Abstract
Oral squamous cell carcinoma (OSCC) ranks as the fifth most common cancer worldwide with poor prognosis. Recently, tumor necrosis factor receptor-associated factor 4 (TRAF4) has attracted increasing attenuation due to its overexpression in certain cancers. However, its function and underlying mechanism in OSCC remains elusive. In this study, the high expression of TRAF4 mRNA and protein levels was noted in OSCC cell lines. Its overexpression with pcDNA3.1-TRAF4 vector transfection dramatically promoted cell proliferation and inhibited cell apoptosis, indicating a pivotal role of TRAF4 in OSCC cell growth. Simultaneously, TRAF4 elevation also increased cell invasion and migration. Mechanism analysis confirmed that TRAF4 up-regulation induced the expression of β-catenin and the downstream target molecules of cyclinD1, c-myc, Bcl-2, MMP-9 and MMP-2, indicating that TRAF4 could induce the activation of Wnt/β-catenin pathway. After pretreatment with β-catenin siRNA, the pathway was remarkably silenced. Simultaneously, cell growth, invasion and migration induced by TRAF4 were strikingly abrogated, suggesting that TRAF4 may promote OSCC cell growth, invasion and migration by Wnt/β-catenin pathway. Together, this study confirmed that TRAF4 acts as an oncogene for the development and progression of OSCC. Therefore, our study may support a promising therapeutic target for the treatment of OSCC.
Keywords: Oral squamous cell carcinoma, TRAF4, cell growth, cell invasion, cell migration, Wnt/β-catenin pathway
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common malignant tumors in the head and neck and ranks as the fifth most common cancer worldwide [1]. OSCC has been identified as a significant public health threat worldwide as its high proliferation and invasive nature [2]. Despite considerable advances being made in the diagnosis and clinical treatment of this tumor, the overall 5-year survival rate of OSCC patients has not markedly improved and remain at less than 50% [3,4]. Though numerous researches have been performed to explore the etiology of OSCC, the accurate molecular mechanism underlying the pathogenesis and progression of OSCC remains undefined. Accordingly, understanding the main mechanism related to OSCC progression is pivotal for development of effective therapeutic strategies for OSCC patients.
Tumor necrosis factor receptor (TNFR)-associated factors (TRAFs) belong to a family of cytoplasmic adaptors and can interact directly or indirectly with TNFR to regulate various signaling events, such as cell growth, invasion, immunity and so on [5-7]. As one of the important member of TRAF family proteins, TRAF4 was initially isolated from breast carcinomas and identified as the first member of the TRAF family to be up-regulated in human carcinomas [8]. Unlike other members of TRAF4 family, the biological function of TRAF4 is still elusive. Recently, TRAF4 has drawn increasing attention due to its critical functions in the development and progression of tumors. Emerging evidences have confirmed a abnormal expression of TRAF4 in certain cancers, including breast cancer, lung carcinoma, prostate cancer [9,10]. It has been demonstrated that TRAF4 nuclear expression is correlated with poor survival in breast cancer patients [11]. Moreover, the elevated TRAF4 expression promotes breast cancer cell invasion, migration and tumor metastasis by regulating the pro-oncogenic TGF-β-induced SMAD and non-SMAD signaling [12]. A recent study indicates that TRAF4 is overexpressed in lung cancer tissues and cells, and that its silencing exhibits inhibitory effects on cell proliferation and tumor development in a xenograft mouse model, indicating as a candidate molecular target for lung cancer prevention and therapy [13]. Although abundant studies on TRAF4 roles in certain cancers have been explored, its roles and underlying molecular mechanism in the development of OSS remain poorly elucidated.
In this study, we examined the expression of TRAF4 on OSCC cell lines and investigated its effect on cell growth, invasion and migration. The underlying mechanisms involved in these processes were also explored.
Materials and methods
Reagents
Unless otherwise mentioned, all substances were purchased from Gibco (Grand Island, NY, USA). The primary antibodies against human β-catenin, Bcl-2, c-myc and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody to cyclinD1 was bought from Abgent (San Diego, CA). Mouse anti-Ki67 monoclonal antibodies were from Sigma (St. Louis, MO). Rabbit anti-human MMP-2 and MMP-9 antibodies were from Chemicon (Temecula, CA).
Cell culture
Human tongue squamous cell carcinoma cell lines Tca8113, SCC-4, SCC-25 and SCC-9 were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in DMEM containing 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 100 U/mL penicillin G and 100 mg/ml streptomycin. The human normal oral keratinocyte cell lines HOK were obtained from the ScienCell Research Laboratories (ScienCell, Ca) and used as “normal”. The obtained HOK cells were incubated with Oral Keratinocyte Medium (OKM) consisted of 500 mL basal medium, 5 ml of oral keratinocyte growth supplement. All cells were maintained at 37°C with 5% CO2 humidified atmosphere.
