Abstract
Purpose: To analyze the correlation between proinflammatory effects of a lectin from Typhonium giganteum Engl. and macrophage. Methods: T. giganteum lectin (TGL) was extracted from the tuber of T. giganteum and purified, and was then identified by using SDS-PAGE gel electrophoresis in combination with mass spectrometry. The morphologic changes of macrophage after being stimulated by TGL were observed with scanning electron microscopy. The influences of such stimulation on neutrophil migration were evaluated by establishing an in vitro macrophage-neutrophil co-culture migration model. By establishing a rat peritoneal macrophage in vitro cultured model, the effects of TGL stimulation on inflammatory factors TNF-α and IL-1β released by macrophage were analyzed. With p65 as the index, the expressions of the NF-κB signaling pathway in the cytoplasm and nucleus were detected before and after TGL stimulation respectively. Furthermore, we also investigated whether the inhibitor for NF-κB signaling pathway BAY11-7082 can block p65 nuclear translocation. Results: After being stimulated by TGL, macrophage had increased volume, number of pseudopodia and gradually cracked cell membrane, accompanied by evidently induced migration of neutrophils due to released inflammatory factors. As the concentration of TGL varied, NF-κB’s monomer p65 had different expression levels in the cytoplasm and nucleus, while BAY11-7082 can indeed block the nuclear translocation of p65. Conclusions: TGL-induced inflammation was closely related to macrophage mediation.
Keywords: Typhonium giganteum Engl. Lectin, macrophage, NF-κB
Introduction
Typhonii Rhizoma (TR), which is the dry tuber of Typhonium giganteum Engl., is known as a toxic herb as it tastes spicy and can irritate the tongue and throat. We have previously confirmed that the tuber of this plant contained T. giganteum lectin (TGL) [1]. Lectins are (glyco) proteins of non-immune origin that interact reversibly and specifically with carbohydrates [2,3]. These proteins are widely distributed in microbe, virus and animal. Lectins can also play a role in pattern recognition [4-6].
The inflammatory response requires rather complex coordination of various types of immune cells such as macrophages, mast cells, lymphocytes, endothelial cells and neutrophils [7,8]. Once challenged by proinflammatory stimuli, these cells can be regulated by chemicals to elicit appropriate inflammatory responses [9,10]. Noticeably, the migration of neutrophils to the sites of inflammation serves as an important step in cellular inflammatory responses [11]. Our recent work found that intraperitoneal injection of TGL can induce the peritoneal migration of neutrophils and argued a proinflammatory role of TGL [12,13]. However, the exact molecular mechanisms remain elusive.
In response to xenogetic stimuli, macrophages can fulfill the role of defending cells and secrete massive chemokines [9,10]. Our previous studies have also found that after being intraperitoneally injected into rats, TGL increased macrophages and elevated the inflammatory factor levels significantly, thereby inducing the migration of neutrophils towards the injection site [12]. These results suggested that macrophages might possibly play a crucial role in TGL mediated inflammatory responses. The macrophage mediated inflammatory responses are highly dependent on NF-kB signaling [14-16] Once activated, the p65 monomer can translocate from cytoplasm to nucleus and regulate the expression of target genes [17-19]. Therefore, monitoring the expression of p65 in cytoplasm and nucleus can indicate whether NF-kB signaling has been activated to further unravel the association between macrophages and TGL-mediated inflammatory responses. Therefore, TGL may induce inflammation, following an unclear mechanism though. Thereby motivated, the morphologic changes of macrophage after being stimulated by TGL were observed by scanning electron microscopy (SEM), and the correlation between TGL-produced inflammatory response and macrophage was analyzed by using a rat peritoneal macrophage-neutrophil co-culture migration model in vitro, ELISA and Western blotting. The results provide valuable evidence for clarifying the toxic action of TR and other toxic herbs in the family Araceae.
Materials and methods
Animals
Male SPF-grade SD rats weighing 200-250 g were provided by the Experimental Animal Center of Zhejiang Province (license No.: SCKK (Z) 2014-0001).
Extraction and purification of TGL [20]
Tuber of T. giganteum (100 g) was cleaned, cut into pieces, and adding 200 mL of deionized water. Then the mixture was centrifuged at 8000 rpm for 0.5 h. Afterwards, the supernatant was collected, added saturated ammonium sulfate to a saturability of 45%, and centrifuged again at 8000 rpm for 15 min. The resulting precipitate was the crude extract of TGL.
The crude extract was then dissolved by adding 0.6 mol/L ammonium sulfate solution, and chromatographed with a lyophobic column filled with Phenyl SepharoseTM High Performance (5 mL of sample each time) to be eluted with 0.6-0 mol/L ammonium sulfate solution within 60 min at the flow rate of 2 mL/min. The eluting peaks were thereafter collected.
The above liquid was transferred to an ion exchange column filled with Phenyl SepharoseTM High Performance, and then eluted with 0-1 mol/L sodium chloride solution within 60 min at the flow rate of 2 mL/min to collect the eluting peaks. Subsequently, the solutions were mixed, dialyzed and lyophilized, yielding the purified TGL.
