Abstract
The cholesterol has been reported to be associated with cancer development. The aim of our study was to investigate the serum cholesterol level (TC) in non-small cell lung cancer (NSCLC) patients and their association with clinicopathologic parameters and the prognosis. The serum cholesterol levels were evaluated in 259 resected NSCLC patients and were correlated with clinicopathologic variables including age, gender, tumor size, regional lymph node metastasis, disease-free survival time and overall survival time. Preoperative serum TC level was significantly associated with gender (P=0.001), smoking history (P=0.016), tumor differentiation (P=0.027), pT stage (P<0.001) and pN stage (P=0.009). Multivariate analyses indicated that decreased serum TC level was an independent prognostic factor for worse outcome. The hazard ratio was 1.89 (95% confidence interval [CI] 1.09-3.26) for disease progression and 2.19 (95% CI 1.01-4.74) for death. Based on this finding, the pretreatment serum cholesterol level is a novel independent prognostic biomarker in resectable NSCLC.
Keywords: Cholesterol, non-small cell lung cancer, prognosis, surgery
Introduction
Lung cancer, contributed to 29% of male and 26% of female cancer estimated deaths, is the leading cause of cancer death in both developed and developing countries [1]. Non-small cell lung cancer (NSCLC) represents 85% of patients diagnosed with lung cancer. Radical surgery is thought to be the single modality that can provide opportunity of cure and long-time survival [2]. However, despite the continuous progress and efforts in the treatment, the five-year overall survival rate is not over 50-60%. Furthermore, nearly 40-50% of patients with newly diagnosed NSCLC have distant organ metastasis. For metastatic or locally advanced NSCLC, radiochemotherapy is the standard method. Yet it remains unknown which patients might benefit from systemic treatment [3-5]. To improve treatment strategies, predictors of the prognosis would be helpful. However, up to now, most of these markers had not been proven to be sufficiently effective [6,7].
Recently, evidence suggested that lipid metabolism is associated with cancer development [8-10]. Some studies found that dietary cholesterol was associated with an increased risk of lung cancer [11,12]. The association between serum total cholesterol (TC) and the risk of lung cancer also remains controversial [13-15]. In a large case-control study, serum total cholesterol (TC) concentration was significant lower in lung cancer patients and in comparison with the control group [16]. But there were no statistically significant differences of TC level between the clinical stages of each histological type. Although TC level widely used to predict prognosis of patients with coronary heart disease, there are a few studies that focus on its role as a prognostic marker in lung cancer [17].
In this retrospective study, this study aimed to investigate the clinical implication of the preoperative serum TC level in patients with NSCLC treated with complete resection at a single institution.
Patients and methods
A total of 298 consecutive NSCLC patients who underwent complete resection (lobectomy and mediastinal lymph node dissection with microscopic examination of margins showing no tumor cells) from January 2011 to July 2014 at the Jiaxing first hospital were enrolled in this study. All patients met the following eligible criteria: (1) all patients did not have previous malignant disease; (2) no patient receive preoperative chemotherapy or radiotherapy; (3) those with concomitant disease suspected of influencing serum TC concentration, such as diabetes, hyperlipidemia, were excluded; (4) patients took any drug known to affect lipid metabolism were excluded. Finally, a total of 259 NSCLC patients enrolled in this study. There were 189 men and 70 women with a median age of 60 years (range: 37-82 years). Histological sub-classification was based on the classification criteria for lung tumors of the World Health Organization and International Association for the Study of Lung Cancer (WHO/IASLC) [18]. The post-operation staging was carried out by exhaustive procedures according to the 2010 7th TNM Classification of Malignant Tumors staging system [19].
The study was approved by the Ethics Committee of Jiaxing people’s hospital. Detailed clinical and pathological information, including patients’ age, gender, smoking status, TNM stage and disease-free survival (DFS), overall survival (OS) data were available for all patients. Disease-free survival (DFS) was defined as the time from surgery to any recurrence. Overall survival (OS) was calculated as the time from the date of surgery to death or censoring. Adjuvant treatment, such as chemotherapy, radiotherapy and target therapy was determined by the clinicopathological stage according to the NCCN guideline. One hundred and twenty-two patients (47.1%) received adjuvant chemotherapy or radioradiotherapy.