Construction of recombinant TRAF4 expression vectors and stable transfection in vitro
The total RNA was extracted by RNeasy Mini Kit with an on-column deoxyribonuclease digestion (QIAGEN, Valencia, CA), followed by the reverse transcription to cDNA using the Promega Reverse Transcription System (Promega, Southampton, UK). Then, human full-length TRAF4 cDNA was amplified with its specific primers to obtain the TRAF4 expression vectors. Following digestion with Xho I and Apa l restriction enzymes, the fragments were then subcloned into the Xho I and Apa l cloning site of the pcDNA3.1 (+) vector (Invitrogen, Carlsbad, CA) to construct the recombinant pcDNA3.1-TRAF4 expression vector. For transfection, cells were cultured to 60% confluence, then transfected with 15 µg of pcDNA3.1-TRAF4 or empty vector in SCC-25 cells using the FuGENE HD transfection reagent (Roche, Indianapolis, IN). The empty vector was performed as a negative control.
Transfection of β-catenin siRNA
Synthetic small interference RNA (siRNA) fragments targeting human β-catenin and control siRNA were obtained from GenePharma (Shanghai, China). For siRNA transfection experiments, TRAF4-overexpressed and control cells were dissociated into single cells in suspension and plated on 12-well plates for 24 h. Then, cells were transfected with 2 μg/mL β-catenin siRNA mixed with 5 μL Lipofectamine RNAi MAX (Invitrogen, Carlsbad, CA). Approximately 24 h later, cells were harvested. The transfection efficiency was analyzed by western blotting.
Quantitative RT-PCR
Total RNA from the above cells were isolated and reverse-transcribed to synthesize the first strand cDNA as described above. Then, the obtained cDNA was subjected to real-time PCR with specific primers for TRAF4 (sense: 5’-AGGAGTTCGTCTTTGACACCATC-3’; anti-sense: 5’-CTTTGAATGGGCAGAGCACC-3’), yielding a product of 162 bps in a total of 20 μL. Quantitative real-time RT-PCR was performed using the SYBR Premix Ex TaqTM II Kit (Takara Bio Inc., Otsu, Japan) on an ABI PRISM 7000 sequence detection system. The reaction conditions were done according to the manufacturer’s instructions. For normalization, β-actin mRNA was used. All samples were tested in triplicate. All results were represented as relative mRNA levels and calculated according to the 2-ΔΔCt method.
Western blot analysis
Cells were lysed by RIPA lysis buffer (100 mM NaCl, 50 mM Tris-HCl pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM b-glycerophosphate, 2 mM sodium vanadate and protease inhibitor) (Sigma). Then, the extracted protein concentration was measured by the Micro BCA protein assay kit from Pierce Chemical (Rockford, IL). Protein electrophoresis was performed to separate the obtained proteins by SDS-PAGE, and was transferred to a PVDF membrane (Schleicher & Schuell, Germany). The membranes were blocked with 5% non-fat milk to block the nonspecific binding. Then, the membranes were incubated with the primary antibodies against TRAF4, β-catenin, cyclinD1, Ki67, c-myc, Bcl-2, MMP-9 and MMP-2, followed by the incubation with the secondary antibodies conjugated with HRP (Jackson Immuno Research). The LumiGLo reagent (KPL, Gaithersburg, MD) was introduced to visualize the bound antibodies. β-actin was used for normalization.
Proliferation assays
Cells were seeded in 96-well plate and MTT was used to evaluate cell proliferation. Following preconditioning with the indicated treatments, the medium was removed from each well and was replaced with PBS solution with 5 mg/ml MTT (Sigma-Aldrich, St. Louis, MO) for further 5 h. Then, the supernatant was replaced with 200 μl isopropanol to dissolve the formazan production. Cell viability was determined by measuring the absorbance of MTT at 590 nm with a micro-ELISA reader (Bio-Rad, Hercules, CA).
Apoptosis assay
To quantitatively assess the rate of apoptosis, annexin V-propidium iodide (AV-PI) staining was conducted. Following pretreatment with the indicated conditions, cells were lysed with lysis buffer (10 mM Tris, 10 mM EDTA, 0.5% Triton X-100, pH 7.5). Then, cells were rinsed with PBS, followed by resuspension in 200 μL of PBS binding buffer including 5 μL FITC-conjugated annexin V, according to the instructions of manufacturers (Beyotime, Shanghai, China). Propidium iodide (PI; KeyGEN) was subsequently added. Cells were analyzed by a FACScan flow cytometer (Becton Dickinson) using FlowJo software (Tree Star Inc.) and the results were expressed as a percentage of total cells counted.
Invasion assay
Cell invasion assay was performed using the QCMTM 24-well Invasion Assay kit (Chemicon International, Temecula, CA) as per the manufacturer’s instruction. Briefly, cells were resuspended in serum-free DMEM medium and then were then added into the interior of the insert containing an 8-mm-pore-size polycarbonate membrane coated with a thin layer of ECMatrix. The medium containing 10% fetal bovine serum was added into the lower compartment as chemoattractant. About 48 h later, the non-invading cells (upper chamber) were gently removed, then cells that passed through the bottom side of membrane were fixed and stained with 0.1% crystal violet, followed by photographed under a light microscope. The quantified analysis was performed by counting six high-powered fields in the center of each well.
