Abstract
We present the case of a 72-year old female with a right cerebellar pilocytic astrocytoma WHO grade I with an Isocitrate dehydrogenase 1 (IDH1) R132H mutation. The patient is recurrence-free 6 years after the initial diagnosis. Only one single case with strikingly similar clinicopathological features has been reported before. Otherwise, IDH1/2 mutations are not seen in pilocytic astrocytomas. The clinical implications of these findings are discussed.
Keywords: IDH1, pilocytic astrocytoma, biomarker
Introduction
Pilocytic astrocytoma is the most common primary brain tumor in children. The tumor entity is quite rare in elderly patients [1,2]. In general it is a benign tumor and after complete resection patients are usually considered cured [3]. However, in very few cases a possible transformation to a more malignant astrocytic tumor has been observed [4] while some argue that the possibility of two distinct neoplasms should also be considered in such rare cases [5]. Mutations in the isocitrate dehydrogenase 1 & 2 (IDH1/2) genes, including the canonical R132H type, occur with a high frequency in WHO grade II and III astrocytomas and oligodendrogliomas [6]. It has been shown that IDH mutations are absent in pilocytic astrocytomas and together with KIAA1549-BRAF fusion transcript analysis, resulting from a somatic duplication of 7q34, serve as a diagnostic tool to distinguish it from diffuse astrocytoma [7]. Such fusion transcripts are seen in high frequency in cerebellar, brainstem and optic pathway pilocytic astrocytomas, while mutations of the proto-oncogene B-Raf (BRAF V600E mutation) are found in less than 10% of the tumors [8]. However, recently the first case of histologically convincing pilocytic astrocytoma harboring an IDH1 R132H mutation has been described [9]. We now present an identical case with strikingly similar clinicopathological features.
Case presentation
We report the case of a 72-year old woman who presented with a history of headaches for several years and an increase in intensity during the last 4 months as well as accompanying vertigo, nausea, gait disturbances and episodic diplopia. Worth mentioning in the patients past medical history was atrial fibrillation and arterial hypertension medicated with metoprolol and phenprocoumon. She had undergone a hysterectomy 27 years earlier due to uterine fibroids. Otherwise, she was taking metoclopramide, zolpidem and betahistine for her current symptoms of vertigo and nausea. A cranial MRI revealed a gadolinium-enhancing nodule of the right paramedian cerebellar hemisphere with a surrounding cyst. On T2-weighted sequences the nodular part was hyperintense (Figure 1). The neuroradiological pre-operative differential diagnosis included cerebellar hemangioblastoma and ganglioglioma as possible entities. Pilocytic astrocytoma, although the morphological characteristics were suggestive, was not primarily considered due to the patient’s age. Extensive tumor staging did not show any primary tumor, which could account for a possible metastatic origin of the intracranial lesion. Based on the uncertainty regarding the tumor type together with the location specific symptoms, the decision for surgical removal was made. After switching phenprocoumon to low molecular weight heparin, the lesion was resected through a median suboccipital craniotomy. A postoperative cranial CT was unremarkable. The patient made an uneventful recovery and reported an improvement regarding the preoperative vertigo and gait disturbances. However, during follow-up visits she stated that her gait remained a bit unsteady and occasional spells of vertigo still occurred. The follow-up images were without signs of tumor recurrence.
Figure 1.
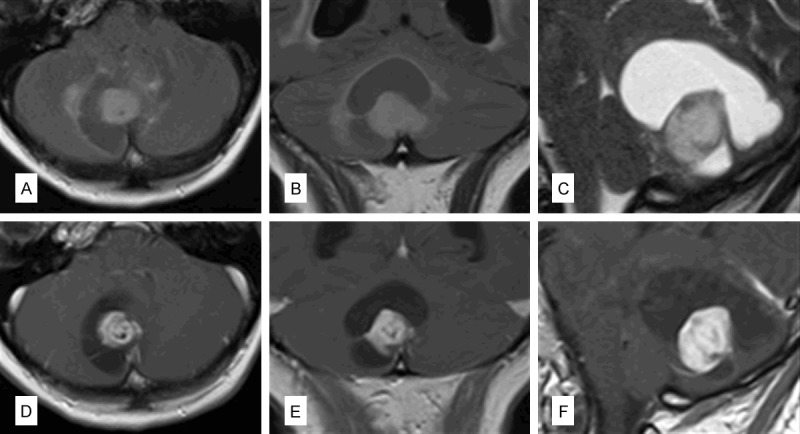
Preoperative imaging: Axial (A), coronal (B) FLAIR images as well as a T2-weighted sagittal scan (C) showed a nodular lesion with a cystic part in the right paramedian cerebellar hemisphere. Postcontrast T1-weighted images revealed contrast enhancement of the nodular portion (D-F show axial, coronal and sagittal orientation, respectively).
