Abstract
We report a case of a 30-year-old woman who had complained of lower abdominal distension. She was noted to have a history of primary mucinous tumor of the left ovary (13.2 × 9.9 × 10.4 cm) that was removed surgically. Two years later she developed the same tumor on her left ovary (8.7 × 6.0 × 6.9 cm) and also had appendiceal mucinous tumor accompanied with acellular PMP. Final pathology revealed two truly independent primary mucinous tumors involving the appendix and ovary accompanied with acellular PMP. We recommend a minimum follow-up of 5 years for the patient to detect any development of mucinous tumors and the acellular pseudomyxoma peritonei.
Keywords: Ovarian mucinous tumor, appendiceal mucinous tumor, independent, acellular PMP
Introduction
The coexistence of mucinous ovarian and appendiceal tumors in association with pseudomyxoma peritonei (PMP) is well established. However, there has been considerable debate regarding to the origin of the tumor in such cases [1,2]. PMP was thought to originate from the ovary or peritoneal cavity; however, recent research has shown that this disease almost exclusively originates from the appendix [3]. If mucinous cells were not identified in the pools of mucin, the PMP was classified as “acellular” [4]. This report describes two truly independent primary mucinous tumors involving the appendix and ovary accompanied with acellular PMP.
Case report
The patient was 30-year-old, she had complained of lower abdominal distension, and visited our hospital for a close evaluation. She was noted to have a history of primary mucinous cystadenoma of the left ovary (13.2 × 9.9 × 10.4 cm) that was removed surgically. At this time, ultrasound examination demonstrated a large multicystic mass extending from the left ovary to pelvis, measuring 8.7 × 6.0 × 6.9 cm. Numerous daughter cysts were noted with no focal solid components or calcification. Blood parameters were all within the normal range.
At laparotomy, the findings were a large cyst arising from the left ovary, with a normal uterus and right ovary. The appendix was seen distended in a saccular form. A left oopherectomy and appendectomy was performed.
Pathologic findings
Macroscopic examination of the specimen revealed a well-circumscribed, gelatinous, polycystic mass emanating from the left ovary and measuring 8.0 × 7.0 × 4.5 cm, with abundant mucin within the lumen. The excised appendix distended with mucous, showed a smooth serosal surface with peritoneal implants.
Histological features were shown in Figure 1. The cysts were lined by tall columnar epitheliums, with slight cellular crowding and stratification. The tumor cells contain abundant cytoplasmic mucin and enlarged hyperchromatic nuclei that are basally located, with minimal atypia (Figure 1A, 1B). The tumor of appendix showed mucinous cystadenoma with mild pleomorphism and nuclear atypia. The peritoneal implants were mucins pools without mucins-producing tumor cells. These appearances are identical with acellular PMP (Figure 1C, 1D).
Figure 1.
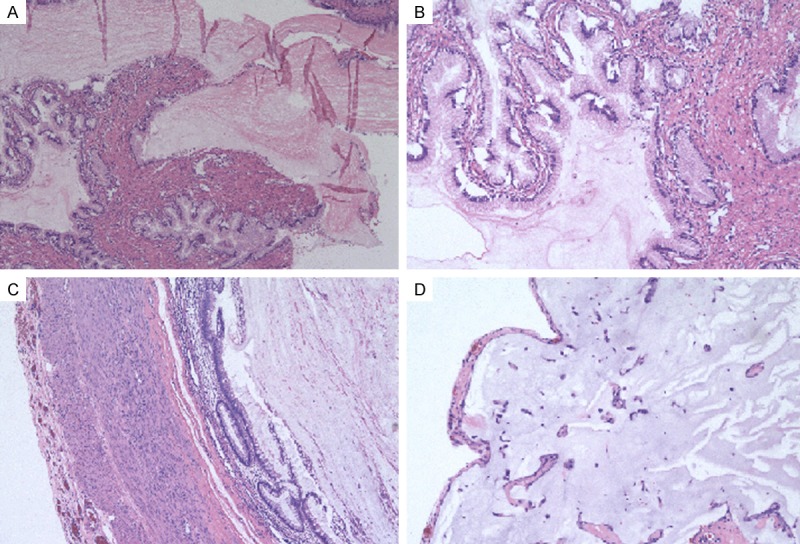
Photomicrograph showing mucinous tumour of uncertain malignant potential with mild pleomorphism and mild nuclear atypia. Left ovary (A, B, 100 ×); Appendix and acellular PMP (C, D, 100 ×).