All patients were followed up for a median of 25 months (range, 3-65) by physical examination, blood evaluation, and chest computer tomography (CT) and other radiological studies when necessary at every 3 months for the first 2 years after operation, a 6-month interval in the third year and annual thereafter.
Peripheral blood samples (3 ml) were primarily obtained in early morning before breakfast from all patients at hospitalization. Blood samples were collected into EDTA-coated tubes and the preoperative serum TC levels were measured automatically by electrophoresis (Architect ci8200 analyzer; Abbott Diagnostics, Wiesbaden, Germany). According to the National Cholesterol and Education Program (NCEP) Adult Treatment Panel III criteria (NCEP-ATPIII) [20], a serum concentration of TC more than 200 mg/dl was defined as hypercholesterolemia. The eligible patients were divided into two groups: the low TC group and the hypercholesterolemia group, according to the above cutoff value.
The preoperative serum TC level was analyzed as a continuous variable and a categorical variable after grouping by the low TC group and the hypercholesterolemia group. Pearson’s chi squared test was used to analyze the relationship between categorical TC groups with clinicopathological characteristics. Progression-free and overall survival was analyzed by Cox proportional hazards regression and Kaplan-Meier curves. All statistical calculations were performed with SPSS 15.0 (Chicago, IL). P<0.05 was considered statistical significance.
Results
Preoperative serum TC level and clinicopathological parameters
The mean ± standard deviation preoperative serum TC level was 235.87±53.46 mg/dL (median: 232 mg/dL, range: 38.93-546.67 mg/dL). The relationship between the preoperative serum TC level and clinicopathological parameters are shown in Table 1. The preoperative serum TC level was found to be significantly higher in female patients than that in male patients (mean: 249.02 mg/dL vs. 231.00 mg/dL, P=0.016), in patients with T1-2 than those with T3-4 (245.70 mg/dL vs. 205.91 mg/dL, P<0.001), in patients without regional lymph node metastasis than those with regional lymph node metastasis (240.91 mg/dL vs. 230.63 mg/dL, P=0.028), in early stages (I-II) than in advantage stages (III-IV) (244.20 mg/dL vs. 217.21 mg/dL, P<0.001). However, no significant differences in mean levels of preoperative serum TC were observed between different age (<60 vs. ≥60) and different histology types (adenocarcinoma vs. non-adenocarcinoma).
Table 1.
Relationship between serum TC level and clinicopathological characteristics in 259 NSCLC
| Variables | Patients, n (%) | Serum TC level | P | |
|---|---|---|---|---|
|
|
||||
| Low (≤200 mg/dL) N (%) | High (>200 mg/dL) N (%) | |||
| Gender | ||||
| Male | 189 | 59 (31.2) | 130 (68.8) | 0.001 |
| Female | 70 | 8 (11.4) | 62 (88.6) | |
| Age, years | ||||
| <60 | 114 | 31 (27.2) | 83 (72.8) | 0.666 |
| ≥60 | 145 | 36 (24.8) | 109 (75.2) | |
| Smoking history | ||||
| Yes | 174 | 53 (30.5) | 121 (69.5) | 0.016 |
| No | 85 | 14 (16.5) | 71 (83.5) | |
| Histology type | ||||
| Adenocarcinoma | 124 | 27 (21.8) | 97 (78.2) | 0.149 |
| Non-adenocarcinoma | 135 | 40 (29.6) | 95 (70.4) | |
| Tumor differentiation | ||||
| Well | 17 | 2 (11.8) | 15 (88.2) | 0.027 |
| Moderate | 137 | 29 (21.2) | 108 (78.8) | |
| Low | 105 | 36 (34.3) | 69 (65.7) | |
| pT stage | ||||
| pT1-2 | 195 | 34 (17.4) | 161 (82.6) | <0.001 |
| pT3-4 | 64 | 33 (51.6) | 31 (48.4) | |
| pN stage | ||||
| pN0 | 132 | 25 (18.9) | 107 (81.1) | 0.009 |
| pN+ | 127 | 42 (33.1) | 85 (66.9) | |
| pTNM stage | ||||
| I-II | 179 | 31 (17.3) | 148 (82.7) | <0.001 |
| III-IV | 80 | 36 (45.0) | 44 (55.0) | |
A total of 67 (25.9%) had low serum TC level (≤200 mg/dL), while 192 (74.1%) had hypercholesterolemia. Preoperative serum TC level was significantly associated with gender (P=0.001), smoking history (P=0.016), tumor differentiation (P=0.027), pT stage (P<0.001), pN stage (P=0.009) and pTNM stage (P<0.001), but not with age (P=0.666) and histology type (P=0.149).
