Abstract
Prdx1 is an important member of peroxiredoxins (Prdxs) regulating various cellular signaling and differentiation. Prdx1 confers an aggressive survival phenotype of cancer cells and drug-resistance, yet its role in hilar cholangiocarcinoma is not fully investigated. In present study, we detected the expression profile of Prdx1 in 88 hilar cholangiocarcinoma by tissue arrays and immunohistochemistry. Prdx1 level was down-regulated by specific Prdx1-shRNA in vitro and the possible mechanism was investigated. Overexpression of Prdx1 was observed in 53 of 88 cases (60.2%). Prdx1 expression was significantly associated with tumor invasion, nodal metastasis, advanced disease stage. Down-regulation of Prdx1 inhibited cell proliferation and colony formation of QBC939 cells and reduced the level of SNAT1 expression. Patients with Prdx1 overexpression had a shorter disease-free survival and overall survival than those without Prdx1 expression. Multivariate analysis showed that Prdx1 was an independent prognostic factor for patients with hilar cholangiocarcinoma. The data indicate that Prdx1 may contribute to the development and progression of hilar cholangiocarcinoma, partially through regulating SNAT1 expression, and may be used as a biomarker in predicting the outcome of patients with hilar cholangiocarcinoma.
Keywords: Hilar cholangiocarcinoma, tissue microarray, Prdx1, SNAT1, prognosis
Introduction
Cholangiocarcinoma is a relatively rare neoplasm with highly aggressive behavior and extremely poor prognosis [1]. Over the past several decades, the rates of cholangiocarcinoma have been continuously rising worldwide. Although great efforts have been made to improve the rate of early diagnosis and treatment efficacy of cholangiocarcinoma, the 5-year survival rates remain unchanged: ranging from 20%-50% for patients with operable tumors and 0% for those with inoperable diseases [2]. Surgical resection offers the only potential chance of cure and chemotherapy or radiation contributes a little to the long-term survival of cholangiocarcinoma cases. However, the cancer is not easily detected until it is advanced and then the majority of patients lost the chance of completely resection when diagnosed. Therefore, exploring novel biomarkers is of great value in predicting tumor recurrence and patients’ outcome and even in developing effective therapeutic strategies.
Reactive oxygen species (ROS) is responsible to multiple physiological and pathological reactions in the mammalian cells [3]. Increasing evidences revealed that ROS plays a critical role in many diseases, including certain cancers [4]. Peroxiredoxins (Prdxs), a family of small (22-27 kDa) peroxidase that could reduce intracellular peroxides (one type of ROS), were highly expressed in various cancer cells [5-7]. Prdxs possess six mammalian isoforms and catalyze peroxide reduction of H(2)O(2), organic hydroperoxides and peroxynitrite [8]. Recent studies reported higher levels of Prdxs in various types of human solid tumors and a strong association with tumor progression, recurrence, and prognosis [9-15]. Thus, Prdxs have been regarded as potential predictive and therapeutic biomarkers in cancer. Among these Prdxs, Prdx1 is an important member regulating various cellular signaling and differentiation [16]. Multiple studies suggest that Prdx1 confers an aggressive survival phenotype of cancer cells and drug-resistance. Most reports revealed elevated levels of Prdx1 in several cancer types, including breast [17], esophageal squamous cell [18], and lung cancer [19]. However, there are opposite reports in esophageal squamous cell carcinoma [20] and cholangiocarcinoma [21].
The above studies identified Prdx1 as a promising candidate biomarker, but the contradictory findings made the potential significance of Prdx1 in hilar cholangiocarcinoma unclear. Therefore, in the present study, we explored the expression of Prdx1 in hilar cholangiocarcinoma and determined its clinical and prognostic values. We further investigated the changes of cell viability and its possible underlying mechanisms by knocking down Prdx1 expression in QBC939 cell lines.
Materials and methods
Cell lines and culture conditions
The hilar cholangiocarcinoma cell line QBC939 was purchased from the Cell Center of Chinese Academy of Sciences, Shanghai, China. QBC939 was maintained in DMEM with 10% fetal bovine serum (FBS) (Invitrogen Corp., Grand Island, NY). The cell lines were cultured in a 37°C humidified atmosphere containing 95% air and 5% CO2.
Tissue samples and tissue microarray construction
Eighty-eight patients with hilar cholangiocarcinoma from the Department of Surgery, Eastern Hepatobiliary Hospital and the Department of Medical Oncology, Changzheng Hospital, Shanghai, China from 2004 to 2008 were enrolled in this study. Hematoxylin and eosin (HE) stained slides were prepared and reviewed by two pathologists to select the typical tissue blocks. These patient characteristics are listed in Table 1. Of the 88 CCA samples, 62 were from male patients and 26 from female patients. Mean age of patients at tumor resection was 55 years old ranging from 31 to 79 years; All these patients received no preoperative treatment, either radiotherapy or chemotherapy. The median follow-up for these patients was 16 months, ranging from 1 to 59 months, while one patient was lost during follow-up. The Institutional Review Board of both Eastern Hepatobiliary Hospital and Changzheng Hospital approved the use of the tissues and clinical information and a inform consent was obtained from each patient or their guardians.