Cell migration assay
Cell migration was determined using a scratch wound healing assay. The treated cells were seeded on a 24-well plate with their respective culture media and grown until confluence. Then, a single scratch wound was generated with 200 μL-disposable pipette tip. After gently washing with serum-free medium, the wounded cell monolayer was allowed to heal in a complete culture medium. The inverted microscope and digital camera were used to photograph the scratch wounds, which was quantitated using the ImageJ software. The results were shown as the percentage of wound closure setting the initial scratch width as 100%.
Statistical analysis
All data were carried out using SPSS software (Ver. 16.0, SPSS Inc., Chicago, IL). The Student’s t-test was used to analyze the statistical significance differences between groups. All results were presented as mean ± SD. P < 0.05 was considered statistically significant.
Results
Expression of TRAF4 was elevated in OSCC cell lines
Given that TRAF4 possesses the critical function in the development of several cancers. However, the related research into TRAF4 and OSCC is still poor understanding. To address this problem, we evaluated the expression levels of TRAF4 in four OSCC cell lines (Tca8113, SCC-4, SCC-25 and SCC-9). Compared with human normal oral keratinocyte cell lines HOK, the obvious up-regulation in TRAF4 mRNA levels was demonstrated in OSCC cell lines, especially in SCC-25 and SCC-4 cells (Figure 1A). Simultaneously, western blotting analysis confirmed the dramatic increase of TRAF4 protein levels in OSCC cell lines. Therefore, these data indicated a notable elevation of TRAF4 in OSCC cells, which would be relevant to the development of human OSCC cancer.
Figure 1.
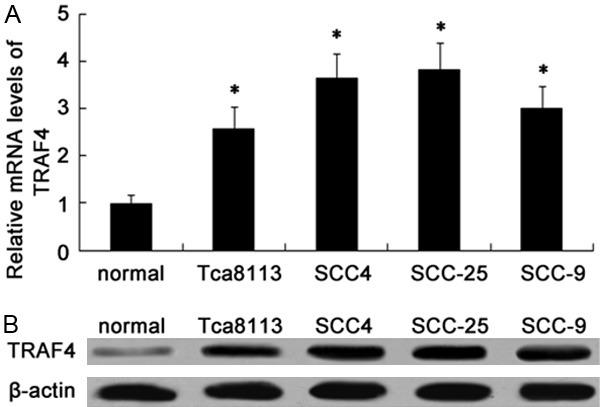
The up-regulation of TRAF4 was observed in OSCC cell lines. The mRNA levels of TRAF4 in four OSCC cell lines (Tca8113, SCC-4, SCC-25 and SCC-9) and human normal oral keratinocyte cell lines HOK (used as normal) were determined by RT-PCR (A). The corresponding protein levels were evaluated by western blotting (B). *P < 0.05 versus normal groups.
Effect of TRAF4 overexpression through rTRAF4 transfection on cell growth
To explore the effect of TRAF4 on the development of OSCC, we evaluated the roles of TRAF4 in SCC-25 cell growth. Firstly, the recombinant pcDNA3.1-TRAF4 (rTRAF4) was transfected into highly malignant SCC-25 cells. As expected, this transfection obviously induced a 4.2-fold increase in TRAF4 mRNA levels, compared with the vector control clone (Figure 2A). Consistently, rTRAF4 transfection also strikingly enhanced TRAF4 protein levels and constructed a stable TRAF4 overexpression clones named SCC25-145 cell line (Figure 2B). Then, we further assessed TRAF4 function on cell growth. As shown in Figure 2C, a significant increase in cell proliferation was observed in TRAF4-overexpressed groups. Additionally, TRAF4 up-regulation remarkably induced the expression of Ki67 protein, a common marker for cell proliferation (Figure 2D). Further apoptosis analysis confirmed that the apoptotic rate was dramatically decreased from 27.5% to 14.6% by Annexin V-FITC and PI staining after rTRAF4 transfection (Figure 2E). Together, these results shown that TRAF4 up-regulation strongly promoted cell growth by enhancing cell proliferation and blocking cell apoptosis, implying as a potential oncogene of TRAF4 in the progression of OSCC.
Figure 2.