Immunohistochemical staining was given in Figure 2. The tumor cells of the left ovary were strongly positive for CK7; negative for CK20 and CDX2 (Figure 2A-C), while the tumor cells of appendix were negative for CK7; strongly positive for CK20 and CDX2 (Figure 2D-F). Acellular PMP was negative for CK7 and CK20 (Figure 2G, 2H).
Figure 2.
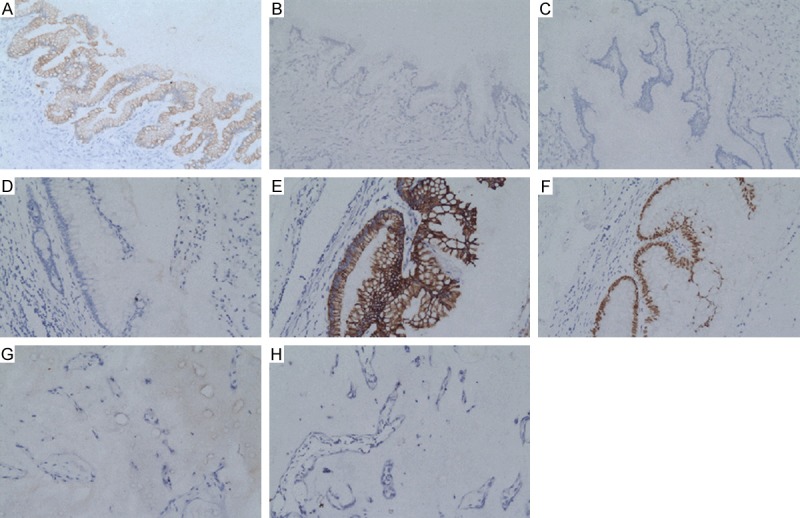
The immunohistochemical photograph of the uterine lesion. Left ovary: CK7 (A, 200×); CK20 (B, 200×) and CDX2 (C, 200×). Appendix: CK7 (D, 200×); CK20 (E, 200×); CDX2 (F, 200×). Acellular PMP: CK7 (G, 200×) and CK20 (H, 200×).
Final pathology revealed two truly independent primary mucinous tumors involving the appendix and ovary accompanied with acellular PMP.
Discussion
In cases of simultaneous tumors, the origin of the ovarian tumor is controversial with some groups’ reporting. Based on clinicopathological, immunohistochemical, and now molecular data, mucinous tumors involving the appendix and ovaries in women with PMP are clonal and derived from a single site, most likely the appendix [4-7].
However, when Seidman et al. [8] analyzed concurrent mucinous ovarian and appendiceal tumors they found that immunoperoxidase reaction for four epithelial antigens in 15 cases showed complete concordance between ovarian and appendiceal lesions in only five cases. Using genetic analysis of microdissected tissue samples, Chuaqui et al. [9] concluded that some synchronous mucinous tumors of the ovary and appendix were appendiceal in origin, whereas others represented independent primaries. They supported an independent origin of the ovarian and appendiceal tumors in most cases and did not favor an origin in a single site but a multi-focal neoplastic process.
The morphology and immunohistochemistry of the ovarian and appendiceal tumors are different in this case. The tumor cells of the left ovary contain abundant cytoplasmic mucin and enlarged hyperchromatic nuclei that are basally located, with minimal atypia as conventional endocervical-type mucinous glands. While tumors of appendix are composed of mild atypical gastrointestinal-type, mucincontaining epithelial cells that show greater proliferation than that seen in benign mucinous tumors. The CK7+/CK20-CDX2- and CK7-/CK20+DX2+ pattern is typical of epithelial ovarian tumors and intestinal tumors, respectively. The pattern of this case indicates that both the mucinous tumor of left ovary and appendix are truly independent primary tumors.
