Abstract
Aim: Non-small cell lung carcinoma is the leading cause of cancer related to death in the world. Squamous cell lung carcinoma (SqCLC) is the second most frequent histological subtype of lung carcinomas. Recently, growth factors, growth factor receptors, and signal transduction system-related gene amplifications and mutations are extensively under investigation to estimate the prognosis and to develop individualized therapies in SqCLC. In this study, besides the signal transduction molecule phosphatidyl inositol-3-phosphate kinase (IP3K) p110α, we explored the expressions of fibroblast growth factor 2 (FGF2) and receptor-1 (FGFR1) in tumor tissue and also their clinical and prognostic significance in patients with early/advanced SqCLC. Materials and methods: From 2005 to 2013, 129 patients (23 early, 106 advanced disease) with a histopathological SqCLC diagnosis were selected from the hospital files of Cukurova University Medical Faculty for this study. Two independent pathologists evaluated FGFR1, FGF2, and PI3K (p110α) expressions in both tumor and stromal tissues from 99 of the patients with sufficient tissue samples, using immunohistochemistry. Considering survival analysis separately for patients with both early and advanced stage diseases, the relationship between the clinical features of the patients and expressions were evaluated by univariate and multivariate analyses. Results: FGFR1 expression was found to be low in 59 (60%) patients and high in 40 (40%) patients. For FGF2; 12 (12%) patients had high, 87 (88%) patients had low expression and for IP3K; 31 (32%) patients had high and 66 (68%) patients had low expressions. In univariate analysis, overall survival (OS) was significantly associated with stage of the disease and the performance status of the patient (P<0.0001 and P<0.001). There was no significant difference in OS of the patients with either low or high expressions of FGFR1, FGF2, and IP3K. When the patients with early or advanced stage disease were separately taken into consideration, the relationship did not differ, either. Any of FGFR1, FGF2 or IP3K expressions was not found predictive for the treatment of early or advanced staged patients. On the other hand, the expressions of both FGFR1 and FGF2 were significantly different with respect to smoking, scar of tuberculosis and scar of radiotherapy (P=0.002; P=0.06 and P=0.05, respectively). Discussion: There has not been identified an effective individualized treatment for SqCLC yet. Therefore, in order to be able to develop such a treatment in the future, it is essential to identify the genetic abnormalities that are responsible for the biological behaviors and carcinogenesis of SqCLC. Although we could not show the prognostic and predictive significance of FGFR1, FGF2 and IP3K expressions in SqCLC, we determined the expression rates of FGFR1, FGF2 and IP3K as a reference for Turkish patients. In conclusion, we want to put some emphasis on the fact that, pulmonary fibrosis which is a late complication of radiotherapy at stage III disease, and the scar of tuberculosis could be associated with FGFR1 and FGF2 expressions.
Keywords: Squamous cell carcinoma of the lung, FGFR1, FGF2, IP3K, prognosis
Introduction
Lung cancer is the leading cause of cancer mortality worldwide, in both men and women [1]. The most prevalent subtype is adenocarcinoma, treatment of which has significantly developed recently [2]. The second most common histological subtype of non-small cell lung carcinoma (NSCLC) is squamous cell carcinoma (SqCLC) [3,4]. This type of carcinoma can develop in scar tissue. Several biochemical and clinical parameters have been investigated to assess their prognostic and/or predictive relevance.
Angiogenesis has a prominent role in NSCLC pathogenesis. Fibroblast growth factor (FGF) is an important molecule involved in angiogenesis. FGF2 or basic FGF (bFGF) and transmembrane domain of FGF receptor (FGFR) tyrosine kinase are signal molecules contributing to different physiologic processes, dysregulation of which lead to cancer development [5,6]. FGF2 is a potent stimulator of angiogenesis, and has a high-affinity for binding to FGFR1. Increased levels of FGF2, FGFR1, and FGFR2 proteins have been demonstrated in NSCLC cell series [7,8]. Some publications have investigated the significance of bFGF and FGFR expression in NSCLC, but the roles of these molecules in early pathogenesis and disease progression are not clarified yet [9-16]. FGFR signal pathway plays a role in the epithelial and mesenchymal transition (EMT) that is necessary for invasion and tumor cell metastasis, as well. They are also believed to be responsible for the resistance developed against drugs used to target at EGFR [17]. While studies showed a positive correlation between smoking and FGFR1 expression, no correlation of FGFR1 to lymph node metastasis, tumor stage, and age was identified [18]. Phosphatidyl inositol-3-phosphate kinase (IP3K) pathway on the other hand is a signal delivery pathway necessary for cellular life cycle, metabolism, motility, and angiogenesis. To date, many researchers have shown that anomalies in IP3K/phosphatase and tensin homologue (PTEN)/serine/threonine-specific protein kinase (Akt)/mammalian target of rapamycin (mTOR) pathways are more common in SqCLC than in adenocarcinomas [19]. Studies report IP3K expression rates around 40% [20,21]. IP3K/PTEN/Akt/mTOR pathway plays a role in cisplatin resistance, indicating that mutations in this pathway might be guiding for drug resistance [22].
Over time, many agents will be used in individualized therapy of SqCLC. Therefore, in this study, we aimed to investigate the FGFR1, FGF2, IP3K expression frequencies and their clinical, predictive, and prognostic significance immunohistochemically in SqCLC, a specific subtype of lung carcinoma.
Materials and methods
Patient recruitment
There were 395 patients at our hospital diagnosed with SqCLC between 2006 and 2013. Pathologic investigation was made in the specimens of 99 of the 129 patients whose complete data could be obtained. Patients with missing follow-up data, insufficient pathology specimens, and diagnosed with transthoracic fine needle aspiration were excluded from the study. Approval of the Ethics Committee of our university was obtained for the study (Approval date: 07.06.2012; Decision No. 9).