Table 1.
Correlation between Prdx1 overexpression and clinicopathological parameters of human hilar cholangiocarcinoma
| Parameters | Prdx1 | ||
|---|---|---|---|
|
| |||
| N | N (%) | P | |
| Age | |||
| ≤55 y | 43 | 33 (76.7) | 0.002 |
| >55 y | 45 | 20 (44.4) | |
| Gender | |||
| Male | 62 | 41 (66.1) | 0.081 |
| Female | 26 | 12 (46.2) | |
| Tumor size* | |||
| ≤3 cm | 30 | 16 (53.3) | 0.247 |
| >3 cm | 56 | 37 (66.1) | |
| T stage | |||
| T1-3 | 14 | 2 (14.3) | <0.001 |
| T4 | 74 | 51 (68.9) | |
| N stage | |||
| No | 29 | 11 (37.9) | 0.003 |
| Yes | 59 | 42 (71.2) | |
| Differentiation | |||
| High/moderate | 67 | 37 (55.2) | 0.087 |
| Poor/un-differentiated | 21 | 16 (76.2) | |
| Disease stage | |||
| I/II | 37 | 17 (45.9) | 0.020 |
| III/IV | 51 | 36 (70.6) | |
Two cases were unavailable for tumor size.
Three paraffin-embedded tissue microarray blocks were created using a manual arrayer (Beecher Instruments, Sun Prairie, WI, USA) as previously described, which contained normal and tumor tissue specimens from these patients.
Immunohistochemistry
Four-micron paraffin-embedded sections from the tissue microarrays were prepared as described previously and processed for Prdx1 (dilution 1:100, EPR5434; EPITOMICS, California, US) and SNAT1 (dilution 1:100, ab59721; Abcam, Cambridge, UK) proteins immunohistochemical staining. An S-p (Streptavidin-Biotin) kit (#KIT-9720, MAIXIN, Fuzhou, China) was used to visualize antibody binding to the tissues. A counterstaining was performed with haematoxylin. Control sections were incubated with PBS only instead of the primary antibodies.
Review and scoring of immunohistochemically stained sections
The stained TMA sections were independently evaluated and scored by two individuals under an Olympus CX31 microscope (Olympus, Center Valley, PA) without any knowledge of clinical information. Any discrepancy was dissolved by re-evaluating the sections by these two pathologists. A mean percentage of positive tumor cells was determined in five areas at × 400 magnifications and assigned from 0% to 100%. The intensity of immunostaining was scored as follows: negative, 0; weak, 1; moderate, 2; and intense, 3. Therefore, a weighted score was generated for each case: ranging from 0 (0 of cells staining) to 3 (100% of the cells staining at 3+ intensity). For convenience in statistical analysis, we defined the score <0.75 as low expression and ≥0.75 as overexpression.
Western blot analysis
CCA cell lines, cancer specimens and matched non-tumor tissues were prepared for Western blot analysis. Standard Western blotting was performed using a rabbit antibody against human Prdx1 (1:1000) and SNAT1 (1:1000) and an anti-rabbit IgG antibody, which was a horseradish peroxidaselinked F(ab’)2 fragments obtained from a donkey (Amersham) as previously described. Equal protein sample loading was monitored by probing the same membrane filter with an anti-β-actin antibody.
Plasmids and transfections
The shRNA-Prdx1 and unspecific scrambled shRNA plasmids were purchased from Genechem Company, Shanghai, China. QBC939 cells were digested and 1 × 105 cells were seeded in six well plates. At 24 hours, transfection was carried out using LipofectamineTM 2000 reagent (Invitrogen, Karlsruhe, Germany) and 5 ng shRNA plasmid per well according to the manufacturer’s instructions.
Cell proliferation assay
Cells were digested and 5000 cells were seeded in 96-well plates at 12 hours after transfection and incubated in medium with 10% FBS. At 24 h, 48 h, and 72 h, CCK8 assay (Dojindo Kumamoto, Japan) was performed to measure the final results. The experiment was repeated three times independently.
Colony formation assay
Cells were digested at 12 hours after transfection and seeded in 6-well plates in triplicate at a density of 500 cells/well for 14 days at 37°C. The colonies were fixed with methanol/acetone (1:1) and stained with crystal violet. Colonies with cell numbers of more than 50 cells per colony were counted.
