Abstract
Intermedin is a proopiomelanocortin-derived peptide before opioid promoting cortical hormone, its main function embodies in mononuclear macrophages and neutrophilic granulocytes to inhibit the proinflammatory cytokines. The aim of this study is to determine intermedin attenuates myocardial infarction and its related mechanisms in a rat model of ischemic heart failure. After rat model of ischemic heart failure was set up, myocardial infarction, blood levels of activities of creatine kinase (CK), the MB isoenzyme of creatine kinase (CK-MB), lactate dehydrogenase (LDH) and cardiac troponin T (cTnT) were effectively reduced by treatment with intermedin. Tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) in a rat model of ischemic heart failure were recovered by pretreatment with intermedin. Administrate of intermedin availably promoted cAMP contents and suppressed caspase-3 protein in ischemic heart failure rat. ERK1/2 and LC3 protein expression were significantly activated and autophagy was significantly promoted by intermedin in a rat model of ischemic heart failure. These results indicate that intermedin protected rat heart, attenuates myocardial infarction from ischemic heart failure in the rat model. The underlying mechanisms may include upregulation of cAMP, ERK1/2 and LC3 protein expression and activating of autophagy.
Keywords: Intermedin, ischemic heart failure, autophagy, cAMP, ERK1/2
Introduction
Coronary artery disease (CAD) is a kind of ancient and serious epidemic disease, which is included in the cardiovascular system. Over the past 20 years, China’s CAD morbidity rate and mortality rate are rising rapidly [1]. Besides, CAD constitutes the fastest-growing disease cause of death for residents, and it has developed into the most dangerous disease threatening public health in China [1]. According to the data collected in 2008, the proportion of the patients who die from the coronary heart disease (CHD) accounts for 22% in the total amount of the patients who die from cardiovascular disease in Chinese urban areas, however, even rural areas the proportion is as high as 13% [2]. Coronary artery provides blood for myocardium. Atherosclerosis of coronary artery caused by a variety of reasons makes coronary stenosis and occlusion, which imposes restrictions on myocardium blood perfusion in corresponding regions [3]. And due to this mentioned above, myocardial imbalance of oxygen supply and demand also occurs in this region. Myocardial ischemia possibly leads to short chest pain, decreased activity tolerance, permanent myocardial infarction, ventricular remodeling and heart failure [4]. A recent statistical data from the United States shows that each year about 670,000 new patients are diagnosed with heart failure, moreover, the mortality rate of the patients who suffer from coronary heart disease featuring ischemic heart failure is increasingly higher [5]. At the same time, medical costs are much higher [6].
Under physiological conditions, autophagy can remove aging organelles and damaged protein in myocardial cells in order to maintain the heart function and the heart shape, thus the autophagy plays a protective role for the heart [7]. However, with the changes in internal environment and the stress conditions (such as the lack of energy, oxidation loss and the opening of the mitochondrial membrane permeability transition pore), autophagy will increase accordingly [8]. Studies show that adults under the condition of hunger, autophagy is of crucial importance to maintain the cardiac function. There are already some studies show that the decrease of saliva secretion level can cause the dysfunction of organelles, such as the accumulation of mitochondria, which is closely related to various diseases [9]. In heart disease, the changes of autophagy level may also cause the accumulation of damaged mitochondria, which affects the heart function [8].
Intermedin is a proopiomelanocortin-derived peptide, mainly produced by hypothalamus, pituitary and a variety of peripheral tissue cells [10]. And intermedin distributes widely. Intermedin is named in accordance with its promoting effect on amphibians’ melanin pigmentation [11]. Intermedin also has lots of other physiological functions. Its main function embodies in mononuclear macrophages and neutrophilic granulocyte which inhibit the secretion of proinflammatory cytokines and promote the secretion of anti-inflammatory cytokines, including the secretion of IL-10 [12]. Intermedin also restrains NO’s generation and migration to the inflammatory parts. Intermedin produced by the central nervous system can activate peripheral beta 2-adrenergic sympathetic nerve so as to play a role in resisting peripheral tissue inflammation [12,13]. Here, we report that intermedin attenuates myocardial infarction through inducing the protective autophagy, and further improving cardiac performance.