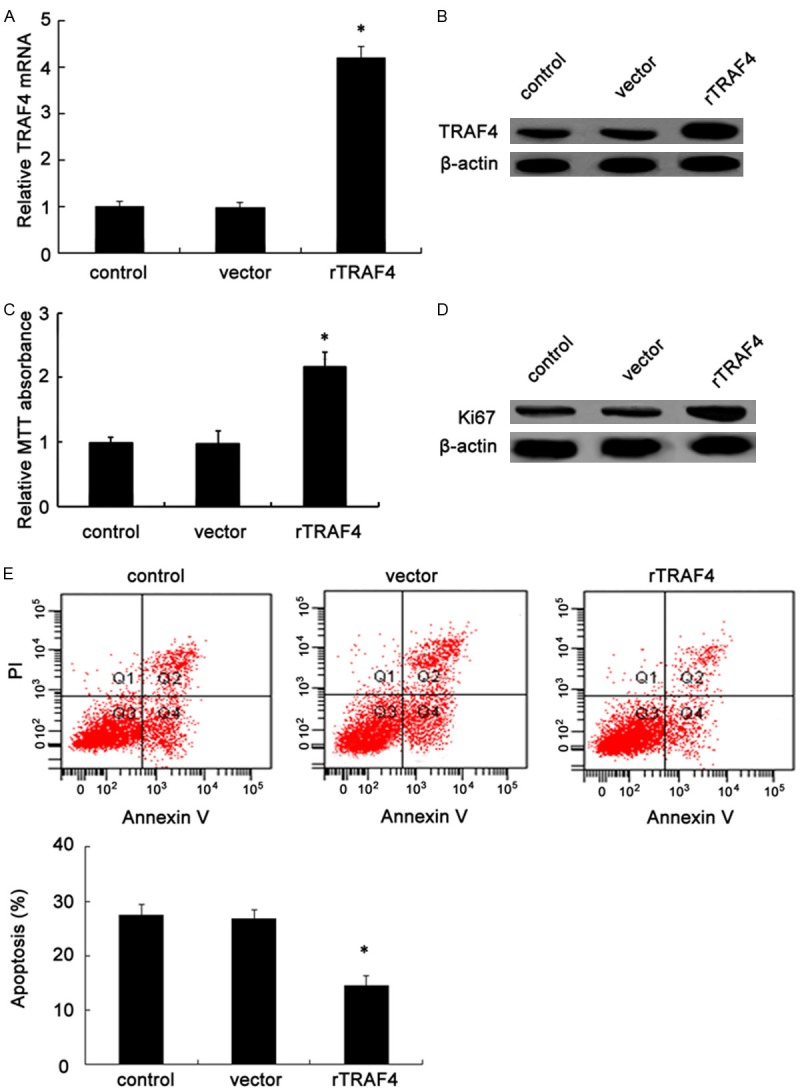
TRAF4 overexpression enhanced cell growth through rTRAF4 transfection. Following transfection with recombinant pcDNA3.1-TRAF4 (rTRAF4) or empty vector, the mRNA (A) and protein (B) levels were detected by RT-PCR and western blotting in SCC-25 cells. Furthermore, the effect of rTRAF4 on cell proliferation was analyzed by MTT assay (C). The corresponding marker molecule of Ki67 was evaluated by western blotting (D). (E) After staining with Annexin V/PI, further effects on cell apoptotic rates were demonstrated by flow cytometry. *P < 0.05.
Overexpression of TRAF4 promoted OSCC cell invasion and migration in vitro
To further validate the involvement of TRAF4 in OSCC metastasis, the functional assay was performed to investigate the roles of TRAF4 on cell invasion and migration. As shown in Figure 3A, ectopic transfection of rTRAF4 resulted in the significant increase in cell invasion. The number of SCC-25 cells invading through the membrane following rTRAF4 transfection was dramatically enhanced from 145 to 211 when compared to cells transfected with the vector control. Further scratch wound healing assay confirmed that TRAF4 up-regulation elevated the rate of cell migration from 24.6% to 36.8% compared with control groups (Figure 3B). Hence, all data suggested that rTRAF4 enhanced OSCC cell invasion and migration, indicating a potential regulatory effect of TRAF4 on OSCC metastasis.
Figure 3.
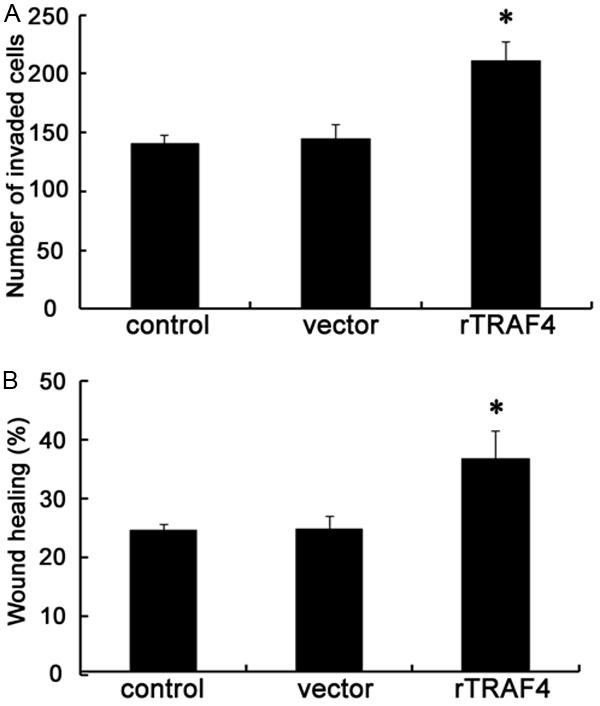
Overexpression of TRAF4 promoted OSCC cell invasion and migration in vitro. Following the stable overexpression of TRAF4 or not, the effect on cell invasive ability was evaluated by Transwell assay (A). Furthermore, the function in cell migration was determined using a scratch wound healing assay (B). *P < 0.05.