Immunohistochemical examination
Tissues fixed in 10% formaldehyde were blocked following the tissue follow-up procedure, and hematoxylin eosin stained slides were prepared using 5-micron serial sections and examined under light microscope. Histological sections were placed on special polylysine slides for immunohistochemical staining. Anti-FGFR-1 (rabbit polyclonal; sc-121, Santa Cruz, 1:50), anti-FGF-2 (rabbit polyclonal; AB1458, Chemicon, 1:200), and anti-IP3K (p110) were applied on sections prepared from the paraffin blocks of the cases included in the study. Two independent pathologists at the Pathology Department, Cukurova University Medical School evaluated the preparations. Cytoplasmic staining in tumor and stromal cells for FGF-2, FGFR-1 and PI3K (p110α) was investigated and both stromal density and intensity scores were recorded. Cytoplasmic staining score for FGFR-1 and FGF-2 were evaluated as 0= negative, 1= weak, 2= moderate, 3= strong. Stroma density scores were determined as 1= low, 2= medium, 3= high and intensity scores were reported as percentages (0-100%) (Figures 1, 2, 3, 4, 5 and 6).
Figure 1.
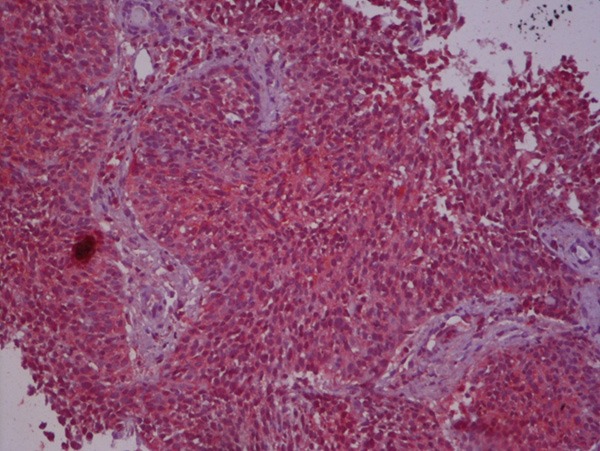
High FGFR1 expression on tumoral tissue.
Figure 2.
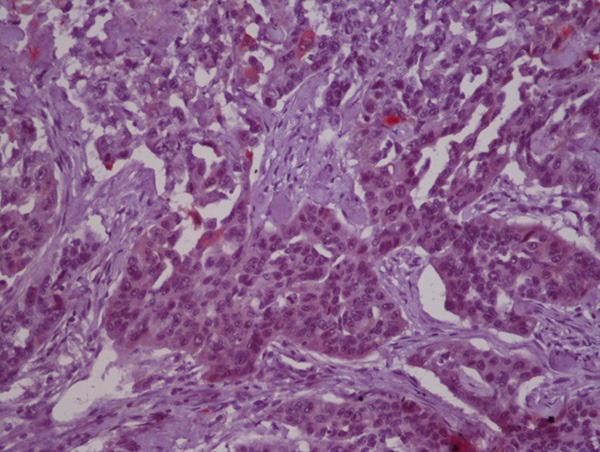
Low FGFR1 expression on tumoral tissue.
Figure 3.
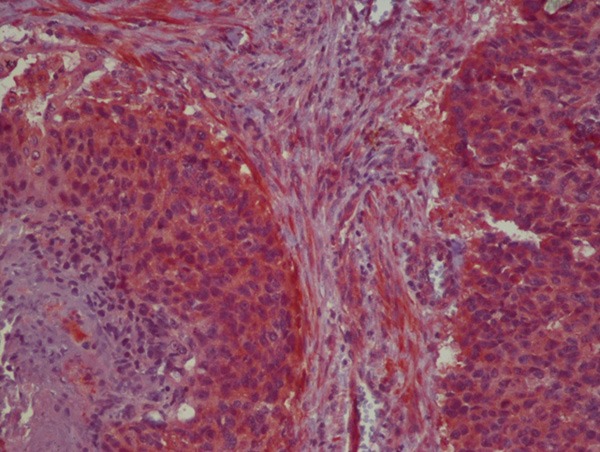
High FGF2 expression on tumoral tissue.
Figure 4.
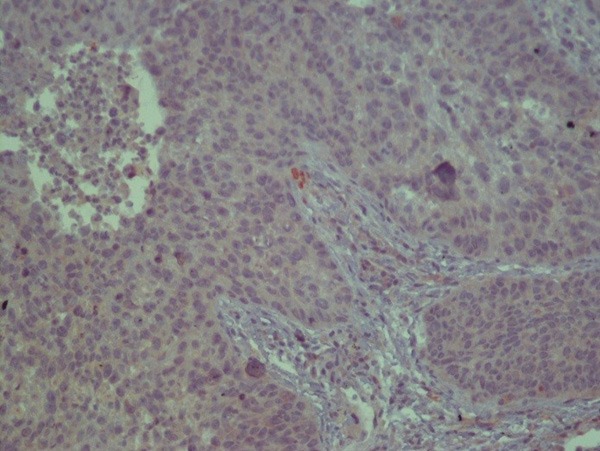
Low FGF2 expression on tumoral tissue.
Figure 5.
High IP3K expression on tumoral tissue.
Figure 6.
Low IP3K expression on tumoral tissue.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 19.0 was used for the statistical analysis of the data. Categorical measurements are summarized as count and percentage (%) and numerical measurements as mean and standard deviation. Between groups comparisons of categorical measurements were made using chi-square test and Fischer’s exact test where appropriate. Because FGFR-1 stained weakly, scores over 1 were considered high in the analysis; and since the cytoplasmic staining of FGF-2 were strong, total scores of 3 or more were considered high. For, IP3K scores of 2 or more were considered high expression. In survival analysis, expected survival curves for disease-free survival and overall survival were obtained with the univariate Kaplan Meier method. The survival curves obtained for different factors were compared using Log rank test. Cox regression model was used for the multivariate analysis of the potential prognostic factors impacting on the outcome variables of overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS). Factors found to have significant impact (P<0.10) in the univariate analysis, were included in the Cox regression model. The effects were expressed as hazard ratio (HR) and 95% confidence interval (CI). Results with P≤0.05 were considered statistically significant.
Results
Demographic and clinical characteristics of the patients are presented in Tables 1, 2 displays the epithelial and stromal expressions of FGFR1, FGF2, and IP3K in paraffin blocks of the patients. Since all of the patients had smoking history, a statistically significant difference could not be observed in FGFR1, FGF2, and IP3K expressions by smoking status. However, a statistically significant association was observed between stromal FGF2 and active smoking when those who had quitted smoking and those who are actively smoking were compared (P<0.05). Stromal FGF2 expression was determined to be lower among active smokers. Still, no difference was observed between quitters and active smokers with regard to other expressions.
Table 1.