SDS-PAGE analysis [1]
An appropriate amount of purified TGL was treated by adding lysate, quantified, loaded, and subjected to SDS-PAGE with 12% resolving gel (pH 8.8), 5% stacking gel (pH 6.8), Tris-glycine (pH 8.3) as the electrode buffer, as well as silver staining. The results showed that the protein was electrophoretically pure.
Mass spectrum identification
The protein band of TGL on SDS-PAGE was cut off and placed in a centrifuge tube (cut into about 1 mm3 gels), with blank gels as the comparison. Then the gels were subjected to decoloration, enzymolysis, extraction, and liquid chromatography-mass spectrometry, referring to database in software BIOWORKS. As proteins from the Araceae family have seldom been studied, NCBI green plant Viridiplantae protein database was employed to enlarge the range of searching and to increase the total number of identifiable proteins. SEQUEST results were filtered with the following parameters: When Charge +1, Xcorr (cross-correlation) ≥1.9; when Charge +2, Xcorr ≥2.2; when Charge +3, Xcorr ≥3.75; if DelCN ≥0.1, the identification results were considered reliable [21]. The extracted and purified protein was demonstrated as TGL.
Extraction and purification of macrophage
A healthy rat was killed by cervical dislocation, immersed in 75% ethanol for several minutes, and intraperitoneally injected with 20 mL of PBS buffer. Then the belly was tenderly massaged for 5 min to allow PBS buffer to be well distributed inside the whole intraperitoneal cavity. The peritoneal fluid was thereafter collected and centrifuged at 1000 rpm for 5 min to obtain the precipitate rich in macrophages. After the supernatant was discarded, the precipitate was re-suspended with RPMI1640 culture medium containing 10% fetal calf serum to the cell density of 1×106/mL. Subsequently, the cells were inoculated into a cell plate and cultured with 5% CO2 at 37°C for 2 h, after which the none-adherent cells were washed by using PBS buffer, leaving the desired macrophages finally. Then the macrophages were cultured in RPMI1640 culture medium without 10% fetal calf serum for another 12 h to reach the serum starvation state.
Scanning electron microscopy
Peritoneal macrophages were inoculated at the density of 1×106/mL into 6-well culture plates equipped with aseptic cover glasses and cultured to the serum starvation state. After sterilization by ultraviolet radiation, TGL solution was prepared by adding PBS buffer. The cultured macrophages were then divided into 7 groups, and the experiments for each group were conducted in duplicate. TGL solutions with the same volumes were added into the model group to keep the final concentrations at 6.25 μg/mL, 12.5 μg/mL and 25 μg/mL (two groups each). The blank group was added PBS buffer with the same volume, and cultured in 5% CO2 at 37°C. Culture was stopped after 0.5 h and 2 h respectively for the model group. After the culture medium was discarded, the macrophages were washed with PBS buffer, and fixed in 2.5% glutaraldehyde overnight at 4°C. The cover glasses bearing fixed macrophages were thereafter dehydrated in ethanol at the mass concentrations of 30%, 50%, 70%, 80%, 90% and 100% respectively to observe and to photograph the morphology of the macrophages by SEM (6000× magnification) after gold spraying.
Transwell assay
Extraction and purification of human neutrophils [22]
Peripheral venous blood of healthy volunteers was collected, into which 6% Dextran-T500 was added to sufficiently precipitate erythrocytes. Afterwards, the mixture was centrifuged at 1500 rpm for 10 min after the supernatant was removed. After the supernatant was discarded again, the precipitate was re-suspended with RPMI1640 culture medium. Percoll stock solution was then mixed with 8.77% sterilized NaCl solution at the ratio of 9:1 as 100% Percoll that was prepared into 60% and 75% Percoll solutions with sterilized normal saline. The 60% and 75% Percoll solutions as well as the cell suspension were added into a centrifuge tube at the volume ratio of 2:2:1, which was then centrifuged at 2000 rpm for 20 min to collect the cell layer between the two Percoll solutions. Eventually, purified neutrophils were obtained by washing the cell layer three times with PBS buffer.
Neutrophil chemotaxis
By using an in vitro transwell co-culture migration model, 100 μL of neutrophil suspension mixed with RPMI1640 culture medium was added in the upper chamber at the density of 1×106/mL, and the lower chamber was divided into 10 groups: (1) RPMI1640 + PBS buffer; (2) pre-cultured macrophage + PBS buffer; (3), (4), (5) and (6) were RPMI1640 + TGL solution prepared by PBS buffer, with the final concentrations of 12.5 μg/mL, 25 μg/mL, 50 μg/mL and 100 μg/mL respectively; and (7), (8), (9) and (10) were pre-cultured macrophage + TGL solution prepared by PBS buffer, with the final concentrations of 12.5 μg/mL, 25 μg/mL, 50 μg/mL and 100 μg/mL respectively. The experiments for each group were performed in triplicate. After being cultured for 1 h in 5% CO2 at 37°C, the transwell chamber was taken out, and residual cells on the inner surface were scraped with cotton swabs. Then the outer surface of the chamber was stained by using rapid Wright’s-Giemsa stain solution, and observed under a 400× microscope to count the number of neutrophils that passed through the microporous membrane.
