Abstract
Hepatocellular carcinoma (HCC) is the most common tumor in worldwide and chemotherapy resistant is a severe obstacle in HCC treatment. Annonaceous acetogenins was a nature compound from Uvaria accuminata and it has show the anti-tumor proliferation activity in many types cancer. In this study, we showed that annonaceous acetogenins is correlated with the drug resistance reversal in human hepatocellular carcinoma BEL-7402/5-FU and HepG2/ADM cell lines. We found that cell apoptosis was improved and cell cycle was arrested, further, multidrug-resistance proteins such as MDR1, MRP1, Topo-IIα, GST-π, cyclin D1, Survivin and bcl-2 are down-regulated, however, intracellular Rh-123 and caspase-3/8 was up-regulated by Annonaceous acetogenins treatment. We also found that there was a decreased activity of NF-κB and Akt in Annonaceous acetogenins treatment groups. Therefore, we demonstrate that Akt/NF-κB pathway was involved in Annonaceous acetogenins reverses drug resistance of human hepatocellular carcinoma cells.
Keywords: Annonaceous acetogenins, hepatocellular carcinoma, drug resistance
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers and now it is the third leading cause of cancer deaths in worldwide [1]. The incidence of hepatocellular carcinoma is highest in Asia and Africa, with an estimated half million new cases diagnosed per year globally, and more than 50% of whom are in China [2]. Hepatocellular carcinoma is generally less sensitivity to chemotherapeutic agents and approximately 67% hepatoma patients treated with chemotherapeutic agents develop rapidly acquired resistance [3]. Multi-drug resistance (MDR) refers to the tumor cells with anticancer drug resistance. MDR is one of the major obstacles in HCCs successful chemotherapy treatment [4]. Thus, development of new chemotheraputic drugs reversal of MDR is essential in treating MDR of HCC.
Variety of natural products have been shown to be excellent and reliable sources for pharmaceutical development, such as Paclitaxel and Curcumin [5,6]. Annonaceous acetogenins (ACGs) are a class of natural polyketides isolated from Annonaceous plants growing in tropical and subtropical regions, which show favorable efficacy in variety of human cancer cell lines in vitro [7]. They have been reported to potently inhibit the activity of NADH-ubiquinone oxidoreductase of the mitochondrial electron transport system [8]. Annonaceous acetogenins phytochemical and pharmacological studies have further revealed a novel therapeutic role as an anticancer agent for these natural products.
Here, we have utilized drug-resistant human hepatocellular carcinoma BEL-7402/5-FU and HepG2/ADM cell lines to investigate the potential reversal drug resistance of human hepatocellular carcinoma, and the nature of the mechanism responsible for reversal multi-drug resistance.
Materials and methods
Cell lines and culture
Human hepatocellular carcinoma cell line HepG2 and BEL-7402 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and mainained in DMEM medium. 5-fluorouracil (5-FU)-selected drug-resistant BEL-7402/5-FU and adriamycin resistant HepG2/ADM cell line was obtained from Nanjing Keygen Biotech. Co. (Nanjing, China). Cells were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin at in 37°C with 5% CO2 incubator.
MTT assay
BEL-7402/5-FU and HepG2/ADM cell lines were plated in 96-well plates respectively. After over-night incubation, cells were treated with various concentrations of Annonaceous acetogenins. After treated for 72 h, 20 μL MTT solution was added to each well. Incubate in 37°C for 4 h, the supernatant was re moved and 100 μL DMSO was added to each well. Samples were then shaken for 15 min. The optical density (OD) was read a t the wavelength of 540 nm. All experiments were performed in triplicates. Each treatment was performed in sextuplicate and each experiment was repeated three times. The half-maximal inhibitory concentration (IC50) was defined as the concentration that caused 50% inhibition of cell proliferation.
Reverse transcriptase PCR
Total RNA was extracted from cells using Trizol Reagent according to the manufacturer’s protocol. RNA was reverse-transcribed using reverse Transcription System (Promega, USA). Primers were obtained from Sangon Biotech (shanghai, China) and the primers sequences were listed in Table 1. The amount of PCR product formed in each cycle was evaluated on the basis of SYBR Green I fluorescence. All amplification reactions were performed using the ABI7500 PCR system with the following cycle conditions: one cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. The mRNA level for e ach gene was normalized to GAPDH.
Table 1.
Primer lists of real-time PCR
| Gene | Sequences (5’-3’) | Tm (°C) |
|---|---|---|
| MRD1 | Forward: GAGCCCATCCTGTTTGACTG | 60 |
| Reverse: GCTGCCCTCACAATCTCTTC | ||
| MRP1 | Forward: GTGGAATTCCGGAACTAC | 58 |
| Reverse: CGGAGGTCGTGCAGGCCG | ||
| Top-IIα | Forward: GGCTCGATTGTTATTTCCAC | 60 |
| Reverse: GGTTGTAGAATTAAGAATAGG | ||
| GST-π | Forward: CTGGAAGGAGGAGGT GGTG | 58 |
| Reverse: GACGCAGGATGGTATTGGAC | ||
| Cyclin D1 | Forward: CAA TGACCCCGCACGATTTC | 60 |
| Reverse: CATGGAGGGCGGATTGGAA | ||
| GAPDH | Forward: GGAGCGAGATCCCTCCAAAAT | 60 |
| Reverse: GAAGATGGTGATGGGATTTC |
Apoptosis assay in vitro
Cells were incubated by 5-fluorouracil for BEL-7402/5-FU cells and adriamycin for HepG2/ADM cells with Annonaceous acetogenins for 24 hr. The percentage of apoptosis was determined by AnnexinV-FITC Apoptosis Detection kit (BD Biosciences) following the manufacture’s instruction. Briefly, cells were washed with ice-cold PBS and resuspended in binding buffer. Annexin V-FITC and PI (BD Biosciences) were added to cells and incubated at room temperature in the dark for 15 min, and then the cells were analyzed by BD FACS can flow cytometer with 488 nm excitation wavelength and 525 nm emission wavelength.