Demographic and clinical characteristics of the patients
| Age (years) | 61.09±9.63 | |
| Sex | male | 124 (96.1%) |
| female | 5 (3.9%) | |
| Stage | I | 4 (3.1%) |
| II | 19 (14.7%) | |
| IIIA | 26 (20.2%) | |
| IIIB | 44 (34.1%) | |
| IV | 36 (27.9%) | |
| LN involvement | N0 | 18 (14%) |
| N1 | 47 (36.4%) | |
| N2 | 52 (40.3%) | |
| N3 | 12 (9.3%) | |
| Metastasis | No | 84 (65.1%) |
| Yes (1 organ) | 33 (25.6%) | |
| Yes (>1 organ) | 12 (9.3%) | |
| Smoking status | exsmoker (<5 years) | 12 (9.3%) |
| exsmoker (>5 years) | 18 (14%) | |
| formersmoker | 99 (76.7%) | |
| Therapy | surgery | 14 (10.8%) |
| CT | 52 (40.3%) | |
| CRT | 38 (29.5%) | |
| CT+RT | 23 (17.8%) | |
| RT | 2 (1.6%) | |
| Modality | neoadjuvant CT | 41 (31.8%) |
| adjuvant CT | 29 (22.5%) | |
| palliative CT | 43 (33.3%) | |
| palliative RT | 2 (1.6%) | |
| Tuberculosis scar | Yes | 17 (13.2%) |
| Radiotherapy scar | Yes | 20 (15.5%) |
| Thrombosis | Yes | 7 (5.4%) |
CT: chemotherapy, CRT: chemoradiotherapy, RT: radiotherapy.
Table 2.
Epithelial and stromal expressions of FGFR1, FGF2, and IP3K in paraffin blocks of the patients
| FGFR1 tumoral | high | 44 (44.4%) |
| low | 55 (55.6%) | |
| FGFR1 stromal | verylow | 24 (24.2%) |
| low | 14 (14.4%) | |
| high | 22 (22.2%) | |
| veryhigh | 39 (39.2%) | |
| FGFR1 total | low | 59 (59.6%) |
| high | 40 (40.4%) | |
| FGF2 tumoral | low | 90 (90.9%) |
| high | 9 (9.1%) | |
| FGF2 stromal | verylow | 19 (19.2%) |
| low | 19 (19.2%) | |
| intermediate | 26 (26.3%) | |
| high | 35 (35.4%) | |
| FGF2 total | high | 12 (12.1%) |
| low | 87 (87.9%) | |
| IP3K total | high | 31 (32%) |
| low | 66 (68%) |
Evaluation of smoking in terms of package/year in 3 groups (0-40 packs/year=1; 41 to 70 packs/year =2; and >70 pack/years =3) yielded that as package/year consumption increased FGFR1 total expression (P=0.002) and FGF2 stromal intensity increased (P<0.05) and FGF2 expression decreased (P=0.001). No statistically significant difference was observed in IP3K total expression by package/year cigarette consumption (P=0.87).
Tumor diameter and disease stage were not significantly different in terms of expressions either: For tumor diameter: FGFR1 total, P=0.64; vs. FGF2 total, P=0.66; vs. IP3K total, P=0.47; FGF2 intensity, P=0.73, FGFR1 intensity, P=0.92; for disease stage: FGFR1 total, P=0.52; FGF2 total, P=0.10; IP3K total, P=0.88; FGF2 intensity, P=0.31; FGFR1 intensity, P=0.54.
Statistically significant difference was not observed between lymph node involvement by expression either but among N3 patients, despite the low number, FGFR was detected to be high in 6 of the total of 9 patients, which was statistically significant (P=0.02). Additionally, among patients with suprarenal metastasis, FGFR1 total expression was high and FGF2 total and IP3K total expressions were low; however, this difference was not statistically different due to small patient number (80% and P=0.18; 100% and P=0.21; 80% and P=0.54, respectively).
Evaluation of the association between tuberculosis scars and expressions showed that those with tuberculosis scars had a significantly increased FGFR intensity (P=0.06). A significant difference was not observed in case of other expressions.
When the association between post-radiotherapy scars and expression was evaluated, significant increase in FGFR tumor expression among those with post-radiotherapy scars was observed (P=0.05).
In patients without history of thrombosis were found to have a lower FGF2 total expression though not statistically significant (P=0.07), compared to those having history of thrombosis.
An evaluation of the patients based on the chemotherapies they received, of the 129 patients, gemcitabine/cisplatin (gemcis) was administered to 69, cisplatin/vinorelbine (cisvino) to 31, docetaxel to 29, carboplatin/gemcitabine (carbogen) to 8, cisplatin/paclitaxel (cispac) to 6, and cisplatin/etoposide (ciseto) to 19 patients.
Univariate overall survival analyses
The distribution of the 125 patients whose survival data are known is as follows: overall survival (OS) of 70.7±7.1 months in Stages I+II; 43.6±9 months in Stage IIIA; 22±3.2 months in Stage IIIB; and 15.2±2.5 months in Stage IV.OS did not show statistically significant difference by tumor diameter (T) (P=0.9). There was no significant difference between active smokers and quitters with respect to OS either (P=0.5). On the other hand, a significant association was found between OS and lymph node involvement (P<0.001). In terms of distant metastasis, single and multiple organ metastasis cases had significantly different OS results (P<0.001). OS outcomes did not vary significantly across patients with different smoking amounts in package/year (P=0.60). Eastern Cooperative Oncology Group (ECOG) performance scores (PS) had a statistically significant relationship with OS (P<0.001). Patients who had pneumonectomy and lobectomy survived longer than those not operated (P<0.001). Neoadjuvant, adjuvant, palliative chemotherapy patients and those who did not receive any chemotherapy had statistically significant differences in OS (P<0.001).
Patients who were administered curative radiotherapy had longer OS than those who received palliative radiotherapy (P=0.002) and those with bone metastasis had longer OS than those who had brain metastasis (P=0.001). With regard to chemotherapy, a comparison of patients who only received gemcis with those who received other chemotherapies did not yield any statistically significant difference in terms of OS (P=0.35).
The patients did not have statistically significant differences in OS based on FGFR1, FGF2, and IP3K total expressions (P=0.46, P=0.75, and P=0.69, respectively). Since there were a small number of patients with high expression in the FGF2 stroma group, low and high density groups were further compared, and those with low FGF2 stroma were observed to survive longer.