ELISA
Rat peritoneal macrophages (500 μL) were inoculated at the density of 1×106/mL into 48-well culture plates and cultured until the serum starvation state. After sterilization by ultraviolet radiation, TGL solution was prepared by adding PBS buffer. The cultured macrophages were divided into 5 groups (experiments for each group were conducted in triplicate), of which 4 groups were given TGL solutions with the same volume but at different concentrations, making the final concentrations at 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL and 50 μg/mL respectively. The blank group was given PBS buffer with the same volume, and cultured for 1 h in 5% CO2 at 37°C after being shaken evenly. The cell supernatant was thereafter collected, in which the levels of inflammatory factors TNF-α and IL-1β were detected according to the instructions of ELISA kit.
Western blotting
Preparation of protein sample from macrophage was made as previously described [23].Macrophages were inoculated at the density of 1×107/mL into a cell culture dish and cultured into the serum starvation state. After sterilization by ultraviolet radiation, TGL solution was prepared by using PBS buffer. The cultured macrophages were divided into 4 groups (experiments for each group were performed in triplicate), of which 3 groups were given TGL solutions with the same volume but at different concentrations, making the final concentrations at 12.5 μg/mL, 25 μg/mL and 50 μg/mL respectively. The blank group was given PBS buffer with the same volume, and cultured for 0.5 h in 5% CO2 at 37°C after being shaken evenly.
6 more groups of cultured macrophages (experiments for each group was performed in triplicate) were further prepared while 5 of which were treated with 0 μM, 1 μM, 5 μM, 10 μM, 20 μM BAY11-7082, respectively and the remaining group was given PBS buffer with the same volume. After 1 h incubation, all 6 groups were treated with 50 μg/mL TGL solutions and cultured for 0.5 h in 5% CO2 at 37°C after being shaken evenly.
After TGL stimulation for 0.5 h, the culture supernatant was discarded, and the macrophages were washed with PBS buffer. Then the macrophages were carefully scraped on ice and centrifuged at 2000 rpm for 5 min. After the supernatant was discarded, the residue was added cytomembrane lysis solution, leaved in ice bath for 5 min, and centrifuged at 14000 rpm for 10 min to collect the supernatant rich in cytoplasmic protein. The precipitate was re-suspended with nuclear lysis solution, leaved in ice bath for 40 min, shaken evenly every 10 min, and centrifuged at 14000 rpm for 15 min to collect the supernatant which contained abundant nuclear protein.
Electrophoresis
SDS-PAGE gel was prepared by using 10% gel formulation. The cytoplasmic and nuclear protein solutions were denatured by being boiled at 100°C for 5 min, and mixed evenly with 5× bromophenol blue loading buffer. Afterwards, 5 μL of protein marker, as well as cytoplasm and nuclear protein samples were loaded (10 μL for each lane) respectively. Electrophoresis was carried out in ice bath until bromophenol blue migrated to the bottom of the gel, under the condition for stacking gel being 10 mA constant current and that for resolving gel being 16 mA.
Membrane transfer and immuno-hybridization
After electrophoresis, protein in the gel was transferred to a membrane with semi-dry transfer method at the constant voltage of 15 V for 20 min. After the transfer, the PVDF membrane was immersed in a 5% skim milk solution and blocked for 3 h. Then the membrane was cut into pieces with appropriate sizes according to the protein marker, and the membranes containing p65 and GAPDH were subjected to immuno-hybridization respectively.
The antibody was diluted with 5% skim milk solution. p65 and GAPDH primary antibodies were diluted 1000 and 1500 times respectively, and goat anti-rabbit secondary antibody was diluted 7500 times.
Antibodies were cultured at 4°C overnight and washed with PBST (3 times, 10 min each) after the antibody solutions were discarded. Secondary antibody was cultured at room temperature for 1 h, and the residual was washed with PBST (3 times, 10 min each).
Gel-imaging and gray value calculation
Preheated ECL chromogenic reagent was mixed with the membrane and cultured for 5 min in dark. With excessive color-substrate solution absorbed, a gel-imaging system was used to expose p65 and GAPDH primary antibodies for 150 s and 25 s respectively.
The exposed image was then imported into Quality one image analysis software. After selection of lanes and bands, the gray value of each band was calculated after the interference of background was deducted, and the ratio of gray value of objective band to that of internal reference band (p65/GAPDH, p65/ GAPDH) was also calculated.
Statistical method
All data were analyzed by statistical software SPSS 16.0 and expressed as (x̅ ± s). Inter-group comparisons were performed by using independent two-sample t-test, and the comparisons among multiple groups employed analysis of variance and test for homogeneity of variance.