Cell cycle distribution analysis
The cells were seeded in 6-wellplates (4 × 105/well). For synchronization experiment, cells were treated with 100 ng/mL of nocodazole for 24 h at 37°C prior to beng treated with 10 μM Annonaceous acetogenins. Following 24 h treatment, floating cells were combined with attached cells, then subjected to centrifugation at 3,000 rpm for 5 min at 4°C. The cell pellets were washed with cold PBS, then fixed in ethanolat -20°C. The cells were washed with PBS, rehydrated and resus-pended in 0.2 mL of RNase A in PBS buffer at 37°C for 30 min. The cells were stained with 0.3 mL propidium iodide in a solution containing 0.1% Triton X-100, 0.1 mM EDTA. Samples were analyzed on a BD FACS can flow cytometer and the percentage of cells in the S, G0-G1, and G2-M phases of the cell cycle was determined using WinMDI 2.8.
Western blot analysis
Treated cells (3 × 106) were harvested by cell scraping, washed once in cold PBS and lysed in 300 μl RIPA lysis buffer (50 mM Tris pH = 8.0, 150 mM NaCl, 0.1% SDS, 1% NP40) containing one tablet of Protease Inhibitor Cocktail (Roche Applied Sciences). Lysates were sonicated and centrifuged at 12,000 r/min at 4°C for 10 min.Protein concentrations were determined by bicinchoninic acid (BCA) protein assay kit. Per lane 40 μg of whole cell lysate was separated using 10% SDS-acrylamide gels, and transferred on PVDF membranes (Amersham Biosciences) blocked by incubation with 5% bovine serum albumin in tween-tris-buffered saline (TTBS) at room temperature for 1 hr. Following incubated overnight at 4°C with different primary antibodies [MDR1, 1:500; MDP1, 1:500; Topo-IIα, 1:200; GST-π, 1:200; Bcl-2, 1:400; Cyclin D1, 1:500; Survivin, 1:400 (all obtained from Santa Cruz); Cleaved caspase-3, 1:1000; Cleaved caspase-8, 1:1000 (from Sigma); β-actin, 1:5,000 (from Sigma)] in 5% fat-free milk prepared in TBST. After rinsing three times in TBST, filters were incubated with secondary antibodies for 1 h at room temperature in TBST containing 1% fat-free milk. After washing, the blots were visualized by horseradish peroxidase (HRP) Western Blot Detection System.
Rhodamine-123 exclusion assay
The cells were seeded in 6-wellplates and incubated with Annonaceous acetogenins for 24 h. They were trypsinized and resuspended at 1 × 106/mL, then 10 μl of the substrate Rh-123 (1 mg/ml) were added to the samples and the cells were incubated for 20 min at 37°C. After washed twice and resuspended in 0.5 ml of PBS, the samples were then immediately detected by flow cytometry. Data were analyzed using FCS Express V3 (De Novo Software).
NF-κB DNA binding activity assay
NF-κB p65 activity was assayed by reporter gene system according to the instruction manual with moderate modification. Briefly, the assays were performed in a 96-well plate. The cells (1.5 to 2 × 106) were seeded into each well in triplicate for 24 h, then transfected each well of the cells with 0.1 μg pGL4.32 [luc2P/NF-kB-RE/Hypro] plasmids (Promega). After 24 h of transfection, the luciferase activity was analyzed by Bright-GloTM Luciferase Assay System (Promega).
Statistical analysis
Quantitative data are presented as the mean ± SD determined from the indicated number of experiments. Statistical analysis was based on one-way ANOVA for comparison of multiple groups.
Result
Cytotoxicity of annonaceous acetogenins
The viability of BEL-7402/5-FU and HepG2/ADM cells were treated with Annonaceous acetogeninsat different concentrations (0, 1, 2, 5, 10, 20, 50 and 100 μM) for 72 h was detemined by MTT assay. Annonaceous acetogenins could inhibited the growth of BEL-7402/5-FU and HepG2/ADM cell lines in a dose-dependent manner in vitro. IC50 of BEL-7402/5-FU cells was 73.7 µg/ml and IC50 of HepG2/ADM cells was 83.7 µg/ml by Annonaceous acetogenins. The results also showed that 10 µg/ml Annonaceous acetogenins was not cytotoxic (inhibition rate < 5%) and 20 µg/ml Annonaceous acetogenins was weakly cytotoxic (inhibition rate 10-15%) for all of BEL-7402/5-FU and HepG2/ADM cell lines. Thus, treatment concentrations of 10 µg/ml and 20 µg/ml Annonaceous acetogenins were chosen to study reversal effect of Annonaceous acetogenins on MDR in BEL-7402/5-FU and HepG2/ADM cells. We found that the IC50 of BEL-7402/5-FU cells by 5-fluorouracil was 311.4 µg/ml and IC50 of BEL-7402/5-FU cells with by 5-fluorouracil 10 µg/ml and 20 µg/ml Annonaceous acetogenins were 22.7 µg/ml and 12.3 µg/ml, the reversal fold (RF = IC50 of MDR cell/IC50 of treatment cell) were 13.7 fold and 25.3 fold, meanwhile, the IC50 of HepG2/ADM cells by adriamycin was 75.7 µg/ml and IC50 of HepG2/ADM cells with by adriamycin 10 µg/ml and 20 µg/ml Annonaceous acetogenins were 7.6 µg/ml and 5.7 µg/ml, the reversal fold (RF = IC50 of MDR cell/IC50 of treatment cell) were 9.7 fold and 13.3 fold.