Materials and methods
Animals and cell culture
Sprague-Dawley (SD) rats (250-320 g) and H9c2 cell lines were obtained from Animal Experimental Center of Soochow University and housed in a specific pathogen-free room at 24°C ± 2°C and humidity (55 ± 10%) and free access to standard rat chow and water. H9c2 cell was cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Logan, UT, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (HyClone, Logan, UT, USA) and 1% (w/v) of an antibiotic-antimycotic preparation at 37°C under 5% (v/v) CO2.
All experiment SD rats were anesthetized by intraperitoneal injection of 7% chloral hydrate (Suzhou, China). Left coronary artery was dissected above the first diagonal branch after left thoracotomy and pericardiotomy executed. Then, the left circumflex arterial origin was immediately ligated with silk thread. Slipknot-induced occlusion proceeded for 30 min, and then myocardium distal was observed darkened. All study protocols were approved by the Medical College of Soochow University Animal Care Committee. Rats were randomly assigned to three different groups: sham group, ischemic heart failure group and intermedin group. In intermedin group, ischemic heart failure model rats were gave with 20 nmol/kg intermedin from caudal vein for 4 weeks. In sham group or ischemic heart failure group, normal rat or ischemic heart failure model rats were gave with the same volume of normal saline.
H9c2 cell was cultured in complete medium under 95% N2 and 5% CO2 (both v/v) at 37°C for 1 h. Control cells were cultured in complete medium at 37°C under 5% (v/v) CO2 for 90 min. In intermedin group, ischemic heart failure model H9c2 cell were gave with 1 μM intermedin from caudal vein for 4 weeks. In sham group or ischemic heart failure group, H9c2 cell and ischemic heart failure model H9c2 cell were gave with DMSO.
Serum biochemical analysis
Blood was collected after intermedin by cardiac puncture and centrifuged at 2000 g for 10 min at 4°C. The serum concentrations of casein kinase (CK), the MB isoenzyme of creatine kinase (CK-MB), lactate dehydrogenase (LDH), cardiac troponin T (cTnT), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) were measured according to the manufacturer instructions (JianCheng Bioengineering Institute, China).
Intracellular cAMP contents
To measure intracellular cAMP level, rats were sacrificed via direct intraventricular KCl injection, and then hearts of rats were removed and rinsed with ice-cold phosphate buffered saline. Frozen ventricle samples were homogenized in protein lysate buffer. The suspension was centrifuged at 2000 g for 10 min at 4°C and assayed for intracellular cAMP content by ELISA (R&D Systems, MN, USA) following the manufacturer’s protocol. The total protein contents were measured by Bradford assay (Bio-Rad).
Western blot analysis
Rats were sacrificed via direct intraventricular KCl injection, and then hearts of rats were removed and rinsed with ice-cold phosphate buffered saline. Frozen ventricle samples were homogenized in protein lysate buffer. The suspension was centrifuged at 2000 g for 10 min at 4°C. The total protein contents were measured by Bradford assay (Bio-Rad). Protein lysate was loaded on 12% SDSPAGE and then transferred to polyvinylidene fluoride membrane (Pierce, Rockford, IL, USA). The membranes were probed with antibodies against caspase-3 (Santa Cruz, CA, USA, 1:1000 dilution), ERK1/2 (Santa Cruz, CA, USA, 1:1000 dilution), LC3B (Santa Cruz, CA, USA, 1:1000 dilution) and β-actin (1:4000, CWBIO, China) respectively, overnight with agitation at room temperature. The membranes were horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz, CA, USA, 1:1000 dilution) enhanced chemiluminescence.
Fluorescence microscopy
H9c2 cell was fixed with 4% paraformaldehyde for 30 min at 4°C and permeabilized using precool methanol for 10 min at 4°C. Then, H9c2 cell fixed was cultured with 5% normal goat serum and 0.3% Triton X-100 for 1 h at darkness. H9c2 cell was incubated with LC3B (Santa Cruz, CA, USA, 1:1000 dilution) by 1 h incubation with Alexa Fluor 488 goat anti-rabbit IgG (Sangon Biotech, Shanghai, China) as a secondary antibody (Santa Cruz, CA, USA, 1:1000 dilution). Then, H9c2 cell was washed with ice-PBS and stimulated using microscope slides with Flouromount-G (SouthernBiotech, Birmingham, AL).
Statistical analysis
All values are reported as means ± SE. Statistical analyses between groups of two were done by unpaired t-test. P < 0.05 was considered to be statistically significant for all tests.