TRAF4 overexpression enhanced the activation of Wnt/β-catenin pathway
Accumulating evidence has shown that the abnormally high activation of the Wnt/β-catenin pathway is required for the initiation and progression of various tumors [14-16]. In order to explore the underlying mechanism involved in TRAF4-induced OSCC cell growth, invasion and migration, we investigated the activation of Wnt/β-catenin pathway. After transfection with rTRAF4, the expression levels of β-catenin were obviously up-regulated and induced an approximate 1.98-fold increase in β-catenin protein levels (Figure 4A). Further western blotting analysis demonstrated the notable up-regulation of cyclinD1, c-myc and Bcl-2 protein when cells were transfected with rTRAF4, all of them are the key targeted molecules in Wnt/β-catenin signaling and related to cell growth [17]. Moreover, the expression levels of its downstream targets MMP-2 and MMP-9 were also strikingly increased following rTRAF4 treatment; both of them are correlated with cell invasion and migration (Figure 4B). Together, these results indicated that TRAF4 could promote the activation of Wnt/β-catenin pathway.
Figure 4.
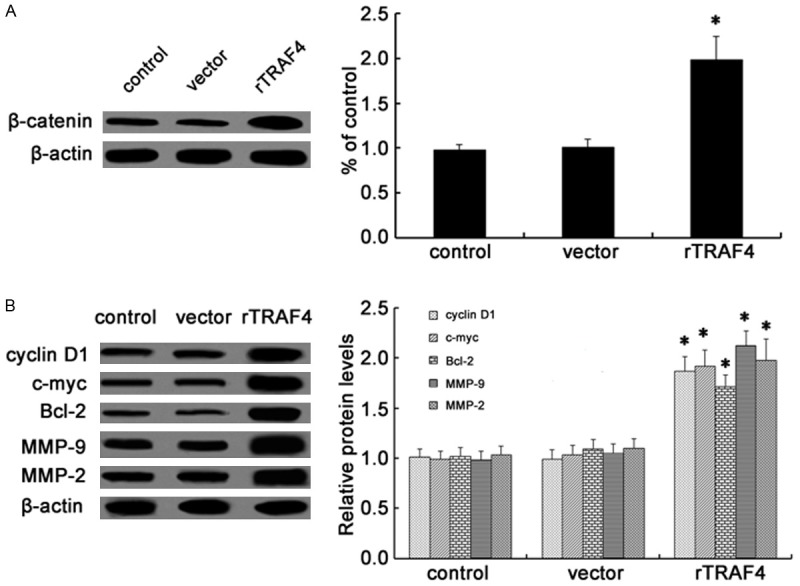
Effect of TRAF4 on the Wnt/β-catenin pathway. Following transfection with rTRAF4, the expression levels of β-catenin were detected by western blotting (A). The expression of downstream targets were also assessed including cyclinD1, c-myc, Bcl-2, MMP-9 and MMP-2 (B). All of the corresponding quantitative analysis of western band densities was analyzed using ImageJ software. *P < 0.05.
Wnt/β-catenin signaling was responsible for TRAF4-induced tumorgenesis
To further investigate whether TRAF4 enhanced OSCC cell growth, invasion and migration by Wnt/β-catenin pathway, we constructed the specific β-catenin siRNA. As shown in Figure 5A, pretreatment with β-catenin siRNA obviously abrogated the expression of β-catenin siRNA in SCC-25 cells. Simultaneously, the increases in downstream targets of cyclinD1, c-myc, Bcl-2, MMP-9 and MMP-9 induced by TRAF4 were remarkably dampened when silencing β-catenin expression (Figure 5B). Importantly, β-catenin siRNA transfection evidently mitigated TRAF4-induced up-regulation of cell viability (Figure 6A). The similar elevated effects on cell invasion (Figure 6B) and cell migration (Figure 6C) triggered by TRAF4 overexpression were also corroborated in cells treated with β-catenin siRNA transfection. Hence, these data suggested that TRAF4 might induce tumorgenesis of OSCC by Wnt/β-catenin signaling.
Figure 5.