Annonaceous acetogenins downregulated the expression of MDR1 and MRP1
MDR frequently results from the incongruous expression of drug-resistant genes, such as multi-drug-resistance gene (MDR1). MDR1 and MRP1 are two major membrane transporter proteins that involved in efflux activities and lead to multiple drug resistance [9]. So we would like to investigate whether Annonaceous acetogenins could downregulate the expression of these proteins to restore the intercellular accumulation of Rhodamine-123. RT-PCR and western blotting results showed that, oppose to the high expression level observed in the parental cells, the MDR1 and MRP1 were decreased in BEL-7402/5-FU and HepG2/ADM cells (Figure 1). Rh-123 is a substrate for P-gp and commonly used as an indicator for evaluating the activities of P-gp. Thus, the activity of the P-gp drug pump can be gauged by the degree of intracellular accumulation of Rodamine-123, which can in turn be determined by the measurement of intracellular fluorescence [10]. So rhodamine-123 accumulation was detected by flow cytometry. As expect, Annonaceous acetogenins could increased the intracellular accumulation of rhodamine-123 (result did not show).
Figure 1.
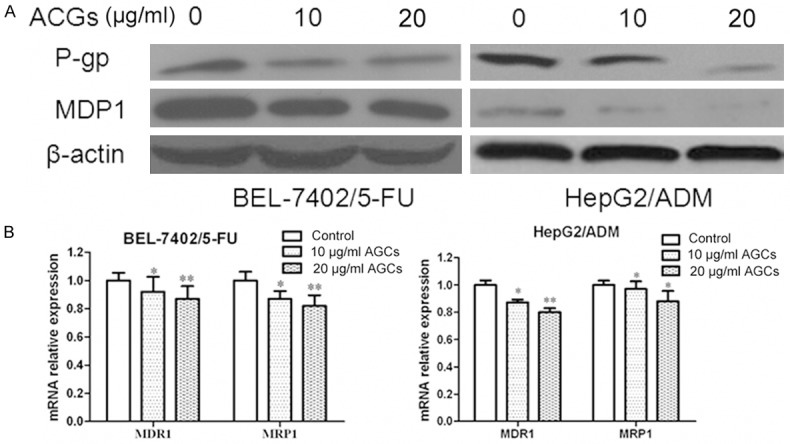
Inhibitory effect of Annonaceous acetogenins on expression of MDR1 and MRP1. A. Western blot analysis of protein extracts obtained from BEL-7402/5-FU and HepG2/ADM cells; B. mRNA expressions of MDR1 and MRP1 genes in BEL-7402/5-FU and HepG2/ADM cells. Data presented are means ± SD values, N = 3. Bars indicate SD. Significant differences from control were indicated by P<0.05 (*) and P<0.01 (**).
Annonaceous acetogenins reverse the MDR by inhibition of other MDR related genes expression
Except MDR1 and MRP1, other MDR related genes might also get involved in MDR of HCC. Topoisomerase IIα (Topo IIα) is an essential nuclear enzyme with a role in the maintenance of DNA topology. Topo IIα is a target for several anticancer drugs and the activity of topoisomerase IIα is important in the development of drug resistance [11]. GST-π is a multifunctional enzyme that plays a critical role in cellular detoxification by catalyzing the conjugation of reduced glutathione to hydrophobic electrophilic compounds and may influence mutagenesis and carcinogenesis [12]. In addition, GST-π is the most important phase II drug-metabolizing enzyme and is involved in the metabolism and detoxification of environmental carcinogens and chemotherapeutics [13]. We next treated BEL-7402/5-FU and HepG2/ADM cells with Annonaceous acetogenins at the concentration of 10 µg/ml and 20 µg/ml for 24 h. Western blot and Real time PCR was used to test genes expression. Topoisomerase IIα (TopoIIα) and glutathione S-transferase π (GST-π) genes, were observed down-regulated in BEL-7402/5-FU and HepG2/ADM cells after Annonaceous acetogenins incubation (Figure 2).
Figure 2.