Results
Intermedin attenuates myocardial infarction in a rat model of ischemic heart failure
To evaluate the cardiac protection of intermedin, myocardial infarction was measured after treatment. 4 weeks after intermedin treatment, myocardial infarction of intermedin treatment rat was lower than that of ischemic heart failure rat (Figure 1).
Figure 1.
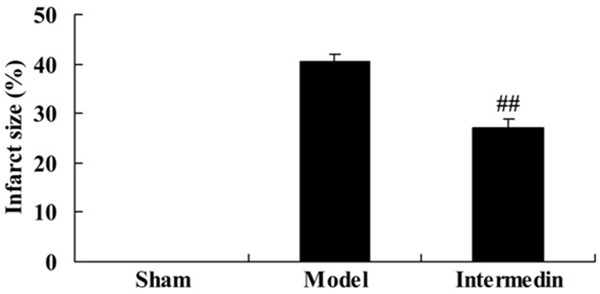
Intermedin attenuates myocardial infarction in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; ##P<0.01 versus the model group.
Intermedin attenuates the serum concentrations of CK, CK-MB, LDH and cTnT in a rat model of ischemic heart failure
We further detected the cardiac protection of intermedin on the serum concentrations of CK, CK-MB, LDH and cTnT in a rat model of ischemic heart failure. In the serum concentrations of CK, CK-MB, LDH and cTnT, we observed that compared with the sham group, the serum concentrations of CK, CK-MB, LDH and cTnT were memorably increased in a rat model of ischemic heart failure (Figure 2). However, there was statistical difference in the serum concentrations of CK, CK-MB, LDH and cTnT among intermedin treatment group (Figure 2).
Figure 2.
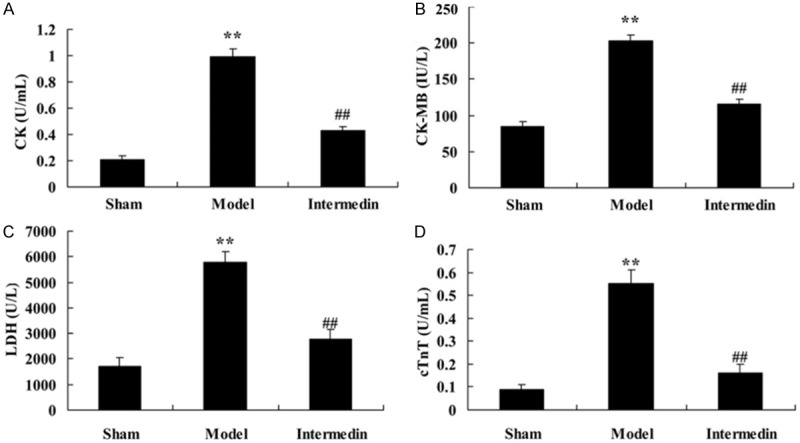
Intermedin attenuates the serum concentrations of CK, CK-MB, LDH and cTnT in a rat model of ischemic heart failure. Intermedin attenuates the serum concentrations of CK (A), CK-MB (B), LDH (C) and cTnT (D) in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; **P<0.01 versus the model group; ##P<0.01 versus the model group.
Intermedin attenuates the serum concentrations of TNF-α and IL-6 in a rat model of ischemic heart failure
We further detected the cardiac protection of intermedin on inflammation in ischemic heart failure rats, the serum concentrations of TNF-α and IL-6 were recorded in very group. In ischemic heart failure rats, the serum concentrations of TNF-α and IL-6 were signally higher than those of sham control group (Figure 3). In addition, the serum concentrations of TNF-α and IL-6 showed decreased after intermedin treatment in ischemic heart failure rats (Figure 3).
Figure 3.
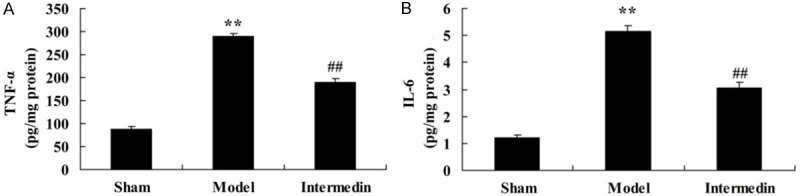
Intermedin attenuates the serum concentrations of TNF-α and IL-6 in a rat model of ischemic heart failure. Intermedin attenuates the serum concentrations of TNF-α (A) and IL-6 (B) in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; **P<0.01 versus the model group; ##P<0.01 versus the model group.