Statistical analysis
Statistical analysis was performed using the SPSS 16.0 statistical software program for Microsoft Windows. Categorical data were analyzed using χ 2 statistics tests. Within-group correlations of variables were assessed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient. The Kaplan-Meier method was used to estimate survival rates and the log-rank and the Wilcoxon rank sum tests were performed to assess survival differences between groups. The Cox proportional hazards model for multivariate survival analysis was used to assess predictors related to survival. The significance of the in vitro results was determined by using the Student t test (two tailed). Two-sided P value <0.05 was considered statistically significant.
Results
Prdx1 expression in patients with hilar cholangiocarcinoma
Immunohistochemistry analysis showed that Prdx1 positive staining was preferentially cytoplasm-localized. The epithelium in normal bile ducts showed negative or weak staining of Prdx1 (Figure 1A). In contrast, Prdx1 was highly expressed in the tumor cells (Figure 2B). This result was further confirmed by Western blot analysis that Prdx1 level was up-regulated in the tumor tissues compared with the adjacent non-cancerous tissues from the same patients (Figure 1E, 1F). The mean values of Prdx1 in tumor tissues were 1.10±0.96, significantly higher than that in normal tissue: 0.40±0.72 (Figure 2A). Among these 88 tumors, 53 (60.2%) showed overexpression of Prdx1 and 35 (39.8%) showed low/absent expression of Prdx1.
Figure 1.

Analysis of Prdx1 expression in human hilar cholangiocarcinoma and adjacent non-cancerous specimens. (A) Normal tissues showed negative staining of Prdx1; (B) Strong positive staining of Prdx1 in the cytoplasm of cancer cells; (C) Weak staining of Prdx1 in cancer cells; (D) Negative staining of Prdx1 in cancer cells; (E) Western blot analysis of three paired cancer specimens (T) and adjacent non-cancerous specimens (N); (F) Relative amounts of protein in these specimens vs β-actin from (E) analyzed by Image J. Original magnification of (A-D) 40 ×; Original magnification of small pictures in (A-D) 200 ×.
Figure 2.
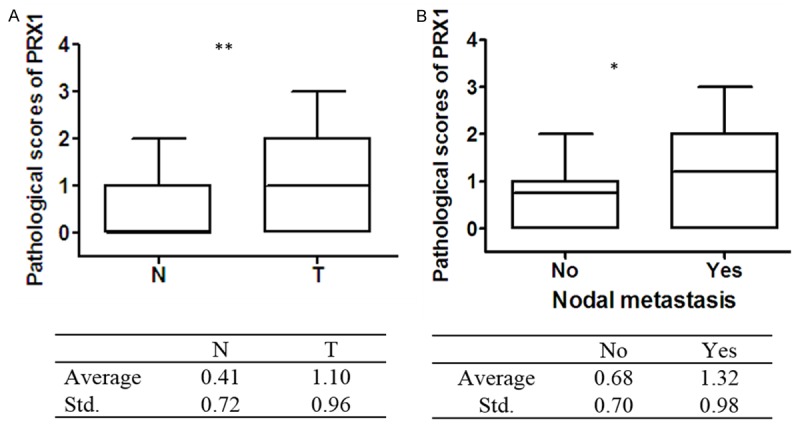
Expression level of Prdx1 in different tissues. A. Higher level of Prdx1 expression in cancer tissues (T) (1.10±0.95) compared with that in normal tissues (N) (0.41±0.72). B. Higher level of Prdx1 expression in cancer tissues with lymph node metastasis (1.32±0.98) compared with that cancer tissues without lymph node metastasis (0.68±0.70). *P<0.05; **P<0.01.
Correlation between Prdx1 expression and clinicopathologic characteristics of hilar cholangiocarcinoma
Table 1 presented the association between Prdx1 overexpression and clinicopathological parameters of hilar cholangiocarcinoma. No significant relationship was observed between Prdx1 expression and gender, tumor size, tissue differentiation. There was a statistically significant association between Prdx1 expression and age, tumor invasion, lymph node metastasis and disease stage. Up-regulation of Prdx1 was more often observed in highly invasive tumors (T4, 68.9%) than less invasive tumors (T1-3, 14.3%; P<0.001). In addition, Activation of Prdx1 occurred more frequently in patients with regional LN metastasis (71.2%) than in N0-stage tumors (37.9%; P=0.003). The mean values of Prdx1 in tumor tissues with nodal metastasis were 1.32±0.98, significantly higher than that without nodal metastasis: 0.68±0.70 (Figure 2B). As for TNM stage, increased expression of Prdx1 significantly correlated with advanced disease stage: 70.6% at stage III/IV and 45.9% at stage I/II (P=0.020).