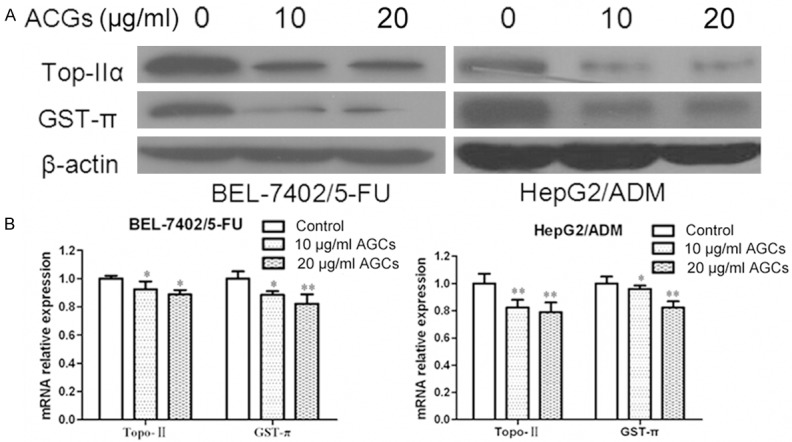
Inhibitory effect of Annonaceous acetogenins on expression of TopoIIα and GST-π. A. Western blot analysis of protein extracts obtained from BEL-7402/5-FU and HepG2/ADM cells; B. mRNA expressions of TopoIIα and GST-π genes in BEL-7402/5-FU and HepG2/ADM cells. Data presented are means ± SD values, N = 3. Bars indicate SD. Significant differences from control were indicated by P<0.05 (*) and P<0.01 (**).
Annonaceous acetogenins affect cell cycle distribution and downreagulated the expression of cyclin D1
Cell cycle phase distribution was detected by flow cytometry to determine whether there is any difference in cell cycle kinetics between BEL-7402/5-FU and HepG2/ADM cells and their parental cells. BEL-7402/5-FU and HepG2/ADM cells incubated in Annonaceous acetogenins at the concentration of 10 µg/ml and 20 µg/ml for 24 h resulted in had a significant increase in G2/M phase and decrease in the G0/G1 phase compared with control group phase.
Cyclin D1 function as regulators of CDK4 or CDK6, is required for cell cycle G1/S transition, is very important in cell cycle progression [28]. To search for the indication of mechanisms involved in cell cycle,we would like to investigate whether Annonaceous acetogenins could affect cyclin D1 experssion. Real-time PCR and western blotting results showed that, the expression level of cyclin D1 in BEL-7402/5-FU and HepG2/ADM cells obviously decreased compared to their parental cells. These data suggested that Annonaceous acetogenins affect cell cycle distribution may by inhibition of cyclin D1 expression (Figure 3).
Figure 3.
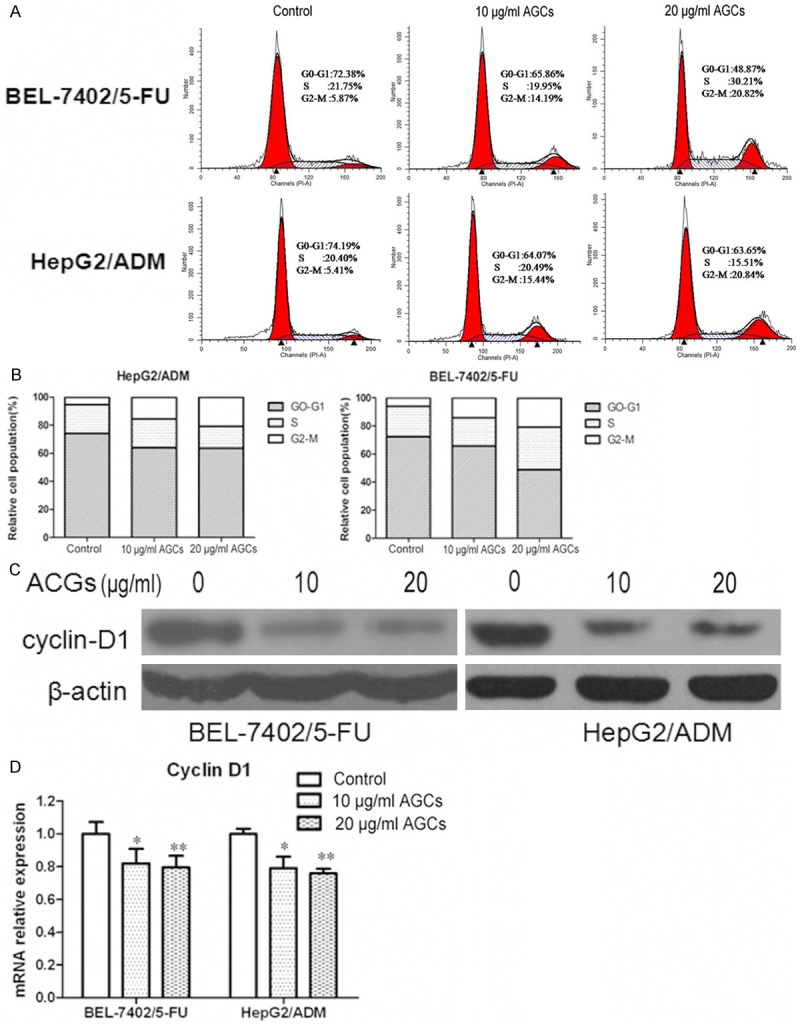
Annonaceous acetogenins induced the G2/M phase arrest and regulated cell cycle related proteins in BEL-7402/5-FU and HepG2/ADM cells. A. Cells were treated with 10 µg/ml and 20 µg/ml Annonaceous acetogenins for 24 h. PI staining was used to analyze the cell cycle distribution; B. Histogram of cell cycle distribution of BEL-7402/5-FU and HepG2/ADM. C. Western blot analysis of protein extracts obtained from BEL-7402/5-FU and HepG2/ADM cells; D. mRNA expressions of cyclin D1 in BEL-7402/5-FU and HepG2/ADM cells. Data presented are means ± SD values, N = 3. Bars indicate SD. Significant differences from control were indicated by P<0.05 (*) and P<0.01 (**).
