Abstract
Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UCPOGC) is an extremely rare non-endocrine pancreatic tumor. To date, some cases have been reported, however, histogenesis and biologic behavior of UCPOGC remain controversial. We report a case of an UCPOGC in a 54-year-old female, who presented with a three-month history of recurrent abdominal pain without any incentive. Abdominal computed tomography (CT) revealed a large cystic mass of 10.5 × 9.3 cm in the body and tail of the pancreas compressing the adjacent bowel loop and stomach. The preliminary diagnosis was considered as a malignant tumor of body and tail of the pancreas. The patient had open distal pancreatic mass resection with splenectomy and according to the results of histopathological and immunohistochemical studies, the diagnosis of an UCPOGC was established.
Keywords: Osteoclast-like giant cells, undifferentiated carcinoma, pancreas
Introduction
Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UCPOGC) is an extremely rare and aggressive neoplasm that comprises < 1% of non-endocrine pancreatic tumors. UCPOGC is composed of two main cell populations, the multinucleated osteoclast-like giant cells (OGCs) and the pleomorphic mononuclear cells [1]. UCPOGC is frequently seen as a large cystic neoplasm with various degrees of hemorrhage and necrosis. Owing to its rarity, the histogenesis and biologic behavior of UCPOGC remain controversial. As a result from numerous light microscopic, electron microscopic, histomorphologic, immunohistochemical, ultrastructural, and molecular biologic studies, usually performed as single case studies, it has been concluded that UCPOGC originate from epithelial cells, histiocytic cells, and mesenchymal cells [2]. However, UCPOGC is classified by the World Health Organization (WHO) as a rare variant of ductal pancreatic adenocarcinoma (PAC), based on the epithelial origin of its OGCs. Undifferentiated carcinoma of the pancreas may contain OGCs that are positive for CD68 and lysozyme without reactivity to epithelial markers similar to that previously described for giant cell tumor of bone [3]. On the basis of histopathological and immunohistochemical studies, the diagnosis of an UCPOGC was established. In this study, we report a case of UCPOGC and review the previously published literature in order to summarize the characteristic clinical, pathologic and imaging findings which currently describe this rare neoplasm.
Case report
A 54-year-old female presented with a three-month history of recurrent abdominal pain without any incentive. The pain was paroxysmal, staying around the navel area and could be endured. She had an associated 4 kg weight loss over the past 3 months. She had no palpable abdominal mass and jaundice. She didn’t present any vomiting, diarrhea, fever or melena. Her past medical history was insignificant. No allergies or significant social or family history was reported. Physical examination didn’t find any obvious abnormality.
Lab values included the following: hemoglobin 98.0 g/L; fibrinogen 5.45 g/L; Carbohydrate antigen 19-9 41.26 u/ml; D-dimer 0.75 mg/L FEU; pre-globin 150 mg/L; globin 37 g/L; 5’-nucleotidase 9.4 U/L; and inorganic phosphorus 1.40 mmol/L. Gastroscopy revealed chronic superficial gastritis. Ultrasonography (USG) found a large cystic mass in the tail of pancreas.
Abdominal computed tomography (CT) revealed a huge well defined cystic component-based mass (approximately 10.5 × 9.3 cm) in the body and tail of the pancreas compressing the adjacent bowel loop and stomach. Inside the mass, it showed multiple encapsulated irregular vesicles and small calcification on the envelope of the biggest cyst (Figure 1A). For the cystic part, CT value was about 14 HU and no enhancement on contrast image. However, for solid part and envelope, plain scan CT value was 36 HU, and enhanced significantly in artery phase (Figure 1B). Portal venous phase showed continuous enhancement of the solid part and dilatation of right gastro-epiploic vein (Figure 1C). The CT values were 86 HU, 124 HU and 105 HU for artery, portal venous and delayed phases respectively. Moreover, no regional lymphadenopathy, ascites, and distant metastasis were detected on CT. Computed tomographic angiography (CTA) revealed that the mass compressed the splenic artery causing severe luminal stenosis and significant shift. Moreover, it also showed mild compression and displacement of the left branch of the superior mesenteric artery (SMA). The blood supply of the mass was from the left branch of the SMA (Figure 1D) and left gastric artery which were more prominent when entering into the mass. Abdominal aorta and the left renal artery had no obvious abnormalities. Computed tomographic venography (CTV) showed compression of the left renal vein with luminal stenosis. Furthermore, no definite display of splenic vein revealed and formation of collateral circulation was observed (Figure 1E). The preliminary diagnosis was considered as a malignant tumor of body and tail of the pancreas.