Relationship between serum TC level and CRP
The median concentration of preoperative CRP was 5.89 mg/L. An elevated median CRP level was significant for the patients with a low serum TC level (P=0.032). Spearman’s correlation revealed that the serum TC level was inversely correlated with the serum CRP levels (r=0.179, P<0.001) (Figure 1).
Figure 1.
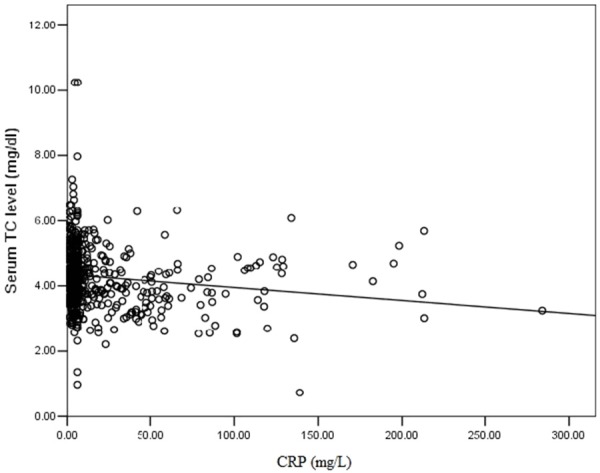
Correlation between pretreatment serum C-reactive protein (CRP) levels and pretreatment serum TC level.
Relationship between preoperative serum TC level and prognosis
The median postoperative follow-up time was 26 months. During the follow-up, 59 patients (22.8%) were reported treatment failure, 18 patients experienced locoregional recurrence, 32 patients developed distant organ metastasis and 9 patients developed both. Overall, 30 patients died from cancer disease. The 3-year disease-free survival and overall survival rates were 67.6% and 67.9%. Patients with hypercholesterolemia had higher 3-year disease-free survival rate (69.1% vs. 61.9%) and overall survival rate (75.1% vs. 88.7%) than those with low serum TC level. The Kaplan-Meier survival curves indicated that patients with hypercholesterolemia had better disease-free survival (P=0.007, Figure 2A) and overall survival (P=0.008, Figure 2B) than patients with low TC level. In univariable analysis, pT stage and serum TC level were all significantly associated with DFS and OS. pN stage was significantly associated with OS (Table 2). In multivariable models, serum TC level was identified as an independent prognostic factor (Table 3). Patients with low preoperative serum TC level had increased risk of cancer progression and death compared with those had hypercholesterolemia. After adjusting for the effect of pT stage and pN stage, patients with low serum TC level had a 1.89-time increased risk of disease progression compared with those with hypercholesterolemia. After adjusting for the effect of pT stage, patients with low serum TC level had a 2.19-time increased risk of death compared with those with hypercholesterolemia.
Figure 2.
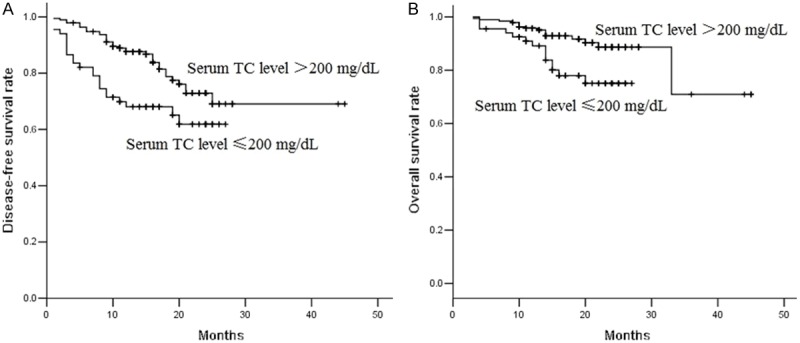
Kaplan-Meier survival curves estimates of 259 patients with NSCLC treated with surgery. Pretreatment serum TC level was categorized according to the threshold of 200 mg/dL. A. Patients with hypercholesterolemia had higher 3-year disease-free survival rate (69.1% vs. 61.9%) than those with low serum TC level. Patients with hypercholesterolemia had better disease-free survival (P=0.007). B. Patients with hypercholesterolemia had higher overall survival rate (75.1% vs. 88.7%) than those with low serum TC level. Patients with hypercholesterolemia had better overall survival (P=0.008).