One case [1] with a CK-7 negative appendiceal cystadenoma unaccompanied by PMP also had a mucinous cystadenoma in her left ovary. This ovarian cyst was CK-7 positive, as would be expected in any primary epithelial tumor of the ovary. This case can be regarded as an example of two truly independent primary mucinous tumors involving the appendix and ovary.
The histology of peritoneal implants was mucins pools, which was similar to that of acellular PMP. They were negative for CK20 and CK7. Study [4] strongly supported the conclusion that mucinous tumors involving the appendix and ovaries in women with PMP were clonal and derived from a single site, most likely the appendix. Thus, we suspect that its occurrence is quite rare and probably originates from the appendix. Recent WHO classification of these tumors as low-grade appendiceal mucinous neoplasms acknowledges their unique morphologic appearance and biologic behavior [10].
In conclusion, based on clinicopathological and immunohistochemical features, this case can be regarded as an example of two truly independent primary mucinous tumors involving the appendix and ovary. For patients with a mucinous epithelial neoplasm of the appendix and ovary accompanied with acellular PMP, we recommend a minimum follow-up of 5 years to detect any development of mucinous tumors and PMP.
Disclosure of conflict of interest
None.
References
- 1.Chuaqui RF, Zhuang ZP, Emmert-Buck MR, Bryant BR, Nogalfes F, Tvassoli FA, Merino MJ. Genetic Analysis of Synchronous Mucinous Tumors of the Ovary and Appendix. Hum Pathol. 1996;27:165–71. doi: 10.1016/s0046-8177(96)90370-6. [DOI] [PubMed] [Google Scholar]
- 2.Guerrieri C, Franlund B, Fristedt S, GillooleyI JF, Boeryd B. Mucinous tumors of the vermiform appendix and ovary, and pseudomyxoma peritonei: histogenetic implications of cytokeratin 7 expression. Hum Pathol. 1997;28:1039–45. doi: 10.1016/s0046-8177(97)90057-5. [DOI] [PubMed] [Google Scholar]
- 3.Panarelli NC, Yantiss RK. Mucinous neoplasms of the appendix and peritoneum. Arch Pathol Lab Med. 2011;135:1261–8. doi: 10.5858/arpa.2011-0034-RA. [DOI] [PubMed] [Google Scholar]
- 4.Szych C, Staebler A, Connolly DC, Wu R, Cho KR, Ronnett BM. Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am J Pathol. 1999;154:1849–55. doi: 10.1016/S0002-9440(10)65442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young RH, Gilks CB, Scully RE. Mucinous tumors of the appendix associated with mucinous tumours of the ovary and pseudomyxoma peritonei: A clinicopatholical study of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991;15:415–429. doi: 10.1097/00000478-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hart WR. Borderline epithelial tumors of the ovary. Mod Pathol. 2005;18(Suppl 2):S33–S50. doi: 10.1038/modpathol.3800307. [DOI] [PubMed] [Google Scholar]
- 7.Guerrieri C, Franlund B, Boeryd B. Expression of cytokeratin 7 in simultaneous mucinous tumors of the ovary and appendix. Mod Pathol. 1995;8:573–6. [PubMed] [Google Scholar]
- 8.Seidman JD, Elsayed AM, Sobin LH, Tavassoli FA. Association of mucinous tumors of the ovary and appendix. A clinicopathologic study of 25 cases. Am J Surg Pathol. 1993;17:2234. doi: 10.1097/00000478-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chuaqui RF, Zhuang Z, Emmert-Buck BR, Bryant BR, Nogales F, Tavassoli FA, Merino MJ. Genetic analysis of synchronous mutinous tumors of the ovary and appendix. Hum Pathol. 1996;27:165–71. doi: 10.1016/s0046-8177(96)90370-6. [DOI] [PubMed] [Google Scholar]
- 10.Misdraji J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod Pathol. 2015;28:S67–S79. doi: 10.1038/modpathol.2014.129. [DOI] [PubMed] [Google Scholar]