Figure 1.
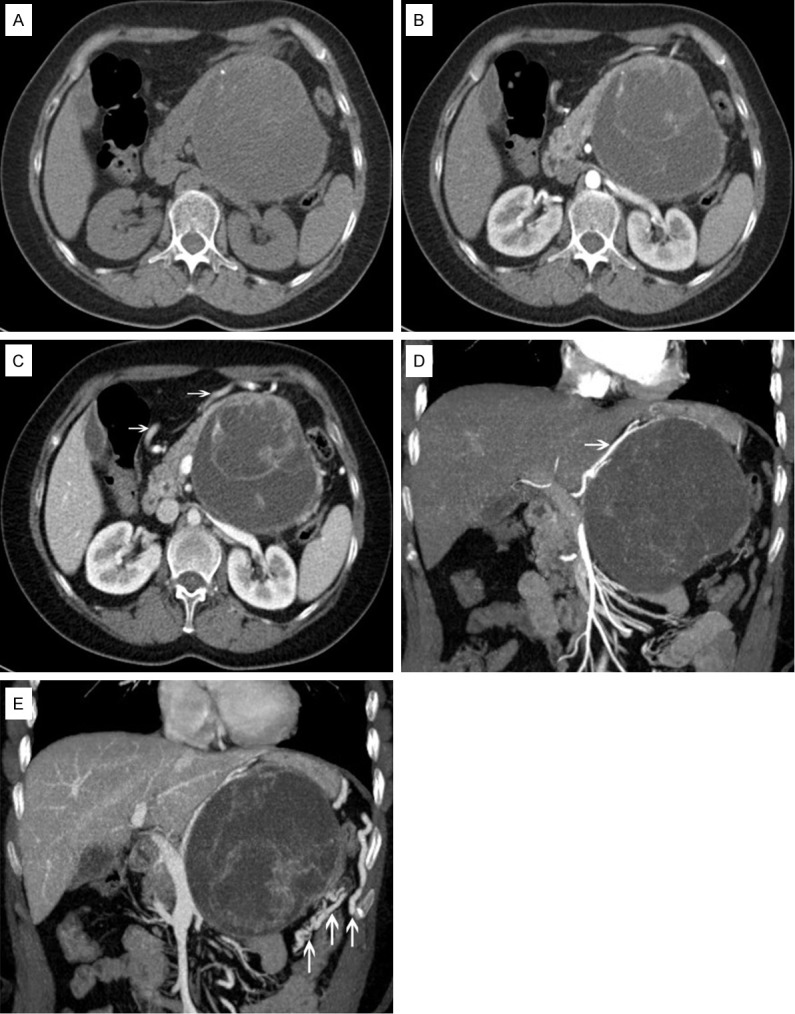
A: A huge well-defined cystic component-based mass in the body and tail of the pancreas compressing the adjacent bowel loop and stomach. Inside the mass, it showed multiple encapsulated irregular vesicles and small calcification on the envelope of the biggest cyst. B: Artery phase with significant contrast enhancement of solid part and envelope. C: Portal venous phase showed continuous enhancement of the solid part and dilatation of right gastro-epiploic vein (shown by arrows). D: Artery phase with multiple projection reconstructions showed the blood supply of mass was from the left branch of the SMA (shown by arrow) and left gastric artery. E: Portal venous phase with multiple projection reconstructions showed compression of the left renal vein with formation of collateral circulation (shown by arrows).