Annonaceous acetogenins induced cell apoptosis and downreagulated the expression of Survivin and Bcl-2
To investigate whether the growth inhibition of Annonaceous acetogenins was caused by apoptosis, annexin V-FITC/PI double staining was employed. The percentage of apoptotic cells was 13.5% and 21.3% in Control group of BEL-7402/5-FU and HepG2/ADM cells, respectively. When exposed to 10 µg/ml and 20 µg/ml of Annonaceous acetogenins for 24 h, the percentage increased to 45.9% and 65.5%, 61.4% and 75.8%, respectively. In summary, these results demonstrated that Annonaceous acetogenins treatment raised the apoptosis percentage of BEL-7402/5-FU and HepG2/ADM cells.
Survivin, a member of the family of inhibitor of apoptosis proteins, functions as a key regulator of mitosis and programmed cell death. Survivin expression is also highly regulated by the cell cycle and is only expressed in the G2-M phase [14]. Bcl-2 family proteins regulate and contribute to programmed cell death or apoptosis.Bcl-2 suppresses apoptosis in a variety of cell systems including factor-dependent lymphohematopoietic and neural cells [15]. Regulates cell death by controlling the mitochondrial membrane permeability. We are in great interest to know if these proteins were downreagulated by Annonaceous acetogenins in BEL-7402/5-FU and HepG2/ADM cells. The western blotting results showed that these proteins obviously decreased when treated with Annonaceous acetogenins. Real-time PCR analyses results were in concord with those from western blotting (Figure 4).
Figure 4.
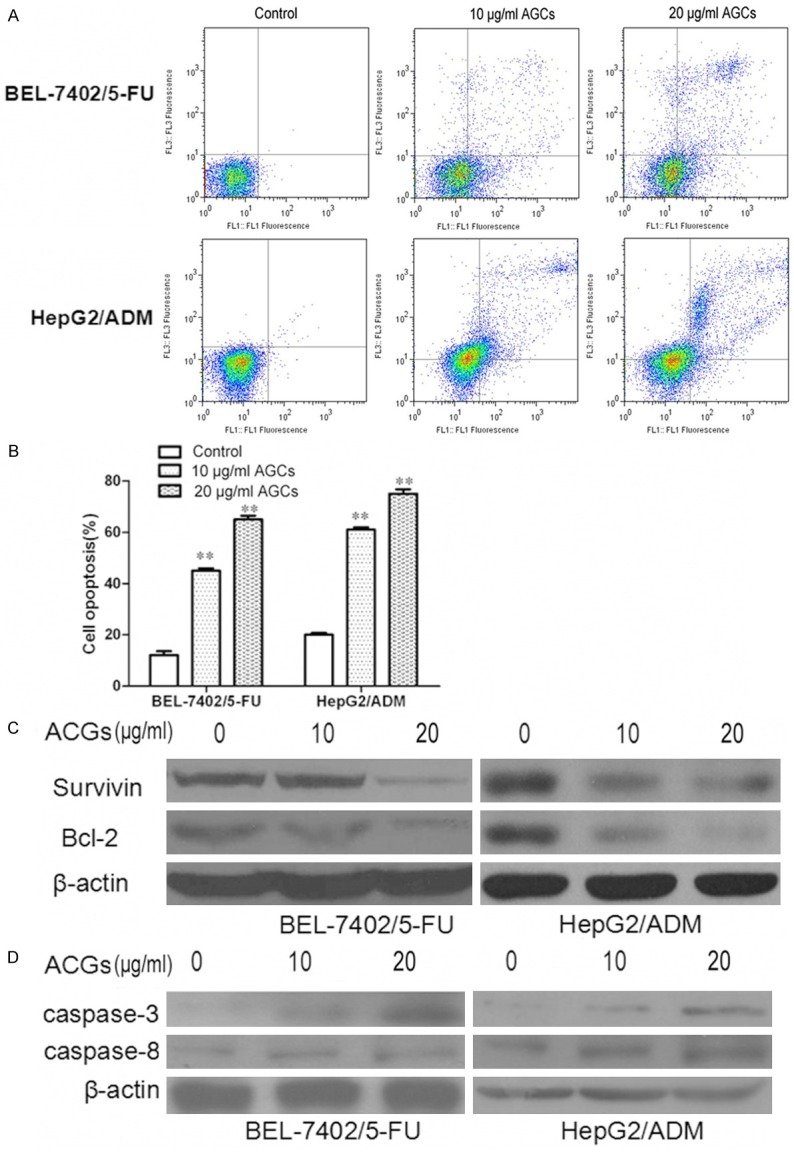
Annonaceous acetogenins induced cell opoptosis in BEL-7402/5-FU and HepG2/ADM cells. A. Cells were treated with 10 µg/ml and 20 µg/ml Annonaceous acetogenins for 24 h. Annexin V-FITC/PI analysis by FCM was used to analyze the percentage of apoptotic cells in BEL-7402/5-FU and HepG2/ADM cells; B. Histogram of the percentage of apoptotic cells in BEL-7402/5-FU and HepG2/ADM cells. C. The expression of survivin and Bcl-2 proteins were determined by western blot in BEL-7402/5-FU and HepG2/ADM cells; D. mRNA expressions of survivin and Bcl-2 in BEL-7402/5-FU and HepG2/ADM cells. Data were presented as the mean ± SD and were representative of an average of three independent experiments per concentration. Significant differences from untreated control are indicated by P<0.05 (*) and P<0.01 (**).