Multivariate overall survival analyses
The multivariate analyses were conducted to investigate if there was a difference in OS of patients who differed by disease stage, ECOG performance score, lymph node involvement, metastasis, radiotherapy, therapies received, package/year of smoking, and whether or not receiving only gemcis chemotherapy or another chemotherapy via Cox regression analysis, and each of these factors’ impact on survival were examined. The impact of the disease stage observed in univariate analysis on OS disappeared in the multivariate analysis model. However, the mortality risk of those with ECOG PS≥2 increased compared to those with ECOG PS=0 by 3.9 times (minimum and maximum multiples of 1.45 and 10.5, respectively). OS was detected to differ between those with N1 and N2 lymph node involvements (P=0.06; HR=0.89-27.99). The risk decreased for those who received adjuvant chemotherapy compared to those who did not, and they had a longer survival [P=0.019; HR=0.004-0.601)]. While only marginally significant, it was observed that among the chemotherapy receivers, those who received cisplatin/gemcitabin survived for longer than others (P=0.05).
In the multivariate analysis, the tumoral, stromal, and total expressions of FGFR1, FGF2, and IP3K, the cofactors in univariate analysis that significantly affected the survival rate were included: disease stage, ECOG PS, lymph node involvement, therapies received, metastasis, package/year of smoking, being active smoker vs. quitter, and expressions of FGFR1, FGF2, and IP3K. Of these, ECOG PS, treatments received, presence of metastasis, FGF2 intensity, and FGFR1 total expression had statistically significant impact. Those with low ECOG PS had higher risk than those with high ECOG PS (P=0.033; RR (relative risk) =2.7 and HR=1.1-6.7). Those who received chemoradiotherapy were at higher risk than those who received chemotherapy only (P=0.09; RR=2.7; HR=0.83-9.2). Patients with multiple metastases were also at higher risk than those with no metastasis (P=0.07; RR=4.4; HR=0.9-22). Mortality risks increased as total FGFR2 and FGF2 intensity increased (P=0.03; RR=22.3, HR=1.3-372 and P=0.08; RR=3.2; HR=0.85-12, respectively).
Evaluation of the association between OS based on disease stage and expressions of FGFR1, FGF2, and IP3K (Figures 7, 8, 9 and 10)
Figure 7.
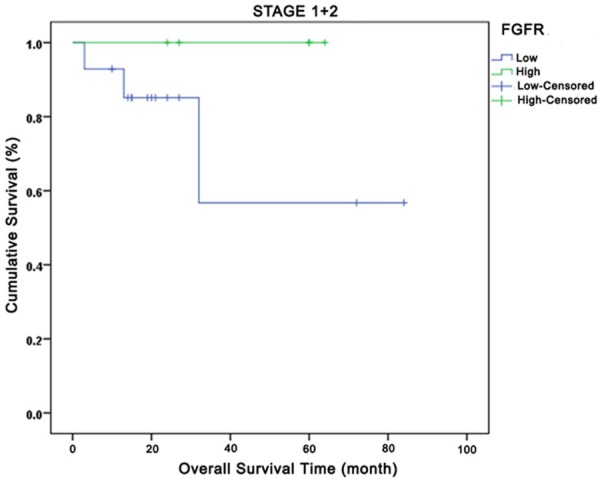
Relationship between total fibroblast growth factor receptors and overall survival in Stages I and II. Green curve: high expression; Blue curve: low expression.
Figure 8.
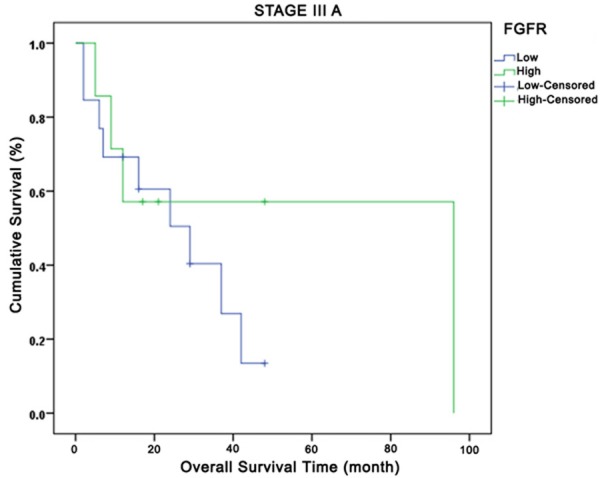
Relationship between total fibroblast growth factor receptors and overall survival in Stage IIIA. Green curve: high expression; Blue curve: low expression.
Figure 9.
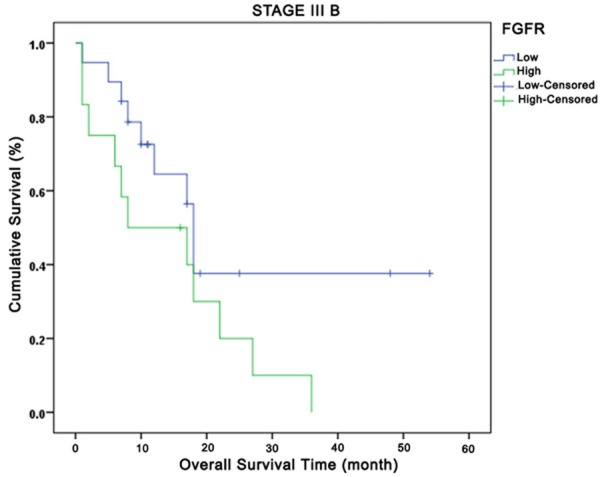
Relationship between total fibroblast growth factor receptors and overall survival in Stage IIIB. Green curve: high expression; Blue curve: low expression.
Figure 10.
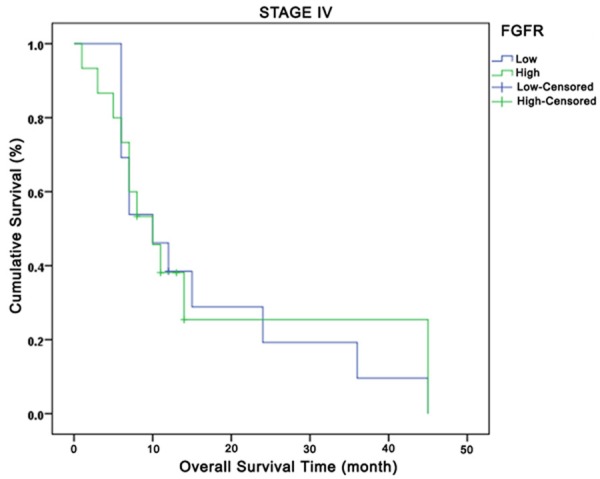
Relationship between total fibroblast growth factor receptors and overall survival in Stage IV. Green curve: high expression; Blue curve: low expression.