The patient had open distal pancreatic mass resection with splenectomy. During laparotomy, an 11 × 12 × 11 cm encapsulated mass was located in the tail of pancreas, and attached to the left side of stomach; the attached part of stomach was swollen. Inside the mass, there was some dark red fluid and necrotic tissues. The mass was also attached loosely to the suspensory ligament of duodenum and retroperitoneum. The mass had a very close relationship with pancreas tail and spleen artery. The mass was separated from the pancreas and removed. The report of quick frozen sample pathological findings during surgery revealed “malignant neoplasm”. Histologically, the tumor was composed of two main cell types, the multinucleated osteoclast-like giant cells and the pleomorphic mononuclear cells. Immunohistologic examinations showed CD68 giant cells (Figure 2A), CK7 (+) (Figure 2B), EMA (+), Vimentin (+), p53 (+), CK20 (-), CEA (+), Ki67 25% (+). According to the results of histopathological and immunohistochemical studies, the diagnosis of an UCPOGC was established.
Figure 2.
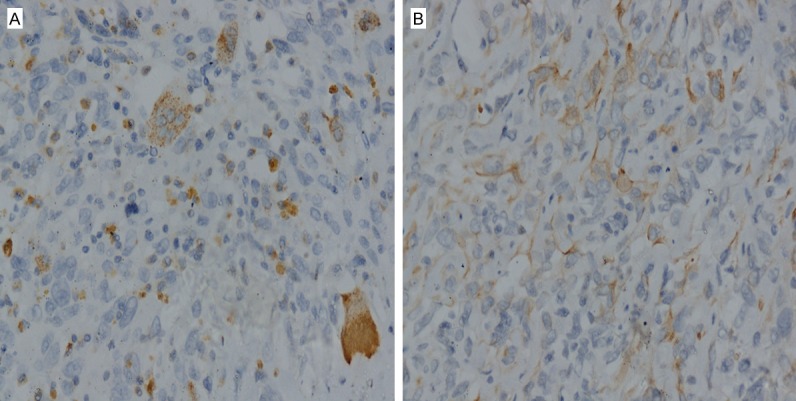
Histomorphologic findings: The immunohistochemical findings show CD68 expression in OGCs (A, magnification × 40), and positive staining by CK7 (B, magnification × 40).
The patient remained stable throughout the operation and had an uneventful recovery. She was discharged from hospital on post-operative day 9. No adjuvant treatment was offered.
Discussion
Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UCPOGC) was formerly known as an osteoclast-like giant cells (OGCs) tumor, or pleomorphic carcinoma of the pancreas with OGCs. Similar tumors with osteoclast-like giant cells have also been reported in various other organs, including the breast, thyroid, parotid gland and soft tissues. Recently, it has been shown that the OGCs found in association with extraskeletal tumors show some similarities and differences with osteoclasts. Although these cells are indistinguishable with the light microscope, OGCs do not possess a complex ruffled border with a surrounding clear zone which is a characteristic ultrastructural feature of the osteoclast [9]. The ability to resorb bone is a common function of both cell types. However, bone resorption stimulated by parathyroid hormone and not inhibited by calcitonin is a function of OGCs and not of osteoclasts. OGCs found in extraskeletal tumors appear to be a specific type of macrophage polykaryon which is distinct from both osteoclasts and foreign-body giant cells.
The giant cells of UCPOGC morphologically resemble the skeletal giant cells tumors. Similar to skeletal giant cells tumors, UCPOGC is also an aggressive tumor that usually invades adjacent organs. However, a literature review of the previously published pancreatic cases suggested that UCPOGC is a less aggressive neoplasm than conventional ductal adenocarcinoma [10]. Moreover, lymph node involvement and distant metastasis are rarely observed in UCPOGC. UCPOGC also tends to be more extensively vascularized than other cystic neoplasms of the pancreas.
Owing to its rarity, the histogenesis and biologic behavior of UCPOGC remain controversial. Epithelial, histiocytic, and mesenchymal metaplasia have all been reported. Review of the literature revealed that UCPOGC usually affects patients in their sixth to eighth decades of life, although younger patients have also been reported [11]. Moreover, there is no gender predilection. The clinical manifestations include non-specific abdominal pain, anorexia, fatigue, weight loss, jaundice and a palpable mass. UCPOGC can arise from any portion of the pancreas, although it frequently develops from the body and tail of the pancreas. In our case, the mass was also located in the body and tail of the pancreas.