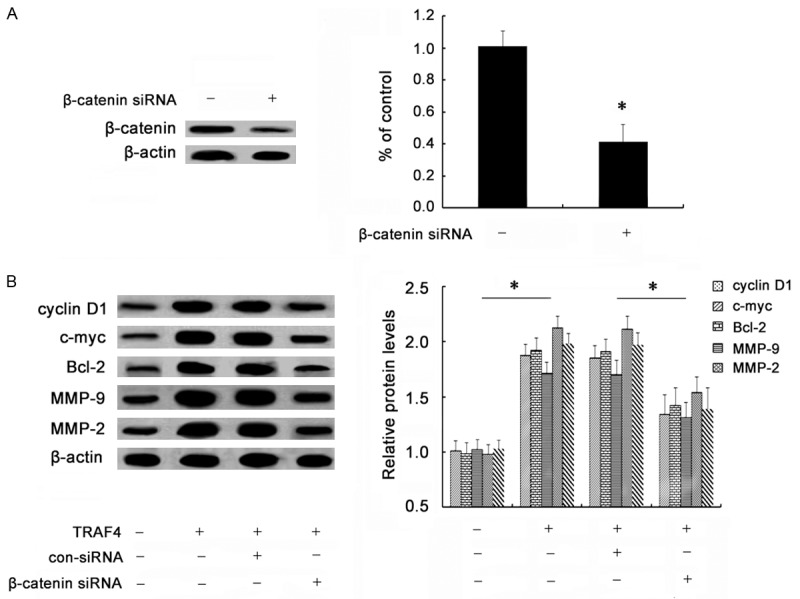
Effect of β-catenin siRNA on TRAF4-induced activation of Wnt/β-catenin pathway. Cells were pretreated with specific β-catenin siRNA. Then, cells were transfected with rTRAF4. The silencing effect on β-catenin levels was validated by western blotting (A). The effect of β-catenin siRNA transfection on TRAF4-triggered activation of the Wnt/β-catenin pathway was corroborated by determining the expression of its downstream targets via western blotting (B). *P < 0.05.
Figure 6.
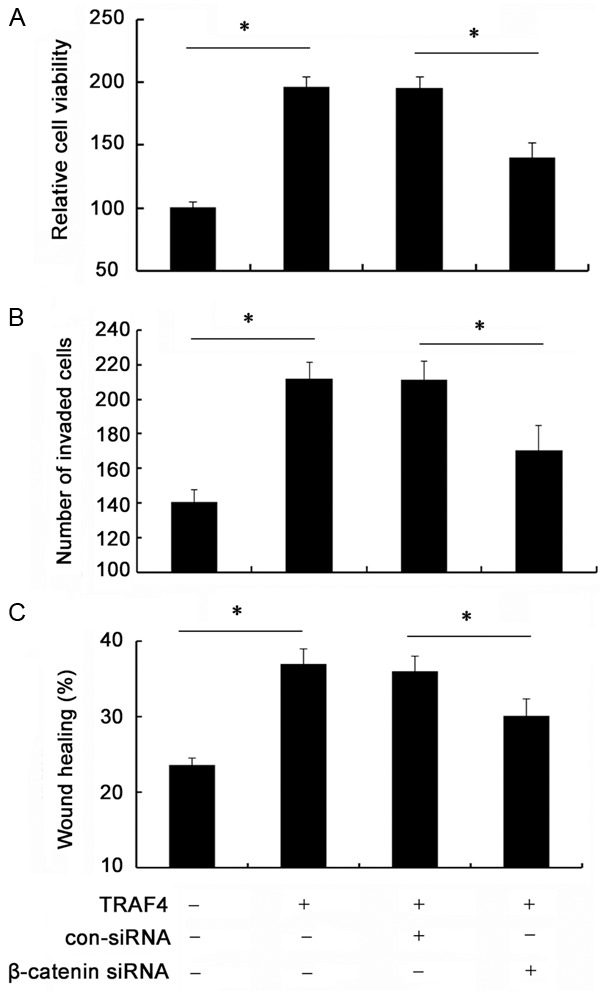
Wnt/β-catenin signaling was responsible for TRAF4-induced tumorgenesis. The TRAF4-transfected cells were pretreated with β-catenin siRNA. Then, cell proliferation was analyzed by MTT assay (A). Moreover, β-catenin silencing on TRAF4-induced cell invasion (B) and migration (C) was also performed. *P < 0.05.
Discussion
Oral squamous cell carcinoma (OSCC) accounts for 95% of oral cavity carcinomas and has a 5-year survival rate of only about 50% [1]. Despite all the advances in modern medicine, the incidence remains high and constitutes a major health problem in developing countries, representing a leading cause of death. This study presents the important finding that TRAF4 was notably up-regulated in several OSCC cell lines. Importantly, TRAF4 overexpression promoted OSCC cell growth, invasion and migration through the activation of Wnt-β-catenin pathway. Accordingly, this study may corroborate an important function of TRAF4 in the development and progression of OSCC.