Table 2.
Univariate analysis for clinicopathological variables affecting DFS and OS in 259 NSCLC
| Variables | Category | DFS | OS | ||
|---|---|---|---|---|---|
|
|
|||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender | Male vs. Female | 1.07 (0.64-1.91) | 0.809 | 0.98 (0.44-2.21) | 0.963 |
| Age | ≥60 vs. <60 | 1.42 (0.84-2.41) | 0.195 | 2.56 (1.13-5.01) | 0.035 |
| Smoking history | Yes vs. No | 1.11 (0.64-1.92) | 0.710 | 0.97 (0.45-2.07) | 0.927 |
| Histology type | Non-ade vs. ade | 1.27 (0.76-2.12) | 0.368 | 2.23 (1.02-4.88) | 0.044 |
| Tumor differentiation | Low vs. well and moderate | 1.31 (0.78-2.18) | 0.306 | 1.10 (0.54-2.27) | 0.790 |
| pT stage | pT3-4 vs. pT1-2 | 2.71 (1.62-4.55) | <0.001 | 3.32 (1.61-6.89) | 0.001 |
| pN stage | pN+ vs. pN0 | 2.14 (1.25-3.65) | 0.005 | 1.77 (0.84-3.73) | 0.134 |
| Serum TC level | Low vs. High | 2.02 (1.20-3.42) | 0.008 | 2.59 (1.25-5.36) | 0.011 |
DFS: disease-free survival; OS: Overall survival.
Table 3.
The impact of serum TC level on DFS and OS in 259 NSCLC patients by multivariate Cox regression analysis
| DFS | HR | 95% CI | P |
|---|---|---|---|
| Serum TC level >200 mg/dL | 1 | ||
| Serum TC level ≤200 mg/dL | 1.89 | 1.09-3.26 | 0.023 |
| OS | |||
| Serum TC level >200 mg/dL | 1 | ||
| Serum TC level ≤200 mg/dL | 2.19 | 1.01-4.74 | 0.047 |
DFS: Disease-free survival; OS: Overall survival; HR: Hazard ratio; CI: Confidence interval.
Discussion
NSCLC remains a highly lethal disease with a poor prognosis. Although several clinicopathologic variables, such as TNM stage system, have been the standards for determining the clinical prognosis of NSCLC patients, some patients with similar clinical stage have remarkably different survival prognosis. Therefore, it is necessary to identify effective biologic markers which are associated with advanced tumor progression for the discovery of a therapeutic target. In current study, there are two main findings according to our data. First of all, the preoperative serum TC level in NSCLC patients was significantly correlated with advanced tumor progression and aggressive clinicopathologic characteristics. Second, the influence of preoperative serum TC level on patients’ prognosis was assessed by Kaplan-Meier analysis, and the results showed that NSCLC patients with low preoperative serum TC level had shorter survival time (DFS and OS) than those with high preoperative serum TC level. Moreover, both univariate and multivariate analyses clearly revealed that the preoperative serum TC level was a statistically significant risk factor affecting DFS and OS of patients with NSCLC.