Comparison of OS based on FGR1 tumor expressions by disease stage yielded the following results: no OS difference in-between all stages (P=0.43), but better survival outcomes for patients with high FGFR1 expression in Stages I+II, Stage IIIA, and Stage IV and poorer results for those in Stage IIIB. At the 24th month at Stage IV, while 20% of the low FGFR1 tumor expression cases had survived, approximately 40% of the high FGFR1 tumoral expression cases had survived. When OS was compared based on expressions of total FGF2, FGFR1 intensity, and FGF2 intensity by disease stage, no difference was observed (P=0.24, P=0.86 and P=0.30, respectively). OS comparison based on disease stage, FGFR and FGF2 total expressions yielded no significant results for OS across all stages (P=0.42 and P=0.23, respectively). However, high total FGF2 expression in Stages IIIA, IIIB, and IV were observed to be associated with early deaths. A significant OS difference was not observed in investigation of IP3K (p110α) total expression by disease stage.
Disease-free survival (DFS)
Median DFS in Stages I and II was 32 months. No statistically significant difference was observed between DFS and FGFR1 tumor, tumoral FGF2, FGF2 intensity, FGFR1 intensity, FGFR1 total, FGF2 total, and IP3K total expressions in Stage I and II (P=0.71, P=0.94, P=0.33, P=0.54, P=0.41, P=0.73, and P=0.34, respectively). No significant results were obtained in the multivariate Cox regression analysis comparing disease stage, treatment, and expressions.
Progression-free survival (PFS)
Median PFS was detected to be 6.5 months among advanced stage patients, with a median of 4 months. Stage-wise expressions and PFS were not found to have a significant association.
Discussion
NSCLC is the leading cause of cancer mortality worldwide, in both men and women [23]. Prognostic and/or predictive impacts of many biochemical and clinical characteristics have been investigated. The genomic mutations detected in SqCLC are of great importance because of the possibility that they can determine the prognosis because the average survival in lung carcinoma is still 8-10 months [24]. FGFR1 amplification plays role in squamous cell lung carcinogenesis through IP3K, MAPK pathway and FGF2 on initiation (smoking as an etiologic trigger) and progression (angiogenesis, metastasis). FGFR1 and FGF2 amplifications have been proven to affect disease stage and survival in SqCLC and to play a role in predicting the benefit of adjuvant chemotherapy in early stages, chemoradiotherapy in local advanced disease, and chemotherapy in advanced disease.
FGFR1 amplification in SqCLC was detected to be between 16-22% in different studies [25,26]. While some studies did not demonstrate an OS advantage, others associated it with poor OS and relapse free survival (RFS) based on the nuclear or membranous nature of the staining, and was even associated with better OS in a platinum receiving subgroup analysis in a study [14,16,26-29]. In our study, FGFR1 amplification was, detected as 40%, higher than in other studies. However, the association between FGFR1 expression and smoking has not been clearly identified, as majority of the patients in our study were active or former heavy smokers. We determined that the FGFR1 expression increased as the package/year of smoking increased. With increased duration of smoking, lung carcinoma risk increases and this effect is reflected upon FGFR1.
The role of smoking in the development of especially small and squamous CLC is not clearly defined. Among these two cancer types that are directly associated with smoking, detection of high FGFR1 expression in SqCLC patients is actually an indicator of the fact that FGFR1 is not a direct and sole contributor of the association between smoking and lung carcinoma. FGFR1 amplification might have been detected as high in our patient group because there were no patients without smoking history. Moreover, the expression percentage (%) within the Turkish population is not known, because this is the first study conducted in Turkey on this topic. A significant difference was not observed between lymph node involvement and FGFR1 expression either but among the N3 patients, 6 of the 9 patients had high FGFR1 expression, which was close to the level of statistical significance. Additionally, patients with suprarenal metastasis had high total FGFR1 expression, which could not reach the level of statistical significance most probably due to low number of patients. A similar finding was presented in a recent study as well, in which visceral metastases were observed to increase as FGFR1 expression increased [30]. This finding also explains the association between high FGFR1 expression and poor prognosis. Though the intermediate pathways of carcinogenesis are not clearly defined, high expression in early stages, considering the high expression in cases with suprarenal metastasis and as the FGFR/FGF angiogenesis association is also known, FGFR1 may be speculated to contribute to development of distant metastasis. Therefore, drugs targeting these molecules can be further studied in order to prevent progression and metastasis.
An association between tuberculosis scars and SqCLC has been mentioned. An investigation of the relationship between tuberculosis and FGFR1 expression among the patients in our study led to detection of an increased FGFR1 intensity among cases with tuberculosis scars and that this increase was close to the level of significance.
Though not many, there are studies in the medical literature pointing out that tumor necrosis factor-α, vascular endothelial growth factor, and FGF play a role in radiotherapy-related lung damage [31-33]. In our study, when investigated the relationships between post-radiotherapy scar and expressions, we have also identified high FGFR1 tumoral expression among those with scars. Moreover, while Stage IIIB patients receiving chemoradiotherapy as part of their treatment who had low FGFR expression survived longer than 20 months, the other group failed to survive. High FGFR1 expression may predict chronic toxicity of radiotherapy (post 20 months).
No significant difference was observed in OS of our patients with respect to FGFR1 total expression. Multivariate analyses did not detect a significant difference in other parameters with respect to FGFR1 expression either.
FGF2 expression in NSCLC was defined first by Takanami et al. [34,35]. However, its prognostic significance is still disputable [34,36-39]. In our study, the pathologic evaluations showed that epithelial staining was stronger for FGFR1 and stromal staining was stronger for FGF2. With the few publications examining FGFR1 and FGFR2 expression together, this finding has not been explained in the literature. A study conducted with resected NSCLC patients reported an association between immunohistochemically increased tumoral FGF2 expression and reduced 5-year survival, and between increased stromal expression and increased 5-year survival [40].