Relationship of Prdx1 expression with poor outcome in patients with hilar cholangiocarcinoma
The single cohort consisted of 62 male (70.5%) and 26 female (29.5%) patients with the median age of 55.5 y (range, 31-79 y) and the median survival duration of 16 mo. Factors that associated with tumor recurrence in univariate analysis were tumor invasion (52.3 mo for T1-T3 tumors vs. 19.8 mo for T4 tumors; P<0.001), surgical types (38.4 mo for R0 vs. 16.9 mo for R1-2; P<0.001), lymph node metastasis (19.0 mo for LN-positive tumors vs. 37.4 mo for LN-negative tumors; P=0.002), tissue differentiation (29.4 mo for well/moderate differentiated tumors vs. 15.9 mon poor/un-differentiated tumors; P=0.017) and TNM stage (33.3 mo for stages I and II disease vs. 20.5 mo for stage III and IV disease; P=0.019). Specifically, patients with Prdx1-low/absent expression tumors had a future recurrence of 36.0 mo, whereas patients with Prdx1-overexpression tumors had a recurrence of 17.6 mo (P=0.001) (Figure 3). Multivariate analysis using the Cox proportional hazards model for all significant variables in univariate analysis showed that tumor invasion and lymph node metastasis was independent prognostic factors, while Prdx1 was not (P=0.469) (Supplementary Table 1).
Figure 3.
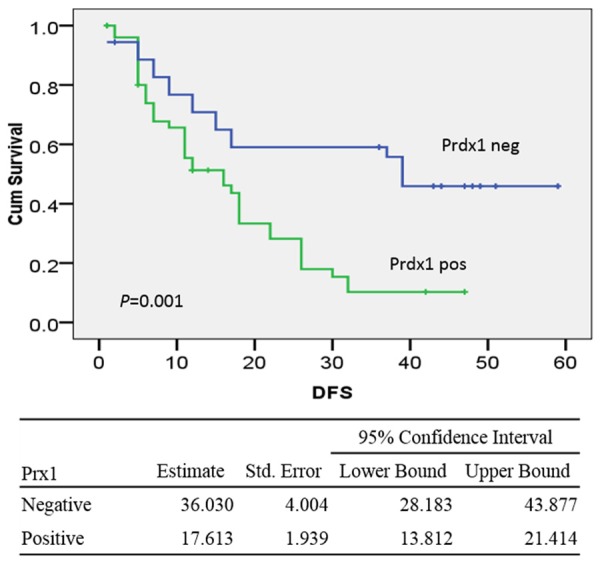
Kaplan-Meier curves of disease-free survival durations in patients with hilar cholangiocarcinoma according to the expression of Prdx1. Patients with Prdx1 overexpression had shorter time to recurrence than those without Prdx1 expression (17.6 mo vs. 36.0 mo; P=0.001).
As for overall survival, the valuable factors in univariate analysis were tumor invasion (52.4 mo for T1-3 tumors vs. 23.4 mo for T4 tumors; P<0.001), LN metastasis (22.5 mo for LN-positive tumor vs. 41.1 mo for LN-negative tumors; P=0.002), surgical types (41.9 mo for R0 vs. 20.4 mo for R1-2; P<0.001), tissue differentiation (32.6 mo for well/moderate differentiated tumors vs. 18.6 mo for poor/un-differentiated tumors; P=0.017) and TNM stage (36.5 mo for stages I and II disease vs. 23.7 mo for stage III and IV disease; P=0.016). Overexpression of Prdx1 correlated with decreased survival durations. Patients with Prdx1-low/absent tumors had a median survival of 41.8 mo, whereas patients with Prdx1-overexpression tumors had a median survival of 20.2 mo (P<0.001) (Figure 4). Multivariate analysis by Cox Regression showed that Prdx1 overexpression and tumor invasion were independent prognostic factors (Table 2).
Figure 4.
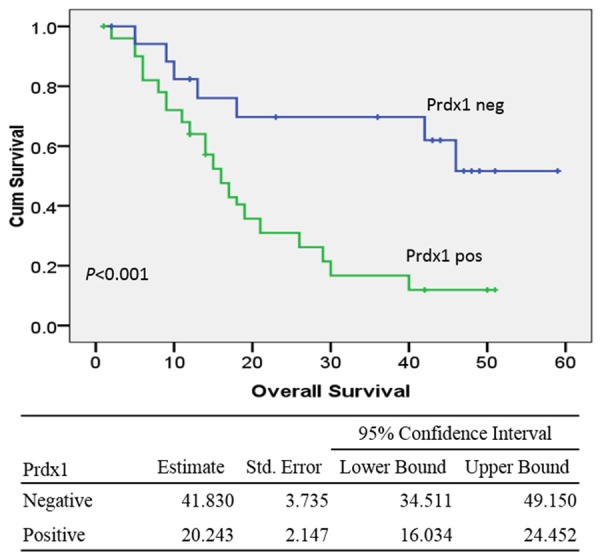
Kaplan-Meier curves of overall survival durations in patients with hilar cholangiocarcinoma according to the expression of Prdx1. Patients with Prdx1 overexpression had shorter overall survival duration than those without Prdx1 expression (20.2 mo vs. 41.8 mo; P<0.001).
Table 2.