Despite the advanced age of the patient, the histopathological evaluation of the cerebellar biopsy specimen unveiled a pilocytic astrocytoma. H&E sections showed moderate pleomorphic ovoid to polygonal nuclei with strong eosinophilic cytoplasm. The tumor cells were embedded in a microcystic partially fibrillary matrix with marked Rosenthal fibers and single eosinophilic granular bodies (Figure 2A, 2B). No mitotic activity or necrosis was observed. The biphasic tumor focally exhibited myxoid areas (Figure 2C). Intraoperative smears showed piloid tumor cells with long, biopolar cell processes (Figure 2D). Immunohistochemistry confirmed the low proliferation index with less than 2% of tumor cells on MIB-1 (E3 ubiquitin-protein ligase 1) staining (Figure 2E). Expression of the glial fibrillary acidic protein (GFAP) (Figure 2F) was appreciated in the more compact areas while S-100 (Neural crest cell origin marker) was more evenly distributed throughout the tumor tissue. MAP2 (Microtubule-associated protein 2) staining showed merely a few singular positive tumor cells. Immunohistochemistry for p53 (Tumor suppressor protein) and BRAFV600E mutation were negative. No nuclear loss of “alpha thalassemia/mental retardation syndrome X-linked” protein (ATRX) was seen in the tumor. However, IDH1 R132H staining was positive (Figure 2G) which was confirmed by sequencing of exon 1 of the IDH1 gene, showing a heterozygous G>A substitution at nucleotide position 395 (Figure 2H). In addition we were able to exclude the common KIAA1559-BRAF fusion transcripts in the current tumor using RT-PCR as previously described [10].
Figure 2.
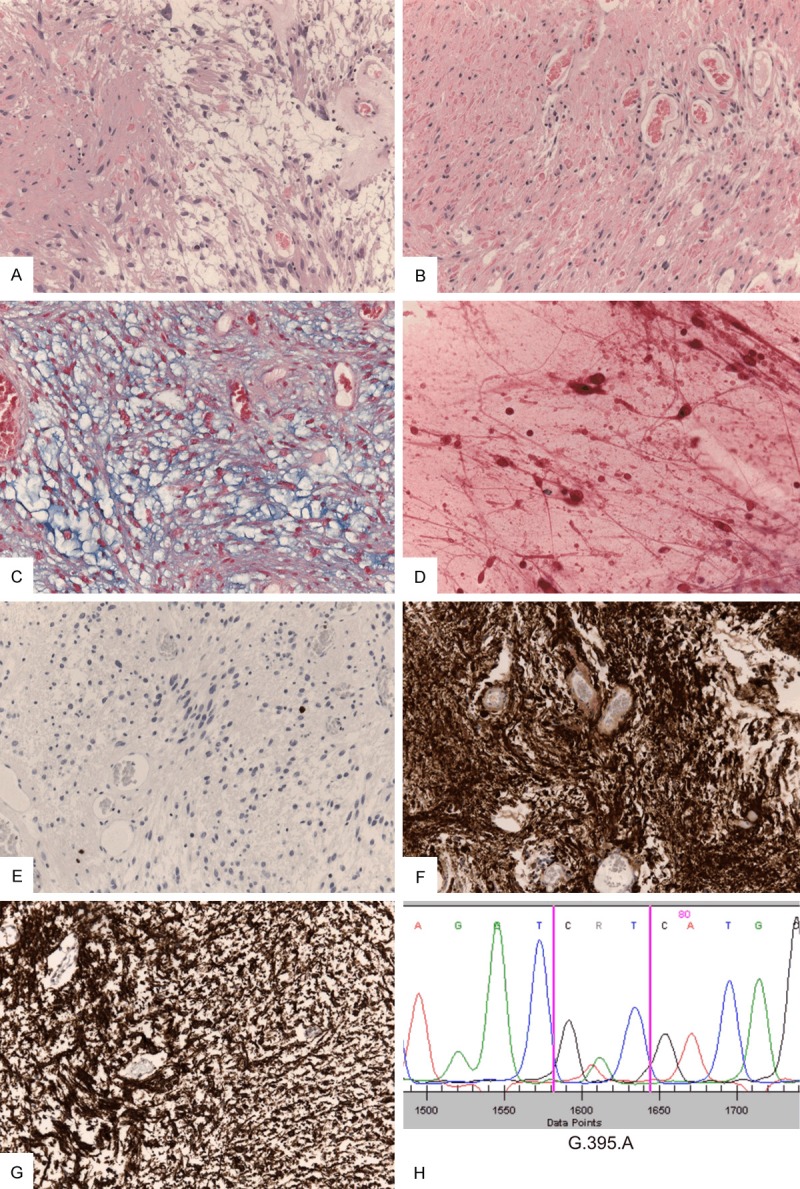
Histological and molecular work-up of the tumor diagnosed as pilocytic astrocytoma: Biphasic tumor areas consisting of compact and loosely arranged tumor cells with mild pleomorphism can be seen (A) as well as numerous Rosenthal fibers and eosinophilic droplets (B). Alcian blue stain highlights focally myxoid tumor components (C). Long bipolar tumor cell processes are seen in smear preparations (D). MIB-1 immunhistochemistry confirms the low proliferative activity (E) and strong GFAP staining highlights the astrocytic nature of the tumor (F). IDH1 immunreactivity is shown in all tumor cells (G) and IDH1 R132H mutation is confirmed through Sanger sequencing showing the relevant amino acid exchange at position 395 (H).
Discussion