In our study, tumoral FGF2 expression was observed to be high in 9 (9.1%) patients and low in 90 (90.9%) patients. Stromal FGF2 expression was very low in 19 (19.2%) patients, low in 19 (19.2%) patients, moderate in 26 (26.3%) patients, and high in 35 (35.4%) patients. Based on a review of the total FGF2 expression, both epithelial and stromal, 12 (12%) patients had high and 87 (88%) patients had low expression. A comparison of the total FGF2 expression by disease stage demonstrated no difference in OS across all stages. However, keeping the statistically low patient count in mind, high total FGF2 expression was observed to be associated with early deaths in Stages IIIA, IIIB, and IV. While disease stage is the most significant prognostic factor, OS was not observed to be associated with stage-FGF2 together, but FGF2, independent of stage, was observed to be associated with death.
As an investigation of the effect of active smoking, a statistically significant association was observed between FGF2 stroma and active smoking, but not in quitters. Stromal FGF2 expression was low among active smokers. Though FGF2 stroma intensity increases as package/year smoking increased, total FGF2 expression decreased. This finding was in accordance with the findings of another study in the medical literature [41]. Nicotine use increases FGF2 secretion from endothelial cells [41]. This effect on angiogenesis is believed to occur via endothelial nicotinic acetylcholine receptors. There is a synergistic interaction between angiogenetic growth factor receptors and endothelial nicotinic acetylcholine receptors.
Thrombosis is observed at a 10% rate in lung cancer. Comparison of the cases with and without thrombosis history showed that, though not statistically significant, FGF2 total expression was lower among those without thrombosis history. According to the findings of a former study, the FGFR1 pathway-related increased FGF2 expression on walls of varicose veins affected the enzymes in extracellular matrix metabolism and plays a role in vein wall remodeling and disease pathogenesis [42]. Not many other studies investigated this relationship.
The multivariate analysis of our data failed to demonstrate OS association with disease stage, ECOG PS, lymph node involvement, chemotherapy, metastasis, radiotherapy, therapies received, package/year of smoking, and receiving gemcisonly or another chemotherapy. However, we determined that mortality risk increased as FGF2 intensity increased. Interestingly, increased stromal FGF2 expression was associated with increased 5-year survival in some studies in literature (70% and 53%; and this finding remained significant in multivariate analysis as well [40].
IP3K (p110α) amplifications and mutations, detected in various rates in NSCLC, are observed in SqCLC more often than in adenocarcinoma. Literature reports amplification rates varying between 33-43% [20,21,43,44]. Increased stromal IP3K expression has been determined to be associated with good prognosis [45]. IP3K mutation and amplification, among squamous cell subtype cases among Japanese lung carcinoma patients, were observed more in males compared to females and in smokers [20]. Observed at higher rates among early stage patients, it can be considered a curative target especially in case of patients not suitable for traditional molecular targeted therapies. Yet, its role in carcinogenesis in earlier stage lesions such as dysplasia and atypical adenomatous hyperplasia is still not clear [20].
Another study reported that activation of IP3K/Akt pathway was increased among higher grade and advanced stage patients. This study also demonstrated that p110α, a catalytic subtype of IP3K, was the main determinant of Akt pathway activation [46]. Increased IP3K expression in NSCLC was demonstrated in metastases as well, and the expression is believed to increase as tumor stage increases. PI3K/AKT pathway is targeted in NSCLC in order to increase survival, reduce chemo- and radiotherapy resistance, and to prevent metastasis [47].
In our study, a review of the total IP3K expression, both tumoral and stromal, revealed high expression in 31 (32%) and low expression in 66 (68%) patients. A statistically significant difference was not observed between IP3K total expression and package/year of smoking. Moreover, low IP3K total expression was detected among cases with suprarenal metastasis but this finding was not statistically significant due to low number of cases (80%, P=0.54). An association of IP3K with tumor diameter and disease stage was not observed. Total IP3K expressions in our patients did not have a significant difference with OS.
In conclusion, the recent lung carcinoma studies in medical literature are on either SCLC or NSCLC. However, following developments of targeted therapies in adenocarcinoma, amplifications and mutations related to tumorigenesis only in SqCLC cases have been investigated to a great extent. Studies investigating especially FGFR1, FGF2, and IP3K among them and their relationships with other prognostic and clinical parameters have controversial findings. In our study with early and advanced stage SqCLC patients, we investigated both the percentages (%) of these expressions and if they are prognostic and/or predictive or not.
We determined the relationships of FGFR1, FGF2, and IP3K with clinical parameters among early and advanced stage SqCLC patients were to be significant only within clinical subgroups with specific characteristics (active smokers, cases with tuberculosis scars, cases with radiotherapy scars). A predictive significance of expressions was not observed among early or advanced stage cases, and those received treatment. A statistical difference was not observed with regard to survival (OS, DFS, and PFS) either.
We failed to statistically point out a prognostic significance of expressions in this study. Though not statistically significant, high FGF2 was determined to be associated with poor survival among advanced stage cases. However, it was observed that FGFR expression could have an (predictive) association with smoking, tuberculosis scar, and long-term fibrosis associated with radiotherapy. Future studies are needed on this matter; and clinical trials about agents inhibiting these pathways are at yet at phase 1. Over the years, targeted treatments inhibiting these pathways in SqCLC may be introduced to routine clinical practice.
Disclosure of conflict of 谋nterest
None.