Intermedin attenuates the intracellular cAMP contents in a rat model of ischemic heart failure
To verify the cardiac protection of intermedin on intracellular cAMP contents in ischemic heart failure rats, we inspected intracellular cAMP contents with or without ischemic heart failure rat-treated with intermedin. Intracellular cAMP content of ischemic heart failure group was lower than that of sham group (Figure 4). In addition, the ischemic heart failure-induced intracellular cAMP content was obviously elevated compared with ischemic heart failure group (Figure 4).
Figure 4.
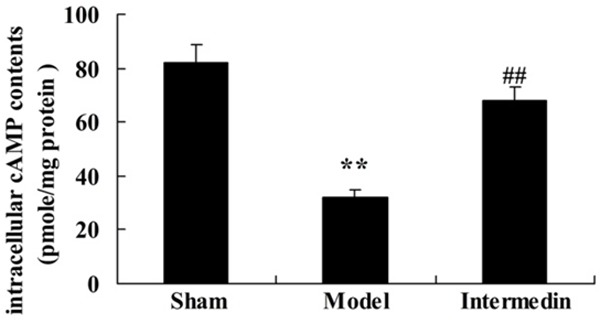
Intermedin attenuates the intracellular cAMP contents in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; **P<0.01 versus the model group; ##P<0.01 versus the model group.
Intermedin attenuates the caspase-3 protein in a rat model of ischemic heart failure
To check on the cardiac protection of intermedin on apoptotic in ischemic heart failure rat, the caspase-3 protein was measured using Western blot analysis. Ischemic heart failure aroused cell apoptosis and induced caspase-3 protein expression in rats, compared with sham group (Figure 5). Interestingly, pre-incubation with intermedin, a cardiac protection, inhibited caspase-3 protein expression of ischemic heart failure rat (Figure 5).
Figure 5.
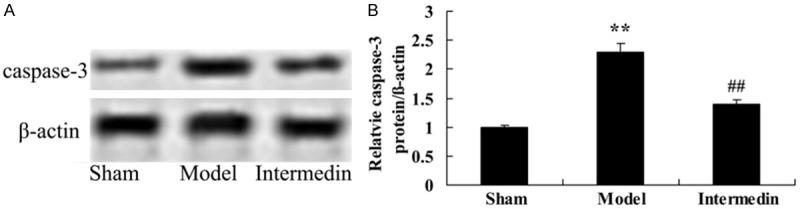
Intermedin attenuates the caspase-3 protein in a rat model of ischemic heart failure. Intermedin attenuates the caspase-3 protein using wstern blot analysis (A), statistical analysis of caspase-3 expression (B) in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; **P<0.01 versus the model group; ##P<0.01 versus the model group.
Intermedin increases the ERK1/2 protein in a rat model of ischemic heart failure
To reveal the functional relationship between cardiac protection of intermedin and apoptosis, ERK1/2 protein also analyzed using Western Blot Analysis. Our results showed that ERK1/2 protein expression was distinctly weakened in ischemic heart failure rat than that of sham group (Figure 6). Pretreatment with intermedin distinctly increased the inhibition of ERK1/2 protein expression in ischemic heart failure rat (Figure 6).
Figure 6.
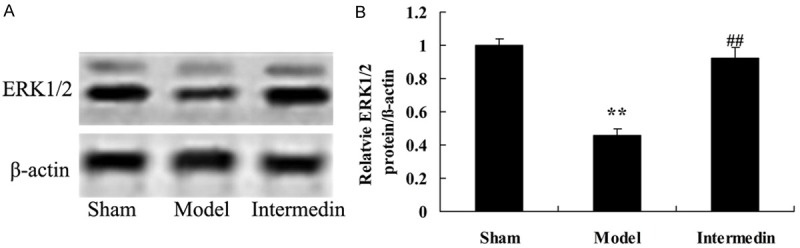
Intermedin increases the ERK1/2 protein in a rat model of ischemic heart failure. Intermedin attenuates the ERK1/2 protein using wstern blot analysis (A), statistical analysis of ERK1/2 expression (B) in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; **P<0.01 versus the model group; ##P<0.01 versus the model group.