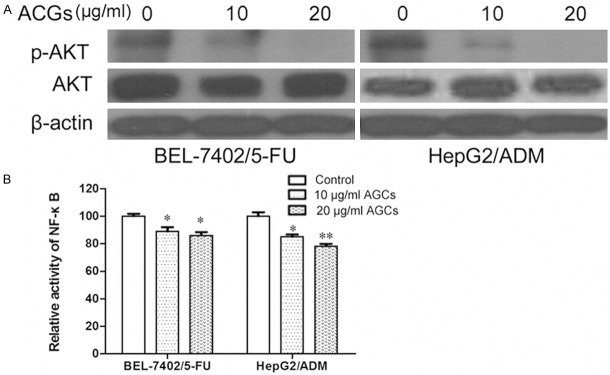
Annonaceous acetogenins inhibits AKT/NF-κB signaling pathway in BEL-7402/5-FU and HepG2/ADM
It has been reported that activation of NF-κB followed by Akt phosphorylation plays a role in regulation of cell survival, apoptosis and drug resistance [16]. We wondered if intracellular AKT/NF-κB signaling pathway was involved in the action of Annonaceous acetogenins. To determine whether Annonaceous acetogenins could alleviate drug resistance via the AKT/NF-κB signaling pathway, BEL-7402/5-FU and HepG2/ADM cells were cultured in the presence of Annonaceous acetogenins, Then ex-pressions of the proteins AKT and NF-κB were evaluated. Results indicated that Annonaceous acetogenins could decrease phosphorylation of AKT, but didn’t affect Total-Akt expression. And the expression levels of NF-κB were also decreased by ACGs (Figure 5). These results suggest that Annonaceous acetogenins in-duced apoptosis results in inactivation of NF-κB via Akt/NF-κB pathway.
Figure 5.
Annonaceous acetogenins affect on AKT/NF-κB Signal Pathway Molecules in BEL-7402/5-FU and HepG2/ADM cells. A. The total cell lysate was separated by SDS-PAGE gel followed by Western blot with anti-p-Akt antibody, anti-total-Akt antibody. The experiment shown is epresentative of three independent experiments with similar results; B. Cells were transfected with pNF-κB-luc and the transcriptional activation was detected by luciferase reporter assay. Data were presented as the mean ± SD and were representative of an average of three independent experiments per concentration. Significant differences from untreated control are indicated by P<0.05 (*) and P<0.01 (**).
Discussion
Hepatocellular carcinoma is the fifth most common malignancy worldwide with a poor long time survival. Multidrug resistance (MDR) is one of the major obstacles in the chemotherapeutic treatment of many human cancers [17]. Although, people have been devoted to search for effective and clinically applicable MDR modulators in the past three decades and various mechanisms have been proposed to explain the multidrug resistance of hepatocellular carcinoma, they are complicated and remain unclear [18]. According to reports, the mechanism of resistance to chemotherapy may be associated with the classical drug-resistant pathway, which is mediated by P-gp, and the inhibition on cell apoptosis, are the two mostly investigated mechanisms in research for MDR of tumor. To find more effective, low-toxicity modulators to inhibit MDR become more and more urgent. In this study, we found that drug resistance of BEL-7402/5-FU and HepG2/ADM cells were reversed by Annonaceous acetogenins.
Annonaceous acetogenins (ACGs) are a class of natural polyketides isolated from Annonaceous plants, which show significant cytotoxicity to human cancer cell lines in vitro. However, reports on whether ACGs can reverse thechemo therapeutic drug resistance in hepatocellular carcinoma are rare. In order to investigate the reversal effect of ACGs on BEL-7402/5-FU and HepG2/ADM cells, we chose the weakly cytotoxic concentrations of 10 μM. Firstly, cell viability was detected by MTT. The results showed that the proliferation of drug-resistant cell line BEL-7402/5-FU and HepG2/ADM was significantly inhibited after cells were pretreated with ACGs. FCM results showed that ACGs could promote cell apoptosis in drug-resistant cell line BEL-7402/5-FU and HepG2/ADM cells. Western blotting results showed that the expression of P-gp, MRP1, cyclin D1, Bcl-2, Survivin, NF-κB, phosphorylation of Akt were decreased in drug-resistant cell line BEL-7402/5-FU and HepG2/ADM compared with their control group. Based on this, we further investigated the potential molecule mechanism of reversing the resistance effect of chemotherapeutic drug resistance hepatocellular carcinoma cell line BEL-7402/5-FU and HepG2/ADM by Annonaceous acetogenins, possibly it is via decreasing the function of P-gp and the mitochondrial apoptosis pathway.