References
- 1.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) based on November 2011 SEER data submission. Bethesda, MD: National Cancer Institute; 2012. [Google Scholar]
- 3.Ettinger DS. Tenyears of progress in non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:292–5. doi: 10.6004/jnccn.2012.0029. [DOI] [PubMed] [Google Scholar]
- 4.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd edition: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl):e1S–29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlyingdifferential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–47. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Ribatti D, Vacca A, Rusnati M, Presta M. The discovery of basic fibroblast growth factor/fibroblast growth factor-2 and its role in haematological malignancies. Cytokine Growth Factor Rev. 2007;18:327–34. doi: 10.1016/j.cytogfr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–37. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn H, Kopff C, Konrad J, Riedel A, Gessner C, Wirtz H. Influence of basic fibroblast growth factor on the proliferation of non-small cell lung cancer cell lines. Lung Cancer. 2004;44:167–74. doi: 10.1016/j.lungcan.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Takanami I, Tanaka F, Hashizume T, Kikuchi K, Yamamoto Y, Yamamoto T, Kodaira S. The basic fibroblast growth factor and its receptor in pulmonary adenocarcinomas: an investigation of their expression as prognostic markers. Eur J Cancer. 1996;32:1504–9. doi: 10.1016/0959-8049(95)00620-6. [DOI] [PubMed] [Google Scholar]
- 10.Takanami I, Tanaka F, Hashizume T, Kodaira S. Tumor angiogenesis in pulmonary adenocarcinomas: relationship with basic fibroblast growth factor, its receptor, and survival. Neoplasma. 1997;44:295–8. [PubMed] [Google Scholar]
- 11.Volm M, Koomagi R, Mattern J, Stammler G. Prognostic value of basic fibroblast growth factor and its receptor (FGFR-1) in patients with non-small cell lung carcinomas. Eur J Cancer. 1997;33:691–3. doi: 10.1016/s0959-8049(96)00411-x. [DOI] [PubMed] [Google Scholar]
- 12.Guddo F, Fontanini G, Reina C, Vignola AM, Angeletti A, Bonsignore G. The expression of basic fibroblast growth factor (bFGF) in tumor-associated stromal cells and vessels is inversely correlated with non-small cell lung cancer progression. Hum Pathol. 1999;30:788–94. doi: 10.1016/s0046-8177(99)90139-9. [DOI] [PubMed] [Google Scholar]
- 13.Shou Y, Hirano T, Gong Y, Kato Y, Yoshida K, Ohira T, Ikeda N, Konaka C, Ebihara Y, Zhao F, Kato H. Influence of angiogenetic factors and matrix metalloproteinases upon tumour progression in non-small-cell lung cancer. Br J Cancer. 2001;85:1706–12. doi: 10.1054/bjoc.2001.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki A, Kuwahara M, Yoshinaga Y, Shirakusa T. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic indicators in NSCLC. Eur J Cardiothorac Surg. 2004;25:443–8. doi: 10.1016/j.ejcts.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–58. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Behrens C, Lin HY, Lee JJ, Raso MG, Hong WK, Wistuba II, Lotan R. Immunohistochemical Expression of Basic Fibroblast Growth Factor and Fibroblast Growth factor Receptors 1 and 2 in the Pathogenesis of Lung Cancer. Clin Cancer Res. 2008;14:6014–22. doi: 10.1158/1078-0432.CCR-08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S, Modrusan Z, Lin CY, O’Neill V, Amler LC. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–98. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 18.Goeke F, Franzen A, Menon R, Goltz D, Kirsten R, Boehm D, Vogel W, Göke A, Scheble V, Ellinger J, Gerigk U, Fend F, Wagner P, Schroeck A, Perner S. Rationale for treatment of metastatic squamous cell carcinoma of the lung using FGFR inhibitors. Chest. 2012;142:1020–6. doi: 10.1378/chest.11-2943. [DOI] [PubMed] [Google Scholar]
- 19.Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol. 2012;13:e418–26. doi: 10.1016/S1470-2045(12)70291-7. [DOI] [PubMed] [Google Scholar]
- 20.Okudela K, Suzuki M, Kageyama S, Bunai T, Nagura K, Igarashi H, Takamochi K, Suzuki K, Yamada T, Niwa H, Ohashi R, Ogawa H, Mori H, Kitamura H, Kaneko T, Tsuneyoshi T, Sugimura H. PIK3CA mutation and amplification in human lung cancer. Pathol Int. 2007;57:664–71. doi: 10.1111/j.1440-1827.2007.02155.x. [DOI] [PubMed] [Google Scholar]
- 21.Ji M, Guan H, Gao C, Shi B, Hou P. Highly frequent promoter methylation and PIK3CA amplification in non-small cell lung cancer (NSCLC) BMC Cancer. 2011;11:147. doi: 10.1186/1471-2407-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu JL, Wang ZW, Hu LM, Yin ZQ, Huang MD, Hu ZB, Shen HB, Shu YQ. Genetic variants in the PI3K/PTEN/AKT/mTOR pathway predict platinum-based chemotherapy response of advanced non-small cell lung cancers in a Chinese population. Asian Pac J Cancer Prev. 2012;13:2157–62. doi: 10.7314/apjcp.2012.13.5.2157. [DOI] [PubMed] [Google Scholar]
- 23.Sant M, Aareleid T, Berrino F, BielskaLasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H, Möller T, Verdecchia A, Capocaccia R, Gatta G, Micheli A, Santaquilani M, Roazzi P, Lisi D EUROCARE Working Group. EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol. 2003;14(Suppl 5):v61–118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- 24.Farhat FS, Tfayli A, Fakhruddin N, Mahfouz R, Otrock ZK, Alameddine RS, Awada AH, Shamseddine A. Expression, prognostic and predictive impact of VEGF and bFGF in non-small cell lung cancer. Crit Rev Oncol Hematol. 2012;84:149–60. doi: 10.1016/j.critrevonc.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, Ullrich RT, Menon R, Maier S, Soltermann A, Moch H, Wagener P, Fischer F, Heynck S, Koker M, Schöttle J, Leenders F, Gabler F, Dabow I, Querings S, Heukamp LC, Balke-Want H, Ansén S, Rauh D, Baessmann I, Altmüller J, Wainer Z, Conron M, Wright G, Russell P, Solomon B, Brambilla E, Brambilla C, Lorimier P, Sollberg S, Brustugun OT, Engel-Riedel W, Ludwig C, Petersen I, Sänger J, Clement J, Groen H, Timens W, Sietsma H, Thunnissen E, Smit E, Heideman D, Cappuzzo F, Ligorio C, Damiani S, Hallek M, Beroukhim R, Pao W, Klebl B, Baumann M, Buettner R, Ernestus K, Stoelben E, Wolf J, Nürnberg P, Perner S, Thomas RK. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62–93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heist RS, Mino-Kenudson M, Sequist LV, Tammireddy S, Morrissey L, Christiani DC, Engelman JA, Iafrate AJ. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol. 2012;7:1775–80. doi: 10.1097/JTO.0b013e31826aed28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HR, Kim DJ, Kang DR, Lee JG, Lim SM, Lee CY, Rha SY, Bae MK, Lee YJ, Kim SH, Ha SJ, Soo RA, Chung KY, Kim JH, Lee JH, Shim HS, Cho BC. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J. Clin. Oncol. 2013;31:731–7. doi: 10.1200/JCO.2012.43.8622. [DOI] [PubMed] [Google Scholar]