Intermedin activates the autophagy in a rat model of ischemic heart failure
To explore the functional relationship between cardiac protection of intermedin and autophagy, H9c2 cell was used to structure the ischemic heart failure model for this study. However, there was no statistical difference in autophagy microscopy among sham group and model group. 4 weeks after intermedin, autophagy microscopy was distinctly observed in H9c2 cell-treated with intermedin (Figure 7).
Figure 7.
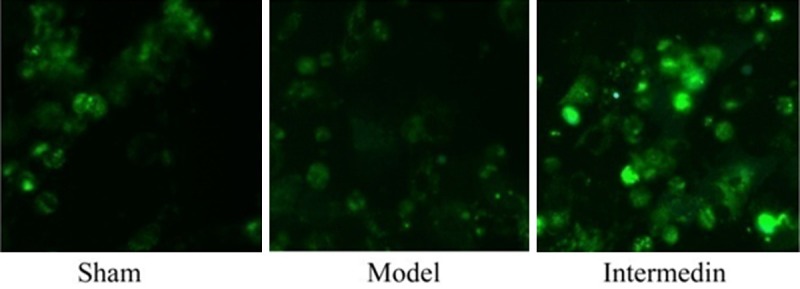
Intermedin activates the autophagy in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group.
Intermedin activates the LC3 protein in a rat model of ischemic heart failure
We further detected the effect of intermedin on cardiac protection by the LC3 protein expression in a rat model of ischemic heart failure. The LC3 protein expression was observe decrease in a rat model of ischemic heart failure, compared with sham group (Figure 8). However, treatment with intermedin clearly increased the LC3 protein expression in rat with ischemic heart failure (Figure 8).
Figure 8.
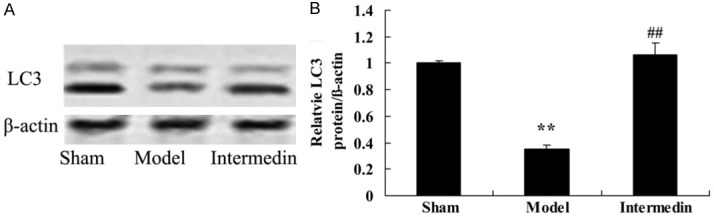
Intermedin activates the LC3 protein in a rat model of ischemic heart failure. Intermedin attenuates the LC3 protein using wstern blot analysis (A), statistical analysis of LC3 expression (B) in a rat model of ischemic heart failure. Sham, Sham group; Model, ischemic heart failure model group; Intermedin, Intermedin treated group; **P<0.01 versus the model group; ##P<0.01 versus the model group.
Discussion
Heart failure is a progressive disease, even if there is no new myocardial damage and it is at the stable stage from the clinical sense, but the heart failure itself still can develop into a deteriorate state [6]. In the process of the development of heart failure, there is a complex series of changes of myocardial structure, functions and phenotype, which are caused by molecules and cellular mechanisms [3]. Its features include pathological cardiomyocyte hypertrophy resulting in the decrease of myocardial cell’s contractility and lifetime; myocardial apoptosis, which is a turning point for heart failure from compensatory state to decompensated state; excessive fibrosis or degradation of myocardial cells’ matrix [14]. At the same time, heart failure causes the excitability increase of the renin - angiotensin- aldosterone system and sympathetic nervous system [15]. Heart failure also leads to the activation of various endogenous neuroendocrine secretion and cytokines. The long-term and chronic activation promotes myocardial remodeling, aggravates damage to myocardium and deterioration of cardiac function; and further activates the neuroendocrine secretion and cytokines, etc., thus all these factors mentioned above form a vicious cycle and aggravates the situation of heart failure [16]. In the present study, we observed that Intermedin attenuates myocardial infarction and the serum concentrations of CK, CK-MB, LDH and cTnT and inhibited the serum concentrations of TNF-α and IL-6 in a rat model of ischemic heart failure.
Under various causes of ischemic heart failure, myocardial contractility is weakened in equal degree, which transforms ATP in the cell into cAMP. The increase of CAMP in the cell strengthens the permeability toward Ca2+ of the cell membrane, and the heart rate will be faster, myocardial oxygen consumption increased correspondingly, therefore, further ischemic myocardial damage will be caused. And cAMP results in malignant ventricular arrhythmia by activating calcium channel, changing the permeability toward Ca2+ of the cell membrane and promoting adipose decompose of myocardial cell. We observed that myocardial infarction, intermedin reduced the intracellular cAMP contents in a rat model of ischemic heart failure. Li et al. suggest that intermedin enhances sympathetic outflow through cAMP/PKA signaling pathway [17].