Cox proportional hazards model analysis of prognostic factors
| B | SE | Wald | Sig. | Exp (B) | 95.0% CI | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Tumor invasion (T1-3 vs. T4) | -2.457 | 0.784 | 9.827 | 0.002 | 0.086 | 0.018 | 0.398 |
| Differentiation (High/moderate vs. poor) | -0.379 | 0.359 | 1.112 | 0.292 | 0.684 | 0.338 | 1.385 |
| Surgical methods (R0 vs. R1-2) | -0.540 | 0.577 | 0.877 | 0.349 | 0.583 | 0.188 | 1.805 |
| TNM (I/II vs. III/IV) | 0.348 | 0.333 | 1.093 | 0.296 | 1.416 | 0.738 | 2.718 |
| Prdx1 (positive vs. negative) | -0.955 | 0.433 | 4.873 | 0.027 | 0.385 | 0.165 | 0.898 |
| Nodal metastasis (Yes vs. No) | 0.353 | 0.196 | 3.230 | 0.072 | 1.423 | 0.969 | 2.092 |
Knockdown of Prdx1 by shRNA inhibits proliferation and colony formation of QBC939 cells
We next assessed the functional significance of QBC939 cells by knocking down Prdx1 using specific Prdx1-shRNA. As shown in Figure 5A and 5B, in QBC939 cells, Prdx1-shRNA leads to a sharp reduction of Prdx1 protein level at 48 hours after the transfection. The knockdown of Prdx1 significantly inhibited cell viability (Figure 5C) as well as colony formation (Figure 5D and 5E) of QBC939 cells. Interestingly, we found that Prdx1 knockdown reduced SNAT1 protein levels in compared with the controls (Figure 5B).
Figure 5.
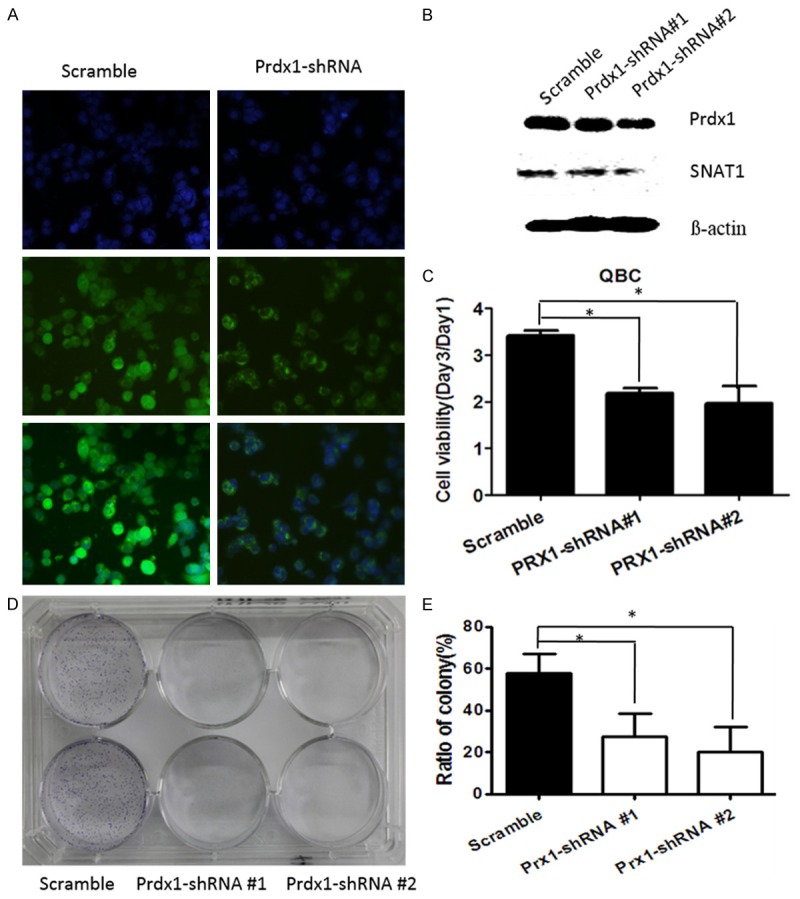
Silencing of Prdx1 by specific Prdx1-shRNA inhibits cancer cell proliferation and colony formation. QBC939 cells were transfected with Prdx1-shRNA for 48 hours and immunofluorescence assay (A) and Western blotting (B) was used to detect the expression status of Prdx1; (C) QBC cells transfected with Prdx1-shRNA and CCK8 assay was performed at 48 hours; *P<0.05, compared with control. (D, E) QBC cells transfected with Prdx1-shRNA were grown in 6-well plates and incubated with 10% FBS for 2 weeks. The numbers of the cell colonies (>50 cells) were obtained and calculated as: colonies/500 × 100. *P<0.05, compared with control.