TRAF4 (also known as RING finger protein 83) belongs to the TNF receptor associated factor (TRAF) family and was originally identified as a gene that is elevated in breast cancer [8]. The subsequent has been validated that TRAF4 exerts anti-apoptotic effects induced by TNF-α in MCF-7 cells. Furthermore, TRAF4 expression enhances breast cancer cell invasion, migration, tumor metastasis and is correlated with poor survival in breast cancer patients [11,12]. Recent researches have demonstrated a abnormal high expression of TRAF4 in several cancers, including breast cancer, lung carcinoma and prostate cancer [9,10,18]. However, numerous studies still focus on its function in breast cancer; its effect on other cancers and the underlying mechanism remains elusive. In this study, we first confirmed a similar high expression of TRAF4 mRNA and protein levels in OSCC cells in contrast to human normal oral keratinocyte cell lines HOK. Moreover, TRAF4 overexpression promoted OSCC cell proliferation and attenuated cell apoptosis, indicating a pivotal role of TRAF4 in OSCC cell growth. Therefore, these data suggest that TRAF4 may act as a potential oncogene during the progression of OSCC.
Wnt/β-catenin pathway ranks as the best understood Wnt signaling pathway and is highly conserved during evolution. Accumulating evidences confer that Wnt/β-catenin pathway possesses multiple roles in regulating diverse physiological processes, including cell proliferation, survival, cell behavior and fate [19,20]. Recently, hyperactivity of the Wnt/β-catenin signaling pathway has been found in many human cancers, such as colorectal cancer, liver cancer and gastrointestinal cancers [21-24]. Wnt/β-catenin signaling has been reported to play vital roles in carcinogenesis by regulating cell growth, cell cycling, cell survival and invasion. Blocking its unrestricted activation will attenuated the development of cancer and thus holds promise for the development of new anti-carcinoma drugs [23,25]. In breast cancer, TRAF4 facilitates the activation of Wnt/β-catenin pathway [26]. To further clarify the molecular mechanism involved in TRAF4-induced OSCC cell growth, we analyzed the expression levels of β-catenin, the central component of this pathway. As expected, TRAF4 up-regulation obviously induced β-catenin expression. It is known that cyclinD1, c-myc and Bcl-2 are common downstream molecules of this pathway, which are proved to be related with cell proliferation and apoptosis [17,27]. Further analysis confirmed a similar effect on the expression of cyclinD1, c-myc and Bcl-2 in TRAF4-overexpressed cells, indicating that TRAF4 induced the activated the Wnt/β-catenin pathway. Importantly, blocking this pathway by β-catenin siRNA transfection evidently mitigated TRAF4-induced up-regulation in cell viability. Together, these results indicated that TRAF4 may promote OSCC cell growth by regulating the Wnt/β-catenin pathway.
The high invasiveness and migration are critical for tumor progression and known as the prominent contributors to morbidity and mortality in cancer patients [28]. To better understand the function of TRAF4 in the development of OSCC, we further analyzed its effect on OSCC cell invasion and migration. In this study, TRAF4 overexpression dramatically enhanced OSCC cell invasion, as well as the rate of cell migration, indicating a critical role of TRAF4 in OSCC metastasis. It is generally believed that Wnt/β-catenin signaling can trigger a cascade of responses, from cell growth to motility and invasion, which drive tumor development and progression [20,21]. Based on the effect of TRAF4 on Wnt/β-catenin activation, we can speculate that TRAF4 may increase cell invasion and migration by Wnt/β-catenin pathway. To further test our hypothesis, we silenced this pathway by β-catenin siRNA transfection. Moreover, the increase in MMP-9 and MMP-2 expression induced by TRAF4 was also notably attenuated, both of these are key regulators of cell invasion [29,30]. Furthermore, the up-regulation in cell invasion and migration triggered by TRAF4 was also obviously ameliorated when inhibiting Wnt/β-catenin pathway, implying that TRAF4 enhance OSCC cell invasion and migration by Wnt/β-catenin signaling.
In conclusion, we first demonstrated that TRAF4 was overexpressed in OSCC cells. Importantly, TRAF4 promoted OSCC cell growth, invasion and migration by activating the Wnt/β-catenin signaling pathway. Therefore, these findings will contribute to our understanding of how TRAF4 regulates the pathogenesis of OSCC, and support a promising therapeutic agent for the future development of anti-OSCC therapy.
Disclosure of conflict of interest
None.