On abdominal CT scan, an irregular solid and cystic mass with strong enhancement are typical of UCPOGC. However, while ductal PAC appears hypovascular on contrast CT scans, UCPOGC appears hypervascular, which is possibly related to the rapid growth of UCPOGC, or the associated inflammatory reaction. Histologically, UCPOGC includes large multinucleated non-neoplastic osteoclast-like giant cells and pleomorphic neoplastic mononuclear cells. Evidence supports that the tumor giant cells are non-neoplastic in nature and of histiocytic origin. The presence of non-neoplastic osteoclast-like giant cells is the histological hallmark of this tumor and the diagnosis is confirmed by histopathological and immunohistochemical studies. Gao L et al. [12] reported that endoscopic ultrasonography (EUS)-guided fine needle aspiration cytology (FNAC) is an effective and accurate means of achieving the cytological diagnosis of UCPOGC.
Osteoclastic giant cells rarely express epithelial markers, but they show staining for histomonocytic markers (CD68). The present tumor contained histiomonocytic cells and osteoclast-like giant cells that were most frequently found toward the periphery of the tumor nodules. Osteoclast-like giant cells may be found within anaplastic pancreatic carcinomas to a variable degree. Because of their immunohistochemical positivity for the histiomonocytic marker CD68, the lack of mitoses and proliferative activity, detected by Ki-67, they were suggested to result from a fusion of mononuclear histiocytes/macrophages, chemoattracted by the tumor cells [13]. With regard to tumor markers, particularly CEA and CA19-9, elevated levels are less common in cases of UCPOGC compared with pancreatic adenocarcinoma. Levels of inflammatory markers, such as WBC, C-reactive protein level, and levels of interleukins, have also been found to be elevated in > 50% of patients with UCPOGC. Table 1 summarizes the characteristic clinical, cytopathologic and imaging findings of UCPOGC.
Table 1.
A literature review of clinical, cytopathologic and imaging findings of undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UCPOGC)
| Author, Date [Ref] | Age/Sex | Symptoms | Location | Size (cm) | Cytopathologic features | Imaging findings |
|---|---|---|---|---|---|---|
| Tezuka et al., 2006 [4] | 68/F | Abdominal pain | Main pancreatic duct (MPD) | Pencil-like tumor | A sheet of spindle cells with scattered OGCs and pleomorphic giant cells (PGCs) | MRI: Hypointense on T2WI |
| Manduch M et al., 2009 [5] | 66/M | Painless jaundice, pruritis, and weight loss | Head of pancreas | 9.5 | OGCs and PGCs | CT: An inhomogeneous mass |
| Hur YH et al., 2010 [6] | 77/F | Abdominal pain and anorexia | Tail of pancreas | 10 | Atypical mononuclear cells and OGCs | CT: A heterogenous enhanced mass invading spleen and adjacent bowel loop |
| Maksymov V et al., 2011 [7] | 68/F | Painless jaundice | Uncinate process | 2.0 | OGCs, PGCs, mononuclear histiocytic cells. And atypical mononuclear cells | CT: A 2.0 cm mass in the uncinate process |
| Gao HQ et al., 2014 [8] | 71/F | Epigastric pain and anorexia | Body and tail of pancreas | 13 | OGCs and PGCs | CT: An irregular solid and cystic mass with strong enhancement |
| Present case | 54/F | Recurrent abdominal pain | Body and tail of pancreas | 10.5 | OGCs and PGCs | CT: A cystic and solid mass with strong enhancement |
More recent molecular studies have demonstrated the presence of KRAS oncogene mutation in the mononuclear cells, which is a typical finding of pancreatic ductal adenocarcinoma [14]. E-cadherin expression within the epithelial component and loss of its expression within undifferentiated tumor cells, which is typical feature of the undifferentiated carcinoma of the pancreas, was also documented [15].
The differential diagnosis of UCPOGC includes cystic lesions, such as pancreatic cystadenomas, cystadenocarcinomas, serous and mucinous cystic tumors, and pancreatic pseudocysts, and solid pancreatic tumors, such as ductal pancreatic carcinomas or neuroendocrine tumors.
Surgery is the treatment of choice. It remains to be demonstrated whether the response of UCPOGC to chemotherapy or radiotherapy will be efficacious. Recent studies on this rare and interesting tumor suggest that outcomes are improving, although survival is very short for some patients.