Results
Surface morphology change of macrophage
After stimulation with 6.25 μg/mL purified TGL for 0.5 h and 2 h respectively, the morphology of macrophage did not change evidently. However, macrophage had increased volume and obvious wrinkles on the surface after being stimulated with 12.5 μg/mL TGL for 2 h, suggesting that the macrophage was activated. After stimulation with 25 μg/mL TGL for 0.5 h, pseudopod was produced on the cell surface, indicating that the macrophage was activated, and it died 2 h later owing to cytomembrane damages (Figure 1). Hence, TGL was able to activate the phagocytic function of macrophage. Besides, the proinflammatory effect of TGL was closely associated with macrophage.
Figure 1.
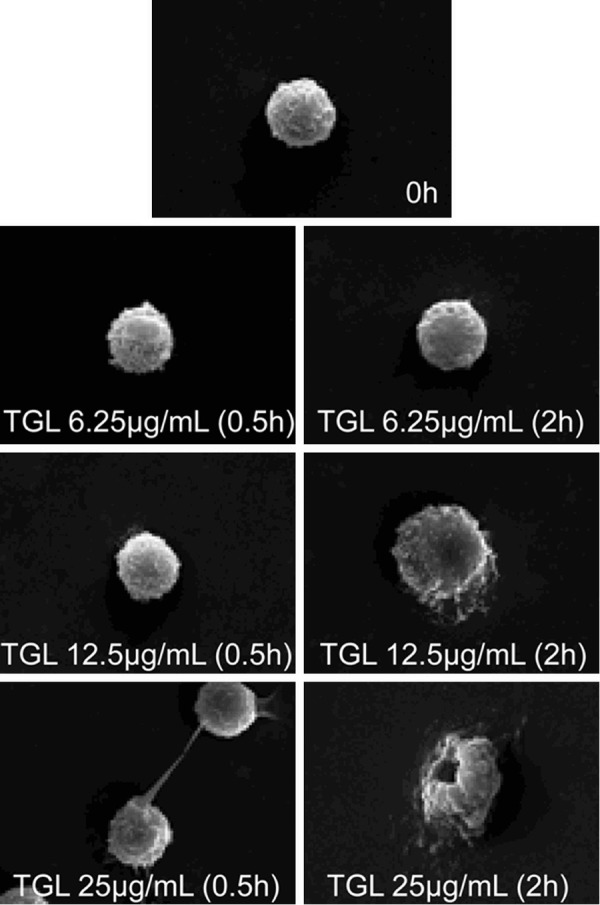
Surface morphology change of macrophage. SEM images showing that After using 6.25 μg/mL purified TGL to stimulate macrophage for 0.5 h and 2 h respectively, there was no evident change in the morphology of macrophage, but after using 12.5 μg/mL to stimulate macrophage for 2 h, the macrophage increased in volume, evident wrinkles were found on surface, after using 25 μg/mL to stimulate macrophage for 0.5 h, pseudopod was produced on cell surface, and it died 2 h later owing to cytomembrane damages.
Neutrophil chemotaxis
After TGL (12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL) stimulation for 2 h, the number of migrating neutrophils increased compared with that of the PBS+MΦ group (P<0.01). Compared with the PBS group, neither the macrophage group nor the TGL group significantly induced migration of neutrophils (Figure 2). Accordingly, in the macrophage-neutrophil co-culture system, TGL stimulation dramatically induced the migration and aggregation of neutrophils through the mediation of macrophage. The migration of neutrophils was the first step of inflammatory response, so the proinflammatory effect of TGL was closely related to macrophage.
Figure 2.
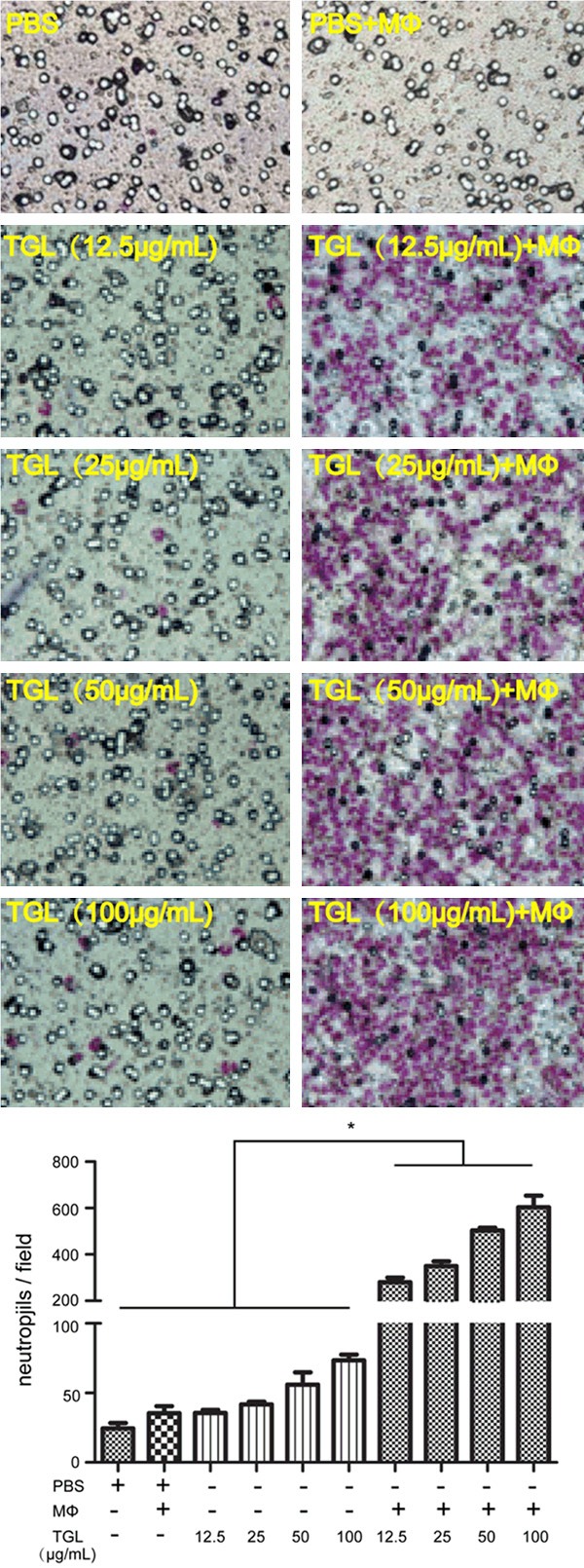
Neutrophil chemotaxis. Transwell images showing that After TGL (12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL) stimulation for 2 h, the number of migrating neutrophils increased compared with that of the PBS+MΦ group. Compared with the PBS group, neither the macrophage group nor the TGL group significantly induced migration of neutrophils; *P<0.01 as compared to the PBS+MΦ group.