References
- 1.Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–399. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 2.Amit M, Yen TC, Liao CT, Chaturvedi P, Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Kreppel M, Zöller J. Improvement in survival of patients with oral cavity squamous cell carcinoma: An international collaborative study. Cancer. 2013;119:4242–4248. doi: 10.1002/cncr.28357. [DOI] [PubMed] [Google Scholar]
- 3.Kessler P, Grabenbauer G, Leher A, Bloch-Birkholz A, Vairaktaris E, Neukam FW. Neoadjuvant and adjuvant therapy in patients with oral squamous cell carcinoma: long-term survival in a prospective, non-randomized study. Br J Oral Maxillofac Surg. 2008;46:1–5. doi: 10.1016/j.bjoms.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Brocklehurst PR, Baker SR, M Speight P. Oral cancer screening: what have we learnt and what is there still to achieve? Future Oncol. 2010;6:299–304. doi: 10.2217/fon.09.163. [DOI] [PubMed] [Google Scholar]
- 5.Conti A, Aguennouz MH, La Torre D, Cardali S, Angileri FF, Buemi C, Tomasello C, Iacopino DG, D’Avella D, Vita G. Expression of the tumor necrosis factor receptor-associated factors 1 and 2 and regulation of the nuclear factor-kB antiapoptotic activity in human gliomas. J Neurosurg. 2005;103:873–881. doi: 10.3171/jns.2005.103.5.0873. [DOI] [PubMed] [Google Scholar]
- 6.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrand JM, Yi Z, Buchta CM, Poovassery J, Stunz LL, Bishop GA. Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol Rev. 2011;244:55–74. doi: 10.1111/j.1600-065X.2011.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasetto C, Regnier C, Moog-Lutz C, Mattei M, Chenard M, Lidereau R, Basset P, Rio M. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21. 3 region of chromosome 17. Genomics. 1995;28:367–376. doi: 10.1006/geno.1995.1163. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri-Broet S, Cremer I, Marmey B, Comperat E, Viguie F, Audouin J, Rio M, Fridman W, Sautes-Fridman C, Regnier C. TRAF4 overexpression is a common characteristic of human carcinomas. Oncogene. 2006;26:142–147. doi: 10.1038/sj.onc.1209762. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed F, Shiraishi T, Vessella RL, Kulkarni P. Tumor necrosis factor receptor associated factor-4: An adapter protein overexpressed in metastatic prostate cancer is regulated by microRNA-29a. Oncol Rep. 2013;30:2963–2968. doi: 10.3892/or.2013.2789. [DOI] [PubMed] [Google Scholar]
- 11.Yi P, Xia W, Wu RC, Lonard DM, Hung MC, O’Malley BW. SRC-3 coactivator regulates cell resistance to cytotoxic stress via TRAF4-mediated p53 destabilization. Genes Dev. 2013;27:274–287. doi: 10.1101/gad.203760.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Zhou F, García de Vinuesa A, de Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li Y, Bauer A, Rousseau A. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell. 2013;51:559–572. doi: 10.1016/j.molcel.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Peng C, Lee MH, Lim D, Zhu F, Fu Y, Yang G, Sheng Y, Xiao L, Dong X. TRAF4 is a critical molecule for Akt activation in lung cancer. Cancer Res. 2013;73:6938–6950. doi: 10.1158/0008-5472.CAN-13-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidya Priyadarsini R, Senthil Murugan R, Nagini S. Aberrant activation of Wnt/β-catenin signaling pathway contributes to the sequential progression of DMBA-induced HBP carcinomas. Oral Oncol. 2012;48:33–39. doi: 10.1016/j.oraloncology.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Lim YC, Kang HJ, Kim YS, Choi EC. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/β-catenin pathway. Eur J Cancer. 2012;48:3310–3318. doi: 10.1016/j.ejca.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Teng Y, Wang X, Wang Y, Ma D. Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392:373–379. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Yu T, Liu K, Wu Y, Fan J, Chen J, Li C, Yang Q, Wang Z. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/β-catenin signaling pathway. Oncogene. 2013;33:5017–5027. doi: 10.1038/onc.2013.448. [DOI] [PubMed] [Google Scholar]
- 18.Rousseau A, Rio MC, Alpy F. TRAF4, at the Crossroad between Morphogenesis and Cancer. Cancers. 2011;3:2734–2749. doi: 10.3390/cancers3022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MD, Monga SP. WNT/β-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 20.Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the Role of Wnt/β-Catenin Signaling in the Survival, Proliferation, and Self-Renewal of Human Embryonic Stem Cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 21.Monga SP. Role of Wnt/β-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021–1029. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dihlmann S, von Knebel Doeberitz M. Wnt/β-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 24.Fodde R, Brabletz T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15:873–887. doi: 10.1517/14728222.2011.577418. [DOI] [PubMed] [Google Scholar]
- 26.Wang A, Wang J, Ren H, Yang F, Sun L, Diao K, Zhao Z, Song M, Cui Z, Wang E. TRAF4 participates in Wnt/β-catenin signaling in breast cancer by upregulating β-catenin and mediating its translocation to the nucleus. Mol Cell Biochem. 2014;395:211–219. doi: 10.1007/s11010-014-2127-y. [DOI] [PubMed] [Google Scholar]
- 27.Abe W, Nasu K, Nakada C, Kawano Y, Moriyama M, Narahara H. miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Hum Reprod. 2013;28:750–761. doi: 10.1093/humrep/des446. [DOI] [PubMed] [Google Scholar]
- 28.Duffy M, McGowan P, Gallagher W. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 29.Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou HF, Guo L, Liu W, Wang SJ, Yu XG. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol. 2012;40:1714–1724. doi: 10.3892/ijo.2011.1320. [DOI] [PubMed] [Google Scholar]
- 30.Fiorentini C, Bodei S, Bedussi F, Fragni M, Bonini SA, Simeone C, Zani D, Berruti A, Missale C, Memo M. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp Cell Res. 2014;323:100–111. doi: 10.1016/j.yexcr.2014.02.025. [DOI] [PubMed] [Google Scholar]