- 28.Craddock KJ, Ludkovski O, Sykes J, Shepherd FA, Tsao MS. Prognostic value of fibroblast growth factor receptor 1 gene locus amplification in resected lung squamous cell carcinoma. J Thorac Oncol. 2013;8:1371–7. doi: 10.1097/JTO.0b013e3182a46fe9. [DOI] [PubMed] [Google Scholar]
- 29.Tran TN, Selinger CI, Kohonen-Corish MR, McCaughan BC, Kennedy CW, O’Toole SA, Cooper WA. Fibroblast growth factor receptor 1 (FGFR1) copy number is an independent prognostic factor in non-small cell lung cancer. Lung Cancer. 2013;81:462–7. doi: 10.1016/j.lungcan.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Preusser M, Berghoff AS, Berger W, Ilhan-Mutlu A, Dinhof C, Widhalm G, Dieckmann K, Wöhrer A, Hackl M, von Deimling A, Streubel B, Birner P. High rate of FGFR1 amplifications in brain metastases of squamous and non-squamous lung cancer. Lung Cancer. 2014;83:83–9. doi: 10.1016/j.lungcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000;10:296–307. doi: 10.1053/srao.2000.9424. [DOI] [PubMed] [Google Scholar]
- 32.Warburton D, Bellusci S. The molecular genetics of lung morphogenesis and injury repair. Paediatr Respir Rev. 2004;5(Suppl A):S283–7. doi: 10.1016/s1526-0542(04)90052-8. [DOI] [PubMed] [Google Scholar]
- 33.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–40. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 34.Takanami I, Tanaka F, Hashizume T, Kikuchi K, Yamamoto Y, Yamamoto T, Kodaira S. The basic fibroblast growth factor and its receptor in pulmonary adenocarcinomas: an investigation of their expression as prognostic markers. Eur J Cancer. 1996;32:1504–9. doi: 10.1016/0959-8049(95)00620-6. [DOI] [PubMed] [Google Scholar]
- 35.Takanami I, Tanaka F, Hashizume T, Kodaira S. Tumor angiogenesis in pulmonary adenocarcinomas: relationship with basic fibroblast growth factor, its receptor, and survival. Neoplasma. 1997;44:295–8. [PubMed] [Google Scholar]
- 36.Volm M, Koomagi R, Mattern J, Stammler G. Prognostic value of basic fibroblast growth factor and its receptor (FGFR-1) in patients with non-small cell lung carcinomas. Eur J Cancer. 1997;33:691–3. doi: 10.1016/s0959-8049(96)00411-x. [DOI] [PubMed] [Google Scholar]
- 37.Guddo F, Fontanini G, Reina C, Vignola AM, Angeletti A, Bonsignore G. The expression of basic fibroblast growth factor (bFGF) in tumor-associated stromal cells and vessels is inversely correlated with non-small cell lung cancer progression. Hum Pathol. 1999;30:788–94. doi: 10.1016/s0046-8177(99)90139-9. [DOI] [PubMed] [Google Scholar]
- 38.Shou Y, Hirano T, Gong Y, Kato Y, Yoshida K, Ohira T, Ikeda N, Konaka C, Ebihara Y, Zhao F, Kato H. Influence of angiogenetic factors and matrix metalloproteinases upon tumour progression in non-small-cell lung cancer. Br J Cancer. 2001;85:1706–12. doi: 10.1054/bjoc.2001.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki A, Kuwahara M, Yoshinaga Y, Shirakusa T. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic indicators in NSCLC. Eur J Cardiothorac Surg. 2004;25:443–8. doi: 10.1016/j.ejcts.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 40.Donnem T, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM. Prognostic impact of fibroblast growth factor 2 in non-small cell lung cancer: coexpression with VEGFR-3 and PDGF-B predicts poor survival. J Thorac Oncol. 2009;4:578–85. doi: 10.1097/JTO.0b013e31819f2e38. [DOI] [PubMed] [Google Scholar]
- 41.Ramaswamy G. Summary of presentation from the targeted therapy in lung cancer meeting. J Thorac Oncol. 2011;6(Suppl 4):S1757–88. doi: 10.1097/JTO.0b013e31822eec73. [DOI] [PubMed] [Google Scholar]
- 42.Cucina A, Corvino V, Sapienza P, Borrelli V, Lucarelli M, Scarpa S, Strom R, Santoro-D’Angelo L, Cavallaro A. Nicotine regulates basic fibroblastic growth factor and transforming growth factor beta1 production in endothelial cells. Biochem Biophys Res Commun. 1999;257:306–12. doi: 10.1006/bbrc.1999.0478. [DOI] [PubMed] [Google Scholar]
- 43.Kowalewski R, Malkowski A, Sobolewski K, Gacko M. Evaluation of aFGF/bFGF and FGF signaling pathway in the wall of varicose veins. J Surg Res. 2009;155:165–72. doi: 10.1016/j.jss.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 44.Stjernström A, Karlsson C, Fernandez OJ, Söderkvist P, Karlsson MG, Thunell LK. Alterations of INPP4B, PIK3CA and pAkt of the PI3K pathway are associated with squamous cell carcinoma of the lung. Cancer Med. 2014;3:337–48. doi: 10.1002/cam4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto H, Shigematsu H, Nomura M, Lockwood WW, Sato M, Okumura N, Soh J, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Date H, Lam WL, Minna JD, Gazdar AF. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008;68:6913–21. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Saad S, Donnem T, Al-Shibli K, Persson M, Bremnes RM, Busund LT. Diverse prognostic roles of Akt isoforms, PTEN and PI3K in tumor epithelial cells and stromal compartment in non-small cell lung cancer. Anticancer Res. 2009;29:4175–83. [PubMed] [Google Scholar]
- 47.Scrima M, De Marco C, Fabiani F, Franco R, Pirozzi G, Rocco G, Ravo M, Weisz A, Zoppoli P, Ceccarelli M, Botti G, Malanga D, Viglietto G. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): new insights on the role of phosphatydil-inositol-3 kinase. PLoS One. 2012;7:e30427. doi: 10.1371/journal.pone.0030427. [DOI] [PMC free article] [PubMed] [Google Scholar]