Nowadays most theories believe that apoptosis is a “waterfall” activation process, which is regulated by the endogenous genes of cell, enzymes and signal conduction. However, the caspase family is the common pathway for all the apoptosis signals, and it is an important symbol of apoptosis. In the human cardiovascular system, the present studies confirm that caspases are expressed in myocardial cells, vascular smooth muscle cells, endothelial cells and fibroblasts. Caspase family consists of 13 members, of which the caspase-3 is the core member because of its apoptosis executive function. The distribution of Caspase-3 is more extensive. However, intermedin attenuates the caspase-3 protein in a rat model of ischemic heart failure. In addition, Du et al. reported that intermedin protected myocardial cell through suppression of caspase-3 in a rat model of severe acute pancreatitis [18].
After the activation of ERK1/2, the activated ERK1/2 shifts into series of transcription factors involving the nucleus phosphorylation and causes cell proliferation and differentiation [19]. Many studies have confirmed that ERK1/2 protein in hypertrophy myocardial tissue is expressed obviously for the patients suffering from hypertension and heart failure [19]. And its expression will progressively increase along with the deterioration of cardiac function. Judging from this point, it is obvious that ERK1/2 is closely related to the pathological myocardial remodeling [20]. In addition, other related researches show that the MEK1-ERK1/2 signal channel has close relationship with physiological cardiac hypertrophy, myocardial function enhancement and antiapoptotic effect, instead of the activation of P38 or JNK [21]. There are related signal pathways between physiological myocardial hypertrophy and pathological myocardial hypertrophy. But the exercise-oriented myocardial hypertrophy is often characterized by strengthening the function of cardiovascular system while pathological myocardial hypertrophy results in the decrease of cardiac function. In this study, intermedin increases the ERK1/2 protein in a rat model of ischemic heart failure. nXiong et al. indicate that intermedin inhibit chronic neuropathic pain injury through ERK1/2 [22].
Based on the present observation results, autophagy also appears in the myocardial cells with heart failure, including dilated cardiomyopathy, valvular heart disease and heart failure caused by ischemic heart disease [9,23]. Ischemic heart disease and dilated cardiomyopathy lead to 0.3% cells in the patients’ myocardial cells to produce cytoplasm of ubiquitin protein polymers in the form of particles at the end stage. And autophagy, apoptosis cells and necrosis cells can be detected in myocardial cells of dilated cardiomyopathy [23]. Besides, autophagy also can be found in animal models. In the animal models of human dilated cardiomyopathy, a typical autophagy vacuole is found in the myocardial cells of ground squirrels [24]. The fact that excessive expression of cathepsin D exists in the myocardial cells, and that cathepsin D existing in the membrane of myocardial cells is positive [23]. According to the facts mentioned above, we can know that the death of myocardial cell is secondary to autophagy. Autophagy also exists in the degradatied myocardial cells influenced by diphtheria toxin of transgenic ground squirrel [9]. We surprised to find Intermedin activates the autophagy in a rat model of ischemic heart failure. Chen et al. indicate that intermedin activated autophagy and suppresses pressure overload cardiac hypertrophy [10]. These results indicated Intermedin attenuates myocardial infarction through activation of autophagy in a rat model of ischemic heart failure.
LC3 is the homologue of ATG8 gene in mammalian cells, and LC3 is located in autophagy bubble and in the membrane surface of autophagy bubble [25]. It involves in the formation of autophagy body. LC3 can be divided into type I and type II. Before the formation of autophagy, the synthesis LC3 in cells develops into the LC3 of I type-the soluble in the cytoplasm after processing. This kind of LC3 belongs to regular expressive forms [26]. When the autophagy occurs, LC3 of type I goes through the processing and modification process of the ubiquitin sample, and gradually combines with phosphatidyl ethanolamine existing in the membrane surface of autophagy, in the end, LC3 of type II is formed [27]. The combination of LC3-II always exists in the membrane of intracellular autophagy body, and the amount of its content is in direct proportion to the number of autophagy bubble [28]. So the expressive intensity of LC3 is closely related to the activity of autophagy. In the present study, we observed that intermedin activates the LC3 protein in a rat model of ischemic heart failure. Chen et al. indicated that intermedin activated autophagy and suppresses pressure overload cardiac hypertrophy through regulating LC3 protein expression [10].