In conclusion, undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (UCPOGC) is an extremely rare and aggressive neoplasm with various clinical characteristics and controversial pathogenesis. Further researches preferably with large cohorts are necessary to clarify the pathogenesis of the neoplasm. Although there are limited data to support the differentiation of pancreatic lesions by CT or MRI alone, an accurate analysis of cross-sectional imaging in conjunction with clinical data may provide valuable insight into a correct diagnosis. However, histopathological and immunohistochemical studies are the gold standard for the diagnosis of UCPOGC.
Acknowledgements
This study was supported by National Key Clinical Specialties Construction Program of China (No. 2013-544).
Disclosure of conflict of interest
None.
References
- 1.Bauditz J, Rudolph B, Wermke W. Osteoclast-like giant cell tumors of the pancreas and liver. World J Gastroenterol. 2006;12:7878–83. doi: 10.3748/wjg.v12.i48.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann F, Esposito I, Michalski CW, Herpel E, Friess H, Schimacher P. Early undifferentiated pancreatic carcinoma with osteoclast-like giant cells: direct evidence for ductal evolution. Am J Surg Pathol. 2007;31:1919–25. doi: 10.1097/PAS.0b013e318067bca8. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RD, Michelassi F, Montag AG. Osteoclast-like giant cell tumor of the pancreas: immunophenotypic similarity to giant cell tumor of bone. Hum Pathol. 1991;22:618–22. doi: 10.1016/0046-8177(91)90243-i. [DOI] [PubMed] [Google Scholar]
- 4.Tezuka K, Yamakawa M, Jingu A, Ikeda Y, Kimura W. An unusual case of undifferentiated carcinoma in situ with osteoclast-like giant cells of the pancreas. Pancreas. 2006;33:304–10. doi: 10.1097/01.mpa.0000235303.11734.2a. [DOI] [PubMed] [Google Scholar]
- 5.Manduch M, Dexter DF, Jalink DW, Vanner SJ, Hurlbut DJ. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: report of a case with osteochondroid differentiation. Pathol Res Pract. 2009;205:353–9. doi: 10.1016/j.prp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Hur YH, Kim HH, Seoung JS, Seo KW, Kim JW, Jeong YY, Lee JH, Koh YS, Kim JC, Kim HJ, Cho CK. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc. 2011;81:146–50. doi: 10.4174/jkss.2011.81.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degreeof pancreas duct involvement. A case report and literature review. JOP. 2011;12:170–6. [PubMed] [Google Scholar]
- 8.Gao HQ, Yang YM, Zhuang Y, Liu P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J Gastroenterol. 2015;21:694–8. doi: 10.3748/wjg.v21.i2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athanasou NA, Wells CA, Quinn J, Ferguson DP, Heryet A, McGee JO. The origin and nature of stromal osteoclast-like multinucleated giant cells in breast carcinoma: implications for tumour osteolysis and macrophage biology. Br J Cancer. 1989;59:491–8. doi: 10.1038/bjc.1989.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewandrowski KB, Weston L, Dickersin GR, Rattner DW, Compton CC. Giant cell tumor of the pancreas of mixed osteoclastic and pleomorphic cell type: evidence for a histogenetic relationship and mesenchymal differentiation. Hum Pathol. 1990;21:1184–7. doi: 10.1016/0046-8177(90)90157-z. [DOI] [PubMed] [Google Scholar]
- 11.Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Li ZS, Jin ZD, Man XH, Zhang MH, Zhu MH. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas diagnosed by endoscopic ultrasonography-guided fine-needle aspiration. Chin Med J (Engl) 2009;122:1598–600. [PubMed] [Google Scholar]
- 13.Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(sici)1097-0142(19980401)82:7<1279::aid-cncr10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Gocke CD, Dabbs DJ, Benko FA, Silverman JF. KRAS oncogene mutations suggest a common histogenetic origin for pleomorphic giant cell tumor of the pancreas, osteoclastoma of the pancreas, and pancreatic duct adenocarcinoma. Hum Pathol. 1997;28:80–3. doi: 10.1016/s0046-8177(97)90283-5. [DOI] [PubMed] [Google Scholar]
- 15.Winter JM, Ting AH, Vilardell F, Gallmeier E, Baylin SB, Hruban RH, Kern SE, Iacobuzio-Donahue CA. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–8. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]