This case report presents the second case of an IDH1 R132H mutated pilocytic astrocytoma in the literature. It was discovered after immunohistochemical assessment of IDH1 mutations in a tissue microarray of 106 pilocytic astrocytomas. All other 105 cases were negative. The radiological diagnosis of a pilocytic astrocytoma, though highly suggestive from the imaging findings, was not primarily considered due to the patient’s age. Instead, other highly vascularized tumors, like a hemangioblastoma, were debated. However, a thorough histological review of the paraffin-embedded tumor tissue and intraoperative smears by three experienced neuropathologists confirmed the diagnosis of a pilocytic astrocytoma.
Infratentorial pilocytic astrocytomas are usually characterized by the presence of a KIAA1549-BRAF fusion transcript or to a lesser extent by BRAF V600E mutations. Genetic alterations in hemispheric pilocytic astrocytomas are less well characterized. IDH1 mutations are considered to be specific for diffuse astrocytic/oligodendroglial neoplasms [11] and in difficult cases mutation analysis usually results in classification either as diffuse astrocytoma (mostly IDH1/2 mutated) or as pilocytic astrocytoma (BRAF fusion present). Recent high-throughput analyses have provided rare exceptions such as the presence of an IDH1 mutation in two medulloblastomas [12].
The current case raises the question, whether these tumors should be treated as pilocytic astrocytomas without KIAA1549-BRAF fusion occasionally observed in adults or as an IDH1 mutated glioma with eventual tumor progression. In our opinion, the following findings argue for the first notion:
1) The tumor did not harbor an ATRX loss, which is almost always associated with the canonical IDH1 mutated diffuse astrocytoma [13].
2) Although cerebellar diffuse gliomas are sometimes seen, infratentorial location is usually associated with IDH1/2 wildtype [14] and genetically more closely related to primary glioblastomas.
3) The clinical course with a stable disease after a 6-year follow up is infrequently observed in diffuse gliomas. Progression to high-grade lesions is usually seen sooner or later [15].
Similar to the case presented by Medress et al. the patient of this case report was an elderly female with a cerebellar pilocytic astrocytoma [9]. Pilocytic astrocytoma with advanced age is by itself a rare entity. In both cases the immunohistochemical IDH1 R132H mutation was confirmed by molecular analysis and the histopathological diagnosis was reviewed. Our findings with the presence of a hotspot IDH1 mutation in a pilocytic astrocytoma suggest that genes involved in the tumorigenesis of the elderly might be different than those of tumors occurring in the pediatric population. It is also possible that a mutation in the IDH1 gene may be an independent secondary event although we found no evidence of BRAF mutation/fusion in the current tumor. Even though the data indicates a possible correlation of increased age with IDH1 mutation in pilocytic astrocytoma, it remains a speculation at this point unless further cases are reported.