Our observation suggests that intermedin attenuates myocardial infarction-induced the serum concentrations of CK, CK-MB, LDH, cTnT, TNF-α and IL-6 in a rat model of ischemic heart failure. These results provide a protective effect of intermedin in ischemic heart failure and may be an interesting new approach for preventing myocardial infarction associated with suppression of cAMP and activation of MAPK/ERK1/2 pathways.
Acknowledgements
The study was supported by Natural Science Foundation young investigator grant program in Jiangsu Province (BK20140296).
Disclosure of conflict of interest
None.
References
- 1.Choi JH, Comess KA, Xu C, Park J, Kim Y. Development of an interactive Coronary Doppler Vibrometry system for detection of coronary artery disease. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:7195–7198. doi: 10.1109/IEMBS.2011.6091818. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu J, Qi Y, Xie C, Zhang Y. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. 2013;65:805–814. doi: 10.1002/art.37815. [DOI] [PubMed] [Google Scholar]
- 3.Bi YF, Mao JY, Wang XL, Hou YZ, Lu YZ, Soh SB, Zhang BL. Clinical epidemiology survey of the traditional Chinese medicine etiology and syndrome differentiation of coronary artery disease: study protocol of a multicenter trial. Zhong Xi Yi Jie He Xue Bao. 2012;10:619–627. doi: 10.3736/jcim20120604. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Xu B, Yang YJ, Zhang RY, Li JP, Qiao SB, Zhang JS, Hu J, Qin XW, Hong T, Chen JL, Huo Y, Shen WF, Gao RL. Sirolimus-eluting cobalt alloyed stents in treating patients with coronary artery disease: six-month angiographic and one-year clinical follow-up result. A prospective, historically controlled, multi-center clinical study. Chin Med J (Engl) 2007;120:533–538. [PubMed] [Google Scholar]
- 5.Burchill LJ, Lameijer H, Roos-Hesselink JW, Grewal J, Ruys TP, Kulikowski JD, Burchill LA, Oudijk MA, Wald RM, Colman JM, Siu SC, Pieper PG, Silversides CK. Pregnancy risks in women with pre-existing coronary artery disease, or following acute coronary syndrome. Heart. 2015;101:525–529. doi: 10.1136/heartjnl-2014-306676. [DOI] [PubMed] [Google Scholar]
- 6.Yan GH, Wang M, Yiu KH, Lau CP, Zhi G, Lee SW, Siu CW, Tse HF. Subclinical left ventricular dysfunction revealed by circumferential 2D strain imaging in patients with coronary artery disease and fragmented QRS complex. Heart Rhythm. 2012;9:928–935. doi: 10.1016/j.hrthm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhao K, Xu XS, Meng X, Li YL, Li JF, Chen WQ. Autophagy of monocytes attenuates the vulnerability of coronary atherosclerotic plaques. Coron Artery Dis. 2013;24:651–656. doi: 10.1097/MCA.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 8.Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Ghavami S, Cunnington RH, Gupta S, Yeganeh B, Filomeno KL, Freed DH, Chen S, Klonisch T, Halayko AJ, Ambrose E, Singal R, Dixon IM. Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis. 2015;6:e1696. doi: 10.1038/cddis.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi M, Saito T. Cytotoxicity of acetaldehyde-derived advanced glycation end-products (AA-AGE) in alcoholic-induced neuronal degeneration. Alcohol Clin Exp Res. 2005;29:220S–224S. doi: 10.1097/01.alc.0000190657.97988.c7. [DOI] [PubMed] [Google Scholar]
- 12.Weiss B. The first 83 and the next 83: perspectives on neurotoxicology. Neurotoxicology. 2009;30:832–850. doi: 10.1016/j.neuro.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers J, London L, Lucchini RG. Neurotoxicology and development: human, environmental and social impacts. Neurotoxicology. 2014;45:217–219. doi: 10.1016/j.neuro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian J, Xu B, Lansky AJ, Yang YJ, Qiao SB, Wu YJ, Chen J, Hu FH, Yang WX, Mintz GS, Leon MB, Gao RL. First report of a novel abluminal groove filled biodegradable polymer rapamycin-eluting stent in de novo coronary artery disease: results of the first in man FIREHAWK trial. Chin Med J (Engl) 2012;125:970–976. [PubMed] [Google Scholar]