P-glycoprotein (P-gp), an ATP-binding cassette (ABC) transporter, which is a 170-kD plasma membrane glycoprotein encoded by the MDR1 gene, is capable of reducing the intracellular accumulation of drugs by excluding a drug outside the cells [19]. P-gp can induce drug resistance due to decrease in the cellular chemotherapeutic drug under effective concentration, through pumping the chemotherapeutic agents out of the cells against the concentration. Its up-regulation helps cancer cells to augment cell survival against the drug and resist the cytotoxic effects of anti-cancer agents. MRP1 is another major membrane transporter protein that involved in efflux activities and lead to multiple drug resistance [20]. Moreover, the ubiquitous NF-κB transcription factor has been reported to induce drug resistance in cancer cells. The inhibition of NF-κB reduced MDR1 mRNA and P-glycoprotein expression in HCT15 cells [21]. The NF-κB pathway responds actively to MDR1 induction due to its activation by the generation of reactive oxygen species, the activation of IκB kinase, and the degradation of IκB [22]. Besides, NF-κB is bound at nucleotide position-159 of the MDR1 promoter mediating the transcription of MDR1, which suggest NF-κB in the regulation of the MDR1 gene expression in cancer cells and in drug resistance. In our study, the MDR1 and MRP1 expression both in protein and in mRNA level detected by western blot and RT-PCR, and down regulations of MDR1, MRP1 and NF-κB were exhibited after treated with Annonaceous acetogenins. Rh-123 is a substrate of P-gp and can be used as an indicator for testing the P-gp functional activities. The intracellular Rh-123 fluorescence was increase in BEL-7402/5-FU and HepG2/ADM cells treated with ACGs, which perhaps due to the down-regulation of P-gp pump. Thus, we can conclude that ACGs reverses MDR by inhibiting the cellular efflux function of P-gp and the expression of MDR1 and MRP1 both in transcription and translation.Apart from MDR1 and MRP1, other molecules may involved in the tumor resistance. So we also detected the expression levels of Topo-IIα and GST-π after treated with Annonaceous acetogenins.
Another mechanisms of drug resistance refer to insensitivity to drug-induced apoptosis. Cell cycle is closely related with the tumor cell proliferation. There is considerable evidence that the anticancer agent suppressed cell cycle progression in different phases and then induced cell apoptosis. In our experiment, Annonaceous acetogenins (ACGs) could caused cell cycle arrest in G2-M phase, accompanied with a decrease in the G0/G1 phase compared with control group. We also confirmed that ACGs induced apoptosis BEL-7402/5-FU and HepG2/ADM cells when treated with 10 µg/ml and 20 µg/ml ACGs for 24 h. These results implied that ACGs induce the apoptosis of BEL-7402/5-FU and HepG2/ADM cells by inhibiting cell cycle progression to reverse drug resistance.
Cyclin D is a member of the cyclin protein family that is involved in regulating cell cycle progression. Suppression of cyclin D1 levels has been shown to potentiate the drug sensitivity of cancer cells [23]. Survivin is the smallest member of the Inhibitor of Apoptosis (IAP) gene family and downregulation of survivin induce apoptosis in tumor cells. Bcl-2, is the founding Bcl-2 family of regulator proteins that regulate apoptosis. Most cellular Bcl-2 is bound to the outer mitochondrial membrane. The down-regulation of Bcl-2 can induce the release of cytochrome-C (cytosol) from mitochondria, trigger the activation of caspases and finally cause cell apoptosis [24]. caspase-3 is an important regulating gene and plays a key role in apoptosis, which cleaves and activates caspases 6, 7, and interacts with caspase-8 and caspase-9. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis [25]. In our experiment, we found that the expression of cyclin D1 and Bcl-2 in BEL-7402/5-FU and HepG2/ADM was significantly lower than their parental cells when treated with ACGs. Suggesting that the mitochondrial receptor pathway may be involved in the formation of drug resistance. ACGs could reverse the chemotherapeutic drug resistant of BEL-7402/5-FU and HepG2/ADM via down-regulation of Cyclin D1, Survivin and Bcl-2 levels.
It is well established that cyclin D1 is a NF-κB target gene. NF-κB can upregulate expression of cyclin D1, thus promoting Rb hyperphosphorylation and cell cycle progression. As known, down-regulation of anti-apoptotic protein is one of the mechanisms that NF-κB takes part in apoptosis and induces apoptosis. The activation of NF-B followed by Akt phosphorylation plays a role in the development of insulin resistance. Akt is also involved in the regulation of NF-kB and apoptosis [26]. Constitutive activation of NF-kB induced by Akt may play a major role in the chemoresistant cells [27]. We postulated that the intracellular AKT/NF-kB signaling pathway was involved in the action of Annonaceous acetogenins. To see whether Akt and NF-kB are the targets of ACGs, their activation in BEL-7402/5-FU and HepG2/ADM cells treated with ACGs was measured. The results showed that Akt phosphorylation and NF-kB transcriptional activation were inhibited by ACGs. And NF-κB was attenuated by Akti-1/2 (an AKT inhibitor) which implies that Akt may play a role in Annonaceous acetogenins. This suggests that ACGs reverses MDR through suppression of the Akt/NF-kB pathway.
In conclusion, Annonaceous acetogenins can effectively reverse the chemotherapeutic drug resistant of BEL-7402/5-FU and HepG2/ADM. The potential mechanisms refer to multiple aspects including inhibition of cell growth, induction of apoptosis and cell cycle arrest, accumulation of rhodamine-123 and decreased expression of P-gp, MRP1, Cyclin D1, Survivin and Bcl-2. Meanwhile, the inhibition of the Akt/NF-kB pathway is a key role in how ACGs functions.
Disclosure of conflict of interest
None.