Co-expression of Prdx1 and SNAT1 in human hilar cholangiocarcinoma
SNAT1 immunostaining was preferentially cytoplasm-localized. Normal bile duct showed negative or weak expression of SNAT1 (Figure S1A), while increased expression of SNAT1 was observed in 53.4% (47/88) cases (Figure S1B). As shown in Supplementary Table 2, there was a significant association between SNAT1 expression and tumor invasion, lymph node metastasis and tissue differentiation. No significant relationship was observed between SNAT1 expression and tumor size and age. Although not reach significant difference, SNAT1 expression was more frequently observed in tumor specimens from patients at stage III/IV than those at stage I/II. Interestingly, further analysis revealed that Prdx1 expression significantly correlated with SNAT1 expression (P=0.001, r=0.335). Coexpression of SNAT1 and Prdx1 were observed in 36 (40.9%) tumors, while 24 (27.2%) tumors showed no expression of both (Table 3). In the majority of the cases, Prdx1 expression co-localized with SNAT1 expression in the same specimens (Figure 6).
Table 3.
The correlation between Prdx1 and SNAT1
| Prdx1 | SNAT1 | R | P | |
|---|---|---|---|---|
|
| ||||
| - | + | |||
| - | 24 | 11 | 0.335 | 0.001 |
| + | 17 | 36 | ||
Figure 6.

Representative pictures showing co-expression of Prdx1 and SNAT1 in human hilar cholangiocarcinoma from the same patients. A, A1: Positive staining of Prdx1 in cancer cells; B, B1: Positive staining of SNAT1 in cancer cells. C: Statistics showed a significant correlation between Prdx1 and SNAT1 (r=0.335, P=0.001). Original magnification of A and B: 40 ×; Original magnification of A1, B1: 400 ×.
Discussion
Hilar cholangiocarcinoma continues to be one of the most high frequency causes of cancer related mortality. In order to prolong the long-term survival of these patients, it is essential to improve the understanding of the pathophysiology of hilar cholangiocarcinoma and to identify proteins and molecular pathways that affect cell proliferation and survival functions. Increasing evidences suggest that Prdx1 may be an important component underlying these mechanisms and up-regulation of Prdx1 various tumors serves as a valuable prognostic factor and a potential therapeutic target. Although Yonglitthipagon et al. reported an inversely association between Prdx1 expression and clinical outcome in cholangiocarcinoma, Prdx1 was overexpressed in cancer tissues compared with normal tissues [21]. Especially, the clinocopathological parameters (lacking tumor invasion, lymph node metastasis, TNM stage, and surgical type) were not sufficient for analyzing the association between Prdx1 expression and characteristics of cholangiocarcinoma, and overall survival in multivariate analysis as well. In this study, we collected the detailed medical information for patients with hilar cholangiocarcinoma and investigated the clinical significance of Prdx1 in these patients. Unexpectedly, our results showed that Prdx1 was up-regulated in cancer cells compared with non-cancerous tissues and over-expression of Prdx1 correlated significantly with tumor invasion, lymph node metastasis, and disease progression. Patients with high expression of Prdx1 had a shorter time to recurrence and poor clinical outcome than those with Prdx1 low/absent expression. Moreover, a high level of Prdx1 expression is an independent and significant prognostic factor for poor survival outcome in patients with hilar cholangiocacinoma. Prdx1 expression level increased the relative risk of death in these patients analyzed by the multivariate Cox proportional hazards model. Multivariate analysis showed that Prdx1 was not an independent factor in predicting tumor recurrence. The current findings, together with the previous results by others, suggest that Prdx1 may play a potential and critical role in the malignant progression of hilar cholangiocarcinoma.
Prdx1 belongs to a family of peroxidases and functions as an antioxidant by protecting cells from DNA damage and mutagenesis induced by reactive oxygen species (ROS) [8,22,23]. Recently, the expression profiles and potential role of Prdx1 in cancers were widely explored, but the results were controversial [24]. Firstly, Prdx1 was regarded as a tumor suppressor since transgenic mice lacking the Prdx1 gene developed several tumors at high frequency [25] and reduced Prdx1 expression correlated with tumor invasion and poor clinical outcome in esophageal squamous cell carcinoma [20] and cholangiocarcinoma [21]. However, re-expression of Prdx1 in cancer cells fails to induce cell death and Prdx1 expression was increased in numerous types of cancers, including lung [13], gallbladder [26], bladder [27], prostate [28], and breast cancer [17]. Interestingly, evaluated expressions of Prdx1 were significantly associated with poor overall survival in these reported tumors. Our present study also indicated that Prdx1 was not only overexpressed in hilar cholangiocarcinoma, but also was an independent prognostic factor, suggesting a strong relevance of Prdx1 with malignancy.