- 15.Chen WR, Qian YA, Chen YD, Shi Y, Yin da W, Wang H, Zhu P, Liu HW, Sha Y. The effects of low vitamin D on coronary artery disease. Heart Lung Circ. 2014;23:314–319. doi: 10.1016/j.hlc.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Hu CL, Li YB, Tang YH, Chen JB, Liu J, Tang QZ, Zhang QH, Huang CX. Effects of withdrawal of Xuezhikang, an extract of cholestin, on lipid profile and C-reactive protein: a short-term time course study in patients with coronary artery disease. Cardiovasc Drugs Ther. 2006;20:185–191. doi: 10.1007/s10557-006-7947-x. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Sun HJ, Han Y, Wang JJ, Zhang F, Tang CS, Zhou YB. Intermedin enhances sympathetic outflow via receptor-mediated cAMP/PKA signaling pathway in nucleus tractus solitarii of rats. Peptides. 2013;47:1–6. doi: 10.1016/j.peptides.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Du X, Cao Y, Xue P, Lin Z, Zeng Z, Xia Q. Protective effect of intermedin on myocardial cell in a rat model of severe acute pancreatitis. Cell Mol Biol Lett. 2011;16:462–476. doi: 10.2478/s11658-011-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 20.Nunes de Carvalho S, Helal-Neto E, de Andrade DC, Costa Cortez EA, Thole AA, Barja-Fidalgo C, de Carvalho L. Bone marrow mononuclear cell transplantation increases metalloproteinase-9 and 13 and decreases tissue inhibitors of metalloproteinase-1 and 2 expression in the liver of cholestatic rats. Cells Tissues Organs. 2013;198:139–148. doi: 10.1159/000353215. [DOI] [PubMed] [Google Scholar]
- 21.Kim B, Abdel-Rahman MH, Wang T, Pouly S, Mahmoud AM, Cebulla CM. Retinal MMP-12, MMP-13, TIMP-1, and TIMP-2 expression in murine experimental retinal detachment. Invest Ophthalmol Vis Sci. 2014;55:2031–2040. doi: 10.1167/iovs.13-13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W, Qiu SY, Xu LY, Zhang CP, Yi Y, Wu Q, Huang LP, Liu SM, Wu B, Peng LC, Song MM, Gao Y, Liang SD. Effects of intermedin on dorsal root ganglia in the transmission of neuropathic pain in chronic constriction injury rats. Clin Exp Pharmacol Physiol. 2015;42:780–787. doi: 10.1111/1440-1681.12416. [DOI] [PubMed] [Google Scholar]
- 23.Cheng J, Qiao L, Xu X, Zhai C, Zhao K, Ji X, Chen W. Lower AMP-activated protein kinase level is associated with the vulnerability of coronary atherosclerotic plaques by attenuating the expression of monocyte autophagy. Coron Artery Dis. 2015;26:322–327. doi: 10.1097/MCA.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 24.Sabe AA, Elmadhun NY, Sadek AA, Chu LM, Bianchi C, Sellke FW. Differential effects of atorvastatin on autophagy in ischemic and nonischemic myocardium in Ossabaw swine with metabolic syndrome. J Thorac Cardiovasc Surg. 2014;148:3172–3178. doi: 10.1016/j.jtcvs.2014.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu XC, Zhang J, Xu BH, Cai L, Ragaz J, Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, Yin YM, Wu CP, Jiang ZF, Wang XJ, Lou GY, Liu DG, Feng JF, Luo JF, Sun K, Gu YJ, Wu J, Shao ZM. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–446. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 26.Du L, Wei H, Li L, Shan H, Yu Y, Wang Y, Zhang G. Regulation of recombinant Trichinella spiralis 53-kDa protein (rTsP53) on alternatively activated macrophages via STAT6 but not IL-4Ralpha in vitro. Cell Immunol. 2014;288:1–7. doi: 10.1016/j.cellimm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- 28.Fisman EZ, Adler Y, Tenenbaum A. Biomarkers in cardiovascular diabetology: interleukins and matrixins. Adv Cardiol. 2008;45:44–64. doi: 10.1159/000115187. [DOI] [PubMed] [Google Scholar]