References
- 1.Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer. 2012;3-4:226–237. doi: 10.1159/000343837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn KA, London WT. The Global Epidemiology of Hepatocellular Carcinoma: Present and Future. Clin Liver Dis. 2011;2:223–243. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CL, Lim YP, Hu ML. Fucoxanthin attenuates rifampin-induced cytochrome P450 3A4 (CYP3A4) and multiple drug resistance 1 (MDR1) gene expression through pregnane X receptor (PXR)-mediated pathways in human hepatoma HepG2 and colon adenocarcinoma LS174T cells. Mar Drugs. 2012;1:242–57. doi: 10.3390/md10010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009;17:2708–2710. doi: 10.4161/cc.8.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Liu XJ, Yang P, Zhao M, Lv LX, Zhang GD, Wang Q, Zhang L. Alkylglyceronephosphate synthase (AGPS) alters lipid signaling pathways and supports chemotherapy resistance of glioma and hepatic carcinoma cell lines. Asian Pac J Cancer Prev. 2014;7:3219–26. doi: 10.7314/apjcp.2014.15.7.3219. [DOI] [PubMed] [Google Scholar]
- 6.Nabekura T. Overcoming multidrug resistance in human cancer cells by natural compounds. Toxins. 2010;6:1207–1224. doi: 10.3390/toxins2061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang GR, Jiang S, Wu YL, Jin Y, Yao ZJ, Wu JR. Induction of cell death of gastric cancer cells by a modified compound of the annonaceous acetogenin family. Chembiochem. 2003;11:1216–1221. doi: 10.1002/cbic.200300677. [DOI] [PubMed] [Google Scholar]
- 8.Landolt JL, Ahammadsahib KI, Hollingworth RM. Determination of structure-activity relationships of Annonaceous acetogenins by inhibition of oxygen uptake in rat liver mitochondria. Chem Biol Interact. 1995;1:1–13. doi: 10.1016/0009-2797(95)03628-y. [DOI] [PubMed] [Google Scholar]
- 9.Wijnholds J. Drug resistance caused by multidrug resistance-associated proteins. Novartis Found Symp. 2012;243:69–79. [PubMed] [Google Scholar]
- 10.Seo SB, Hur JG, Kim MJ. TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases. Mol Cancer. 2010;9:199. doi: 10.1186/1476-4598-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng M, Wang L, Chen X, Cao R, Li P. The association between chemosensitivity and Pgp, GST-π and Topo II expression in gastric cancer. Diagn Pathol. 2013;8:198. doi: 10.1186/1746-1596-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou YT, Li K, Tian H. Effects of vinorelbine on cisplatin resistance reversal in human lung cancer A549/DDP cells. Asian Pac J Cancer Prev. 2013;8:4635–4639. doi: 10.7314/apjcp.2013.14.8.4635. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Konishi H, Ichikawa D, Arita T, Shoda K, Komatsu S, Shiozaki A, Ikoma H, Fujiwara H, Okamoto K, Ochiai T, Inoue J, Inazawa J, Otsuji E. Significance of GSTP1 for predicting the prognosis and chemotherapeutic efficacy in esophageal squamous cell carcinoma. Oncol Rep. 2013;4:1687–1694. doi: 10.3892/or.2013.2606. [DOI] [PubMed] [Google Scholar]
- 14.Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;3322:237–248. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;1:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L, Luo S, Jin C, Ma H, Zhou H, Jia L. FUT family mediates the multidrug resistance of human hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell Death Dis. 2013;4:e923. doi: 10.1038/cddis.2013.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao XD, Qu JH, Chang XJ, Lu YY, Bai WL, Wang H, Xu ZX, An LJ, Wang CP, Zeng Z, Yang YP. Hypomethylation of long interspersed nuclear element-1 promoter is associated with poor outcomes for curative resected hepatocellular carcinoma. Liver Int. 2014;1:136–146. doi: 10.1111/liv.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;2:159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem. 2009;36:4843–4857. doi: 10.2174/092986709789909620. [DOI] [PubMed] [Google Scholar]
- 20.Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;5:621–641. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 21.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;1:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 22.Deng L, Lin-Lee YC, Claret FX, Kuo MT. 2-acetylaminofluorene up-regulates rat mdr1b expression through generating reactive oxygen species that activate NF-kappa B pathway. J Biol Chem. 2001;1:413–420. doi: 10.1074/jbc.M004551200. [DOI] [PubMed] [Google Scholar]
- 23.Hochhauser D, Schnieders B, Ercikan-Abali E, Gorlick R, Muise-Helmericks R, Li WW, Fan J, Banerjee D, Bertino JR. Effect of cyclin D1 overexpression on drug sesitivity in a human fibrosarcoma cell line. J Natl Cancer Inst. 1996;18:1269–1275. doi: 10.1093/jnci/88.18.1269. [DOI] [PubMed] [Google Scholar]
- 24.Qi F, Inagaki Y, Gao B, Cui X, Xu H, Kokudo N, Li A, Tang W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011;102:951–958. doi: 10.1111/j.1349-7006.2011.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CD, Yuan CF, Bu YQ, Wu XM, Wan JY, Zhang L, Hu N, Liu XJ, Zu Y, Liu GL, Song FZ. Fangchinoline inhibits cell proliferation via Akt/GSK-3beta/cyclin D1 signaling and induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pac J Cancer Prev. 2014;2:769–773. doi: 10.7314/apjcp.2014.15.2.769. [DOI] [PubMed] [Google Scholar]
- 26.Sommermann TG, O’Neill K, Plas DR, Cahir-McFarland E. IKKβ and NF-κB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;23:7291–7300. doi: 10.1158/0008-5472.CAN-11-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;6:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Lisanti MP, Liao DJ. Reviewing once more the c-myc and Ras collaboration: converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle. 2011;1:57–67. doi: 10.4161/cc.10.1.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]