. Identification of the JAK2 V617F Mutation in Chronic Myeloproliferative Disorders Using FRET Probes and Melting Curve Analysis. Am J Clin Pathol. 2006;125:625–633. doi: 10.1309/TK0X-L917-XK2V-LRPQ. [DOI] [PubMed] [Google Scholar]
- 10.Gery S, Cao Q, Gueller S, Xing H, Tefferi A, Koeffler H. Lnk inhibits myeloproliferative disorder-associated JAK2 mutant, JAK2V617F. J Leukoc Biol. 2009;85:957–965. doi: 10.1189/jlb.0908575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu H. Pathogenetic Role of JAK2 V617F Mutation in Chronic Myeloproliferative Disorders. J Chin Med Assoc. 2007;70:89–93. doi: 10.1016/S1726-4901(09)70337-5. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez M, De Brasi C, Bianchini M, Gargallo P, Stanganelli C, Zalcberg I, Larripa IB. Improved Diagnosis of the Transition to JAK2V617F Homozygosity: The Key Feature for Predicting the Evolution of Myeloproliferative Neoplasms. PLoS One. 2014;9:e86401. doi: 10.1371/journal.pone.0086401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine R. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–3379. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etheridge S, Roh M, Cosgrove M, Sangkhae V, Fox N, Chen J, López JA, Kaushansky K, Hitchcock IS. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111:2295–2300. doi: 10.1073/pnas.1312148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elnaggar M, Agersborg S, Sahoo T, Girgin A, Ma W, Rakkhit R, Zorrilla I, Leal A. BCR-JAK2 fusion as a result of a translocation (9; 22) (p24; q11.2) in a patient with CML-like myeloproliferative disease. Mol Cytogenet. 2012;5:23. doi: 10.1186/1755-8166-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushansky K. The chronic myeloproliferative disorders and mutation of JAK2: Dameshek’s 54 year old speculation comes of age. Best Pract Res Clin Haematol. 2007;20:5–12. doi: 10.1016/j.beha.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellido M, Te Boekhorst PA. JAK2 Inhibition: Reviewing a New Therapeutical Option in Myeloproliferative Neoplasms. Adv Hematol. 2012;2012:1–6. doi: 10.1155/2012/535709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brière J. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3. doi: 10.1186/1750-1172-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibes R, Mesa R. Targeting hedgehog signaling in myelofibrosis and other hematologic malignancies. J Hematol Oncol. 2014;7:18. doi: 10.1186/1756-8722-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn T, Kremer M, Dechow T, Pfeifer W, Geist B, Perker M, Duyster J, Quintanilla-Martinez L, Fend F. Detection of the Activating JAK2 V617F Mutation in Paraffin-Embedded Trephine Bone Marrow Biopsies of Patients with Chronic Myeloproliferative Diseases. J Mol Diagn. 2006;8:299–304. doi: 10.2353/jmoldx.2006.050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 22.Fantasia F, Di Capua E, Cenfra N, Pessina G, Mecarocci S, Rago A, Cotroneo E, Busanello A, Equitani F, Lo-Coco F, Nervi C, Cimino G. A highly specific q-RT-PCR assay to address the relevance of the JAK2WT and JAK2V617F expression levels and control genes in Ph-negative myeloproliferative neoplasms. Ann Hematol. 2013;93:609–616. doi: 10.1007/s00277-013-1920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxter E, Scott L, Campbell P, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 24.James C, Ugo V, Le Couédic J, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Zhang Q, Luo J, Xing S, Li Q, Krantz S, Fu X, Zhao ZJ. JAK2V617F: prevalence in a large Chinese hospital population. Blood. 2007;109:339–342. doi: 10.1182/blood-2006-03-009472. [DOI] [PMC free article] [PubMed] [Google Scholar]