Cancers are the most complicated diseases because of its genetic heterogeneity and complexity. Recently, obesity, diabetes, and hypercholesterolemia are being considered as important risk factors for cancer [21,22]. However, the mechanism that account for the association between the low TC level and unfavorable outcome in patients with NSCLC remains unclear. So far, as we know, cholesterol plays a very important role in the maintenance of cell membrane and modulation membrane fluidity and function, including transmembrane signaling and cell adhesion to the extracellular matrix. Xu et al found out that the proliferating cancer cells depends on either de novo synthesis in endoplasmic reticulum and acquisition from blood via low-density lipoprotein (LDL) receptor mediated endocytosis [23]. Increased LDL-receptor activity has been observed in patients with lung cancer [24]. Cancer cells need to secrete a hypocholesterolemic factor that stimulates LDL-receptor activity [25]. Moreover, serum TC level was associated with impairing the function of the immune system. For instance, Muldoon et al reported that compared with the high cholesterol group, patients with hypocholesterolemic had significantly fewer circulating lymphocytes, fewer total T cells, and fewer CD8+ cells [26]. Low serum cholesterol may be associated with low serum antioxidant reserve, possibly increasing susceptibility to oxidative stress [27]. As we know, chronic inflammatory was related with NSCLC development and progression. Inflammatory proteins, such as interleukin-6, interleukin-10 or CRP, are reported to be higher in patients with hypocholesterolemia [28]. Additional, an elevated serum interleukin-6 level is reported to be associated with a decreased serum TC level [29]. In our study, preoperative serum TC level was inversely related with patients smoking status. Furthermore, a strong correlation between preoperative serum TC level and CRP was found. Thus, we infer that one possible mechanism is that hypocholesterolemia is an epiphenomenon of the systemic inflammatory response. Based on these findings, the impact of cholesterol on human immune system and inflammatory response may influence the tumor cell proliferation and metastasis. However, further experiments are needed to clarify the mechanism of this association.
This study highlighted important clinical implications of the preoperative serum TC level in patients with operable NSCLC. Patients with low serum TC level exhibited with favorable outcome. However, this study has many limitations. First of all, our study was a retrospective study, and information on post-treatment recurrence was insufficient. Second, the change of serum TC level during the follow-up was not recorded. Furthermore, median follow-up time of 26 months was relatively short, although DFS and OS were significant difference between patients with low serum TC level and those with hypercholesterolemia. A prospective study is required to determine the prognostic and treatment value of cholesterol.
In summary, we found that preoperative serum TC level was related with TNM staging and actually was useful in the identification and follow-up of high-risk groups of recurrence in operable NSCLC patients. Both univariate and multivariate analyses indicated that a low preoperative serum TC level was associated with a shorter DFS and OS. Therefore, consideration of the preoperative serum TC level might provide additional prognostic information for patients with operable NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Peters S, Weder W, Dafni U, Kerr KM, Bubendorf L, Meldgaard P, O’Byrne KJ, Wrona A, Vansteenkiste J, Felip E, Marchetti A, Savic S, Lu S, Smit E, Dingemans AM, Blackhall FH, Baas P, Camps C, Rosell R, Stahel RA ETOP Lungscape Investigators. Lungscape: Resected non-small-cell lung cancer outcome by clinical and pathological parameters. J Thorac Oncol. 2014;9:1675–1684. doi: 10.1097/JTO.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40:716–722. doi: 10.1016/j.ctrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Graziano SL, Lacas B, Vollmer R, Kratzke R, Popper H, Filipits M, Seymour L, Shepherd FA, Rosell R, Veillard AS, Taron M, Pignon JP. Cross-validation analysis of the prognostic significance of mucin expression in patients with resected non-small cell lung cancer treated with adjuvant chemotherapy: Results from ialt, jbr. 10 and anita. Lung Cancer. 2013;82:149–155. doi: 10.1016/j.lungcan.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leong D, Rai R, Nguyen B, Lee A, Yip D. Advances in adjuvant systemic therapy for non-small-cell lung cancer. World J Clin Oncol. 2014;5:633–645. doi: 10.5306/wjco.v5.i4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia M. Biomarkers for personalized oncology: Recent advances and future challenges. Metabolism. 2015;64:S16–21. doi: 10.1016/j.metabol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: Is kras a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J. Clin. Oncol. 2010;28:4769–4777. doi: 10.1200/JCO.2009.27.4365. [DOI] [PubMed] [Google Scholar]