Up-regulation of Prdx1 in cancers makes it an attractive target for therapeutic intervention. Accumulated studies proved that silencing Prdxs inhibited cell proliferation and apoptosis in cancer cells. In present study, we also observed reduced abilities of cell proliferation and colony formation by downregulating Prdx1 using specific shRNA. Our data are in line with a previous study showing that Prdx1 controls cell growth and tumor vasculature in prostate cancer [28]. Taken together, these in vivo and in vitro experiments suggested the Prdx1 is essential in maintaining cell growth and tumor survival.
Prdx1 modulates cell growth by regulating cell signaling via interacting signaling proteins, such as the kinases c-Ab1, JNK, the oncoprotein c-myc, VEGF, COX-2, and the phosphatase PTEN, resulting continuous cell proliferation and oncogenesis [28-32]. Aguilar-Melero et al. reports that silencing Prdx1 in HepG2 cells leads to altered expression of key enzymes of carbohydrate and amino acid metabolism, suggesting a disturbance of the Warburg effect and glutamine utilization in cancer cells [33]. Increased activity of amino acid metabolism is the major characteristic of tumor cells. Recent studies showed that the amino acid carrier plays a pivotal role in energy metabolism and malignant transformation of mammal cell [34]. SANT1 is a glutamine transporter, providing metabolic fuel for glutathione synthesis [35]. Interestingly, SNAT1 expression was found to be increased in human various types of tumors, including hepatic carcinoma [36], breast [37], and hilar changiocarcinoma [38]. In this study, SNAT1 level was downregulated induced by Prdx1-shRNA in QBC939 cells. In line with previous study that oxidative stress significantly enhanced cysteine uptake, which was accompanied by significantly enhanced SNAT1 expression [39], the data highlights the critical role of Prdx1-SNAT1 cross-talk in energy metabolism in cancer cells.
High levels of SNAT1 expression significantly correlated with tumor invasion and clinical stage in some types of tumors including hilar cholangiocarcinoma. A previous study revealed overexpression of SNAT1 in 44.9% patients with hilar cholangiocarcinoma and a significant association with lymph node metastasis. Similarly, our present data showed that SNAT1 was evaluated in 53.4% cases and correlated significantly with tumor invasion, lymph node metastasis, and tissue de-differentiation. Moreover, Prdx1 expression co-localized with SNAT1 expression in the majority of caner tissue specimens. Prdx1(+)/SNAT1(+) was observed in 36 cases, while Prdx1(-)/SNAT1(-) was in 24 cases, accounting 68.2% of all cases. To our knowledge, this is the first report demonstrating the cross-talk between Prdx1 and SNAT1 in cancer cells and its potential role in cell growth. However, the underlying mechanism of this cross-talk promoting cell growth and tumorigenesis needs further investigation.
In summary, Prdx1 was frequently evaluated in human hilar cholangiocarcinoma and its overexpression was associated with disease progression and poor clinical outcome. Silencing SNAT1 inhibited cell proliferation and colony formation by down-regulating SNAT expression. The cross-talk between Prdx1 and SNAT1 provides a novel clue for exploring prognostic biomarkers and potential therapeutic targets for hilar cholangiocarcinoma and other cancers.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250:210–218. doi: 10.1097/SLA.0b013e3181afe0ab. [DOI] [PubMed] [Google Scholar]
- 2.Yubin L, Chihua F, Zhixiang J, Jinrui O, Zixian L, Jianghua Z, Ye L, Haosheng J, Chaomin L. Surgical management and prognostic factors of hilar cholangiocarcinoma: experience with 115 cases in China. Ann Surg Oncol. 2008;15:2113–2119. doi: 10.1245/s10434-008-9932-z. [DOI] [PubMed] [Google Scholar]
- 3.Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Pylvas M, Puistola U, Kauppila S, Soini Y, Karihtala P. Oxidative stress-induced antioxidant enzyme expression is an early phenomenon in ovarian carcinogenesis. Eur J Cancer. 2010;46:1661–1667. doi: 10.1016/j.ejca.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Fujii J, Ikeda Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002;7:123–130. doi: 10.1179/135100002125000352. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 7.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Poynton RA, Hampton MB. Peroxiredoxins as biomarkers of oxidative stress. Biochim Biophys Acta. 2014;1840:906–12. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker HC, Patel D, Howat WJ, Warren AY, Kay JD, Sangan T, Marioni JC, Mitchell J, Aldridge S, Luxton HJ, Massie C, Lynch AG, Neal DE. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer. 2013;109:983–993. doi: 10.1038/bjc.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ummanni R, Barreto F, Venz S, Scharf C, Barett C, Mannsperger HA, Brase JC, Kuner R, Schlomm T, Sauter G, Sültmann H, Korf U, Bokemeyer C, Walther R, Brümmendorf TH, Balabanov S. Peroxiredoxins 3 and 4 are overexpressed in prostate cancer tissue and affect the proliferation of prostate cancer cells in vitro. J Proteome Res. 2012;11:2452–2466. doi: 10.1021/pr201172n. [DOI] [PubMed] [Google Scholar]