Conclusion
Overall, IDH1 mutation in pilocytic astrocytoma is a rarity, which has only been reported twice including this case report. Both cases were exceptionally old regarding the otherwise young patient group suffering from pilocytic astrocytoma. We recommend watchful waiting in such cases, as the clinical course and the molecular profile in these tumors seem to differ from classical diffuse gliomas. We believe that every similar case should be reported to get a better understanding of pilocytic astrocytomas and the astrocytic tumor spectrum in general.
Acknowledgements
We acknowledge the support by Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tuebingen.
Disclosure of conflict of interest
None.
References
- 1.Burkhardt K, Heuberger F, Delavelle J. Pilocytic astrocytoma in the elderly. Clin Neuropathol. 2007;26:306–310. doi: 10.5414/npp26306. [DOI] [PubMed] [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14:1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhard C, Di Patre PL, Schüler D, Schüler G, Yasargil MG, Yonekawa Y, Lütolf UM, Kleihues P, Ohgaki H. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003;98:1170–1174. doi: 10.3171/jns.2003.98.6.1170. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki T, Saito R, Kumabe T, Kanamori M, Sonoda Y, Watanabe M, Tominaga T. Transformation of adult cerebellar pilocytic astrocytoma to glioblastoma. Brain Tumor Pathol. 2004;31:108–112. doi: 10.1007/s10014-013-0154-0. [DOI] [PubMed] [Google Scholar]
- 5.Kanoke A, Kanamori M, Kumabe T, Saito R, Watanabe M, Tominaga T. Metachronous, multicentric glioma of pilocytic astrocytoma with oligodendroglioma-like component and oligodendroglioma through distinct genetic aberrations. J Neurosurg. 2013;118:854–858. doi: 10.3171/2012.9.JNS112353. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wessling P, Reifenberger G, von Deimling A. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 7.Korshunov A, Meyer J, Capper D, Christians A, Remke M, Witt H, Pfister S, von Deimling A, Hartmann C. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 8.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 9.Medress ZA, Xu LW, Ziskin JL, Lefterova MI, Vogel H, Li G. Pilocytic astrocytoma with IDH1 mutation in the cerebellum of an elderly patient. Clin Neuropathol. 2015;34:96–98. doi: 10.5414/NP300810. [DOI] [PubMed] [Google Scholar]
- 10.Hasselblatt M, Riesmeier B, Lechtape B, Brentrup A, Stummer W, Albert FK, Sepehrnia A, Ebel H, Gerss J, Paulus W. BRAF-KIAA1549 fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in adults. Neuropathol Appl Neurobiol. 2011;37:803–6. doi: 10.1111/j.1365-2990.2011.01193.x. [DOI] [PubMed] [Google Scholar]
- 11.Capper D, Reuss D, Schittenhelm J, Hartmann C, Bremer J, Sahm F, Harter PN, Jeibmann A, von Deimling A. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. 2011;121:241–252. doi: 10.1007/s00401-010-0770-2. [DOI] [PubMed] [Google Scholar]
- 12.Snuderl M, Triscott J, Northcott PA, Shih HA, Kong E, Robinson H, Dunn SE, Iafrate AJ, Yip S. Deep sequencing identifies IDH1 R132S mutation in adult medulloblastoma. J. Clin. Oncol. 2015;33:e27–31. doi: 10.1200/JCO.2013.49.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V, Mittelbronn M, Schittenhelm J, Herold-Mende C, Unterberg A, Platten M, Weller M, Wick W, Pfister SM, von Deimling A. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129:133–46. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 14.Ellezam B, Theeler BJ, Walbert T, Mammoser AG, Horbinski C, Kleinschmidt-DeMasters BK, Perry A, Puduvalli V, Fuller GN, Bruner JM, Aldape KD. Low rate of R132H IDH1 mutation in infratentorial and spinal cord grade II and III diffuse gliomas. Acta Neuropathol. 2012;124:449–51. doi: 10.1007/s00401-012-1011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–72. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]