- 8.Riedel S, Abel S, Swanevelder S, Gelderblom WC. Induction of an altered lipid phenotype by two cancer promoting treatments in rat liver. Food Chem Toxicol. 2015;78:96–104. doi: 10.1016/j.fct.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Freter C. Lipid metabolism, apoptosis and cancer therapy. Int J MOL Sci. 2015;16:924–949. doi: 10.3390/ijms16010924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashmi S, Wang Y, Suman DS, Parhar RS, Collison K, Conca W, Al-Mohanna F, Gaugler R. Human cancer: Is it linked to dysfunctional lipid metabolism? Biochim Biophys Acta. 2015;1850:352–364. doi: 10.1016/j.bbagen.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Shekelle RB, Rossof AH, Stamler J. Dietary cholesterol and incidence of lung cancer: The western electric study. Am J Epidemiol. 1991;134:480–484. doi: 10.1093/oxfordjournals.aje.a116119. discussion 543-484. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Zheng W, Sellers TA, Kushi LH, Bostick RM, Potter JD. Dietary cholesterol, fat, and lung cancer incidence among older women: The iowa women’s health study (united states) Cancer Causes Control. 1994;5:395–400. doi: 10.1007/BF01694752. [DOI] [PubMed] [Google Scholar]
- 13.Shekelle RB, Tangney CC, Rossof AH, Stamler J. Serum cholesterol, beta-carotene, and risk of lung cancer. Epidemiology. 1992;3:282–287. doi: 10.1097/00001648-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Scali J, Astre C, Segala C, Gerber M. Relationship of serum cholesterol, dietary and plasma beta-carotene with lung cancer in male smokers. Eur J Cancer Prev. 1995;4:169–174. doi: 10.1097/00008469-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Everatt R, Virviciute D, Kuzmickiene I, Tamosiunas A. Body mass index, cholesterol level and risk of lung cancer in lithuanian men. Lung Cancer. 2014;85:361–365. doi: 10.1016/j.lungcan.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Siemianowicz K, Gminski J, Stajszczyk M, Wojakowski W, Goss M, Machalski M, Telega A, Brulinski K, Magiera-Molendowska H. Serum total cholesterol and triglycerides levels in patients with lung cancer. Int J Mol Med. 2000;5:201–205. doi: 10.3892/ijmm.5.2.201. [DOI] [PubMed] [Google Scholar]
- 17.Sok M, Ravnik J, Ravnik M. Preoperative total serum cholesterol as a prognostic factor for survival in patients with resectable non-small-cell lung cancer. Wien Klin Wochenschr. 2009;121:314–317. doi: 10.1007/s00508-009-1169-8. [DOI] [PubMed] [Google Scholar]
- 18.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallières E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The iaslc lung cancer staging project: Proposals regarding the relevance of tnm in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the tnm classification for lung cancer. J Thorac Oncol. 2009;4:1049–1059. [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: A consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 22.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: Fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mtor activation in endothelial cells. Proc Natl Acad Sci U S A. 2010;107:4764–4769. doi: 10.1073/pnas.0910872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitols S, Peterson C, Larsson O, Holm P, Aberg B. Elevated uptake of low density lipoproteins by human lung cancer tissue in vivo. Cancer Res. 1992;52:6244–6247. [PubMed] [Google Scholar]
- 25.Shiroeda O, Yamaguchi N, Kawai K. Stimulation of low density lipoprotein receptor activity by conditioned medium from a human cancer cell line. Cancer Res. 1987;47:4630–4633. [PubMed] [Google Scholar]
- 26.Muldoon MF, Marsland A, Flory JD, Rabin BS, Whiteside TL, Manuck SB. Immune system differences in men with hypo- or hypercholesterolemia. Clin Immunol Immunopathol. 1997;84:145–149. doi: 10.1006/clin.1997.4382. [DOI] [PubMed] [Google Scholar]
- 27.Muldoon MF, Kritchevsky SB, Evans RW, Kagan VE. Serum total antioxidant activity in relative hypo- and hypercholesterolemia. Free Radic Res. 1996;25:239–245. doi: 10.3109/10715769609149049. [DOI] [PubMed] [Google Scholar]
- 28.Liao C, Yu Z, Guo W, Liu Q, Wu Y, Li Y, Bai L. Prognostic value of circulating inflammatory factors in non-small cell lung cancer: A systematic review and meta-analysis. Cancer Biomark. 2014;14:469–481. doi: 10.3233/CBM-140423. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, Nakagawa K, Oya M, Ohigashi T, Murai M. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007;69:113–117. doi: 10.1016/j.urology.2006.09.039. [DOI] [PubMed] [Google Scholar]