Release of inflammatory factors
After stimulation with TGL at different concentrations (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL and 50 μg/mL) for 1 h, TNF-α and IL-1β contents in the culture supernatants significantly exceeded those of the blank group (P<0.01), which increased dose-dependently (Figures 3 and 4). This result, together with that of migration of neutrophil in vitro mentioned above, further verified that TGL induced evident inflammatory stimulation.
Figure 3.
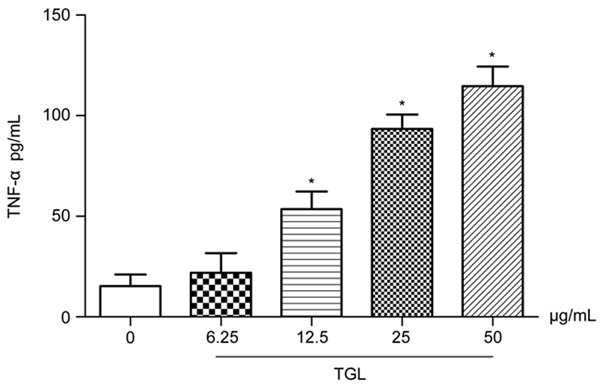
Release of TNF-α. After using TGL of different concentrations (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL and 50 μg/mL) to stimulate macrophage for 1 h, TNF-α in the culture supernatants significantly increased, which rised dose-dependently. *P<0.01 as compared to the blank group.
Figure 4.
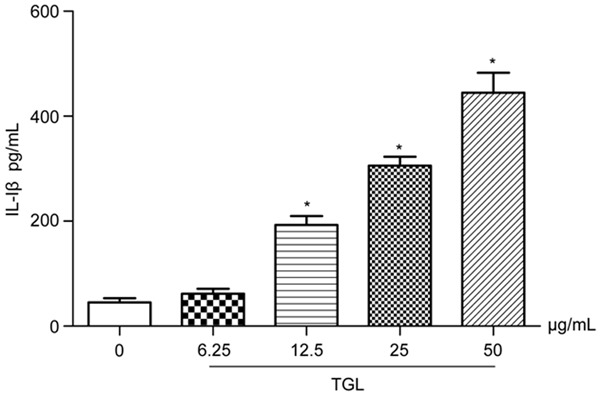
Release of IL-1β. After using TGL of different concentrations (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL and 50 μg/mL) to stimulate macrophage for 1 h, IL-1β in the culture supernatants significantly increased, which rised dose-dependently. *P<0.01 as compared to the blank group.
Expression of p65 in cytoplasm and nucleus
After stimulation with 12.5 μg/mL, 25 μg/mL and 50 μg/mL TGL for 0.5 h, the content of p65 in the macrophage cytoplasm plummeted compared with that of the blank group (Figure 5), but this content in the macrophage nucleus remarkably increased (Figure 6), all showing evident dose-effect relationships. Therefore, TGL stimulation caused transfer of p65 from the macrophage cytoplasm to the nucleus, which suggested the inflammation induced by TGL-stimulated macrophage was associated with the NF-κB signaling pathway.
Figure 5.
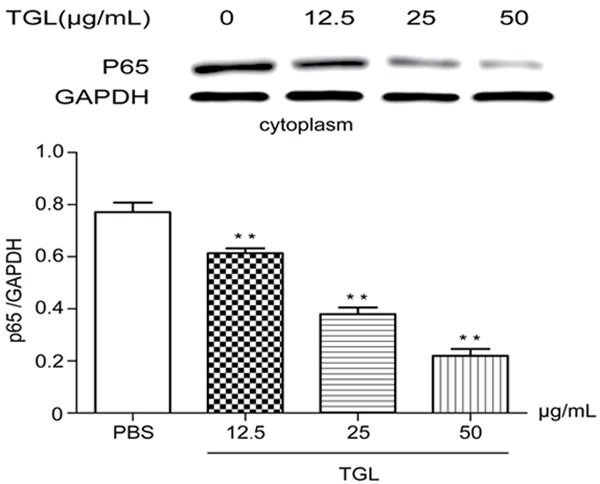
Expressions of p65 in cytoplasm. After stimulation with 12.5 μg/mL, 25 μg/mL and 50 μg/mL TGL for 0.5 h, the content of p65 in the macrophage cytoplasm plummeted compared with that of the blank group (**P<0.01).
Figure 6.
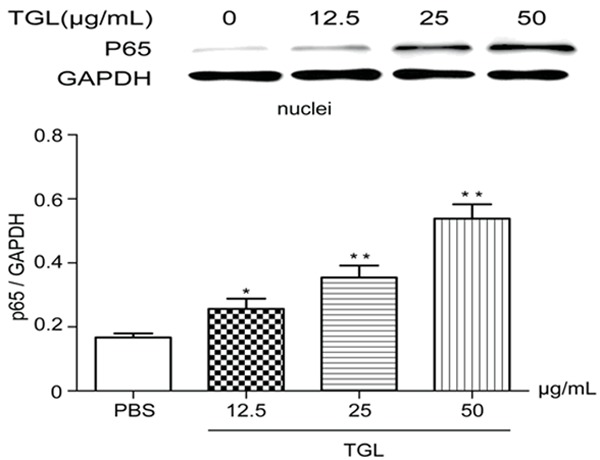
Expressions of p65 in nucleus. After stimulation with 12.5 μg/mL, 25 μg/mL and 50 μg/mL TGL for 0.5 h, the content of p65 in the macrophage nucleus remarkably increased compared with that of the blank group (**P<0.01, *P<0.05).
Expression of p65 in cytoplasm and nucleus under BAY11-7082 treatment
Macrophages were incubated with 1 μM, 5 μM, 10 μM, 20 μM of BAY11-7082 for 1 h and then challenged with 50 μg/mL TGL for 0.5 h. Compared with blank control, the content of p65 in cytoplasm was increased with higher BAY11-7082 concentrations (Figure 7). While the nuclear fraction was significantly decreased proportionally (Figure 8). These results suggested that BAY11-7082 can block nuclear translocation of p65 and effectively attenuate TGL induced macrophage activation. These findings may also establish a correlation between TGL induced macrophage activation and NF-kB signaling.
Figure 7.
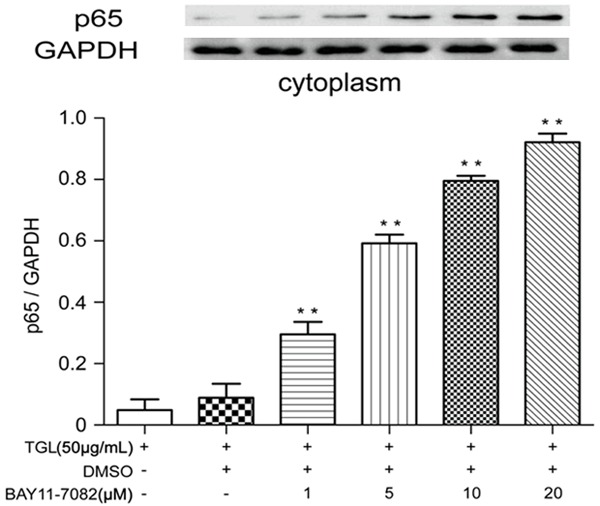
Expressions of p65 in cytoplasm under BAY11-7082 treatment. After incubation with 1 μM, 5 μM, 10 μM, 20 μM BAY11-7082 for 1 h, the content of p65 in the macrophage cytoplasm remarkably increased compared with that of the blank group (**P<0.01).
Figure 8.
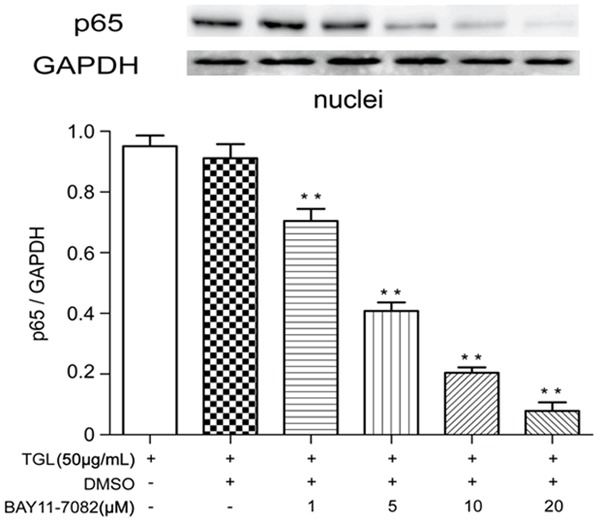
Expressions of p65 in nucleus under BAY11-7082 treatment. After incubation with 1 μM, 5 μM, 10 μM, 20 μM BAY11-7082 for 1 h, the content of p65 in the macrophage nucleus compared with that of the blank group (**P<0.01).
Discussion
Macrophages are immune phagocytes that can swallow bacteria or foreign particles and participate into the defense process of human body, which play important roles in the development of inflammation [11]. Cytokine release and neutrophil migration play key roles in the occurrence and progression of inflammation [11,24]. A large number of literatures have found that plant lectins can stimulate macrophage to release inflammatory mediator nitric oxide, prostaglandin E2, tumor necrosis factor and other cytokines [25-27] to induce the migration of neutrophils to sites of inflammation [28-30]. Similarly, in this study, TGL stimulated macrophage to release inflammatory factors TNF-α and IL-1β, and to mediate the migration and aggregation of neutrophils. These results demonstrated a proinflammatory role of TGL mediated by macrophages. As observed by SEM, when TGL invades human body, it is swollen by macrophages, during which the morphology changes vastly, further arguing that macrophages were critically involved in TGL induced inflammatory responses.
The inflammatory responses depend on the proinflammatory factors secreted by macrophages. Meanwhile, the proinflammatory signaling pathway in macrophages further determines the production of these factors [14,24]. The NF-kB pathway has been reported as one of the important pathways responsible for inflammatory responses and the activation of which can be indicated by p65 nuclear translocation [14-16]. In current study, Western blotting results exhibited that the stimulating effect of TGL on macrophage caused the transfer of p65 from the cytoplasm to the nucleus, the nuclear translocation of p65 was severely attenuated after BAY11-7082 treatment indicating that such inflammation was related with the NF-κB signaling pathway. These results again suggested that macrophages play pivotal roles in TGL induced inflammation. Our current study, to some extent, unraveled the molecular mechanisms of TR induced toxicological effect. The findings herein provide valuable evidence for elucidating the toxic mechanism of TR and other toxic herbs in the family Araceae.
Acknowledgements
This research was supported by National Nature Science Fund of China (30973939, 81173549), Research Fund for the Doctoral Program of Higher Education (20113237120010), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (2011ZYX2-012).
Disclosure of conflict of interest
None.
References
- 1.Yu HL, Zhu FG, Wu H. Toxic Proteins on Raphides from Pinellia ternata and Pinellia pedatisecta. China Journal of Traditional Chinese Medicine and Pharmacy. 2011;26:1037–1042. [Google Scholar]
- 2.Peumans WJ, VDamme EJL. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinik Y, Shatz-Azoulay H, Vivanti A, Hever N, Levy Y, Karmona R, Brumfeld V, Baraghithy S, Artta-Lamdar M, Boura-Halfon S, Bab I, Zick Y. The mammalian lectin galectin-8 induces RANKL expression, osteoclastogenesis, and bone mass reduction in mice. Elife. 2015;4:1–19. doi: 10.7554/eLife.05914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai CZ, Ji HJ, Feng ML, Hao XL, Zhong QM, Cui XD, Wang ZH. Stimulation of dendritic cell maturation and induction of apoptosis in lymphoma cells by a stable lectin from buckwheat seeds. Genet Mol Res. 2015;14:2162–2175. doi: 10.4238/2015.March.27.3. [DOI] [PubMed] [Google Scholar]
- 5.Pujari R, Kumar N, Ballal S, Eligar SM, Anupama S, Bhat G, Swamy SM, Inamdar SR, Shastry P. Rhizoctonia bataticola lectin (RBL) induces phenotypic and functional characteristics of macrophages in THP-1 cells and human monocytes. Immunol Lett. 2015;163:163–172. doi: 10.1016/j.imlet.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Cronstein BN, Weissman G. The adhesion molecules of inflammation. Arthritis Rheum. 1993;36:147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- 7.Malik AB, Lo SK. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol Rev. 1996;48:213–229. [PubMed] [Google Scholar]
- 8.McEver RP. Leukocyte-endothelial cell interactions. Curr Opin Cell Biol. 1992;4:840–849. doi: 10.1016/0955-0674(92)90109-p. [DOI] [PubMed] [Google Scholar]
- 9.Baines KJ, Backer V, Gibson PG, Powel H, Porsbjerg CM. Impaired lung function is associated with systemic inflammation and macrophage activation. Eur Respir J. 2015;45:557–559. doi: 10.1183/09031936.00187514. [DOI] [PubMed] [Google Scholar]
- 10.Wen Z, Fan L, Li Y, Zou Z, Scott MJ, Xiao G, Li S, Billiar TR, Wilson MA, Shi X, Fan J. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: role in posthemorrhagic shock acute lung inflammation. J Immunol. 2014;193:4623–4633. doi: 10.4049/jimmunol.1400899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai DM. Pathology. In: Lai DM, editor. The reasons of Inflammation. Beijing: People’s Medical Publishing House; 2014. pp. 49–52. [Google Scholar]
- 12.Liu XQ. A study on correlation between lectin from Araceae and toxity. Nanjing University of Chinese Medicine. 2012:65. [Google Scholar]
- 13.Liu XQ, Wu H, Yu HL, Zhao TF, Pan YZ, Shi RJ. Purification of a Lectin from Arisaema erubescens (Wall. ) Schott and its pro-inflammatory effects. Molecules. 2011;16:9480–9494. doi: 10.3390/molecules16119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HR, Shin da Y, Chung KH. The role of NF-kappaB signaling pathway in polyhexamethylene guanidine phosphate induced inflammatory response in mouse macrophage RAW264.7 cells. Toxicol Lett. 2015;233:148–155. doi: 10.1016/j.toxlet.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang Y, Lu M, Qiao X, Sun B, Zhang W, Xue D. Modular analysis of bioinformatics demonstrates a critical role for NF-kappaB in macrophage activation. Inflammation. 2014;37:1240–1253. doi: 10.1007/s10753-014-9851-z. [DOI] [PubMed] [Google Scholar]
- 16.Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-kappaB activation and enhancing autophagy. PLoS One. 2014;9:e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 18.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-κB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Pahl HL. Activators and target genes of Rel/NF-kB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 20.Pan YH, Zhang SX, Cao JP, Yang XM, Zhang J, Yin WZ, Huang DF. Purification and separation of and the anti-aphid activity of lectin Pinellia pedatisecta. Prog Nat Sci. 1998;8:502–505. [Google Scholar]
- 21.Xiao F, Chen D, Lu Y, Xiao Z, Guan LF, Yuan J, Wang L, Xi ZQ, Wang XF. Proteomic analysis of cerebrospinal fluid from patients with idiopathic temporal lobe epilepsy. Brain Res. 2009;1255:180–189. doi: 10.1016/j.brainres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Li JF, Liu WL, Shi XJ, Liu W, Han JZ, Wan J, Luo ZQ. Comparison of four common human neutrophil separation method. Int J Pathol Clin Med. 2008;28:277–281. [Google Scholar]
- 23.Kim MK, Chunga SW, Kima DH, Kim JM, Ha YM, Kim YH, No JK, Chung HS, Park KY, Rhee SH, Choi JS, Yu BP, Yokozawa T, Kim YJ, Chung HY. Modulation of age-related NF-κB activation by dietary zingerone via MAPK pathway. Exp Gerontol. 2008;45:419–426. doi: 10.1016/j.exger.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Wang XL. Prarctical Molecular Pharmacology. In: Wang XL, editor. Inflammatory mediators and anti-inflammatory drugs. Beijing: Peking Union Medical College Publishers; 2005. pp. 433–438. [Google Scholar]
- 25.Andrade JL, Arruda S, Barbosa T, Paim L, Ramos MV, Cavada BS, Barral-Netto M. Lectin-induced Nitric Oxide production. Cell Immunol. 1999;194:98–102. doi: 10.1006/cimm.1999.1494. [DOI] [PubMed] [Google Scholar]
- 26.Kang TB, Yoo YC, Lee KH, Yoon HS, Her E, Kim JB, Song SK. Korean mistletoe lectin (KML-IIU) and its subchains induce nitric oxide (NO) production in murine macrophage cells. J Biomed Sci. 2008;15:197–204. doi: 10.1007/s11373-007-9210-2. [DOI] [PubMed] [Google Scholar]
- 27.Figueiredo JG, Bitencourt FS, Mota MR, Silvestre PP, Aguiar CN, Benevides RG, Nascimento KS, De Moura TR, Del-secco D, Assreuy AM, Cunha Fde Q, Vale MD, Cavada BS, Alencar NM. Pharmacological analysis of the neutrophil migration induced by D. rostrata lectin: involvement of cytokines and nitric oxide. Toxicon. 2009;54:736–744. doi: 10.1016/j.toxicon.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Rangel TB, Assreuy AM, Pires Ade F, Carvalho AU, Benevides RG, Simose Rda C, Silva HC, Bezerra MJ, Nascimento AS, Nascimento KS, Nagano CS, Sampaio AH, Delatorre P, Rocha BA, Fernandes PM, Cavada BS. Crystallization and characterization of an inflammatory lectin purified from the seeds of Dioclea wilsonii. Molecules. 2011;16:5087–5103. doi: 10.3390/molecules16065087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Queiroz AF, Moura RM, Ribeiro JK, Lyra IL, Cunha DC, Santos EA, De-Sales MP. Pro-inflammatory effect in mice of CvL, a lectin from the marine sponge Cliona varians. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:216–221. doi: 10.1016/j.cbpc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Alencar NM, Assreuy AM, Havt A, Benevides RG, Te Moura TR, De Sousa RB, Ribeiro RA, Cunha FQ, Cavada BS. Vatairea macrocarpa (Leguminosae) lectin activates cultured macrophages to release chemotactic mediators. Naunyn-Schmiedeberg’s Arch Pharmacol. 2007;374:275–282. doi: 10.1007/s00210-006-0124-8. [DOI] [PubMed] [Google Scholar]