- 11.Kinnula VL, Lehtonen S, Sormunen R, Kaarteenaho-Wiik R, Kang SW, Rhee SG, Soini Y. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002;196:316–323. doi: 10.1002/path.1042. [DOI] [PubMed] [Google Scholar]
- 12.Soini Y, Kinnula VL. High association of peroxiredoxins with lung cancer. Lung Cancer. 2012;78:167. doi: 10.1016/j.lungcan.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Bogner PN, Baek SH, Ramnath N, Liang P, Kim HR, Andrews C, Park YM. Up-regulation of peroxiredoxin 1 in lung cancer and its implication as a prognostic and therapeutic target. Clin Cancer Res. 2008;14:2326–2333. doi: 10.1158/1078-0432.CCR-07-4457. [DOI] [PubMed] [Google Scholar]
- 14.Karihtala P, Mantyniemi A, Kang SW, Kinnula VL, Soini Y. Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003;9:3418–3424. [PubMed] [Google Scholar]
- 15.Wu XY, Fu ZX, Wang XH. Peroxiredoxins in colorectal neoplasms. Histol Histopathol. 2010;25:1297–1303. doi: 10.14670/HH-25.1297. [DOI] [PubMed] [Google Scholar]
- 16.Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8:4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA, Chae HZ. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001;21:2085–2090. [PubMed] [Google Scholar]
- 18.Ren P, Ye H, Dai L, Liu M, Liu X, Chai Y, Shao Q, Li Y, Lei N, Peng B, Yao W, Zhang J. Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol Rep. 2013;30:2297–303. doi: 10.3892/or.2013.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Bogner PN, Ramnath N, Park Y, Yu J, Park YM. Elevated peroxiredoxin 1, but not NF-E2-related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:3875–3882. doi: 10.1158/1078-0432.CCR-06-2893. [DOI] [PubMed] [Google Scholar]
- 20.Hoshino I, Matsubara H, Akutsu Y, Nishimori T, Yoneyama Y, Murakami K, Sakata H, Matsushita K, Ochiai T. Tumor suppressor Prdx1 is a prognostic factor in esophageal squamous cell carcinoma patients. Oncol Rep. 2007;18:867–871. [PubMed] [Google Scholar]
- 21.Yonglitthipagon P, Pairojkul C, Chamgramol Y, Loukas A, Mulvenna J, Bethony J, Bhudhisawasdi V, Sripa B. Prognostic significance of peroxiredoxin 1 and ezrin-radixin-moesin-binding phosphoprotein 50 in cholangiocarcinoma. Hum Pathol. 2012;43:1719–1730. doi: 10.1016/j.humpath.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 24.Neumann CA, Fang Q. Are peroxiredoxins tumor suppressors? Curr Opin Pharmacol. 2007;7:375–380. doi: 10.1016/j.coph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Yang ZL, Ren X, Zou Q, Yuan Y, Liang L, Chen M, Chen S. ILK and PRDX1 are prognostic markers in squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Tumour Biol. 2013;34:359–368. doi: 10.1007/s13277-012-0557-2. [DOI] [PubMed] [Google Scholar]
- 27.Quan C, Cha EJ, Lee HL, Han KH, Lee KM, Kim WJ. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J Urol. 2006;175:1512–1516. doi: 10.1016/S0022-5347(05)00659-2. [DOI] [PubMed] [Google Scholar]
- 28.Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, Gollnick SO. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res. 2011;71:1637–1646. doi: 10.1158/0008-5472.CAN-10-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YJ, Lee WS, Ip C, Chae HZ, Park EM, Park YM. Prdx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006;66:7136–7142. doi: 10.1158/0008-5472.CAN-05-4446. [DOI] [PubMed] [Google Scholar]
- 30.Lou J, Fatima N, Xiao Z, Stauffer S, Smythers G, Greenwald P, Ali IU. Proteomic profiling identifies cyclooxygenase-2-independent global proteomic changes by celecoxib in colorectal cancer cells. Cancer Epidemiol Biomarkers Prev. 2006;15:1598–1606. doi: 10.1158/1055-9965.EPI-06-0216. [DOI] [PubMed] [Google Scholar]
- 31.Huo YY, Li G, Duan RF, Gou Q, Fu CL, Hu YC, Song BQ, Yang ZH, Wu DC, Zhou PK. PTEN deletion leads to deregulation of antioxidants and increased oxidative damage in mouse embryonic fibroblasts. Free Radic Biol Med. 2008;44:1578–1591. doi: 10.1016/j.freeradbiomed.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Bast A, Fischer K, Erttmann SF, Walther R. Induction of peroxiredoxin I gene expression by LPS involves the Src/PI3K/JNK signalling pathway. Biochim Biophys Acta. 2010;1799:402–410. doi: 10.1016/j.bbagrm.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Aguilar-Melero P, Prieto-Alamo MJ, Jurado J, Holmgren A, Pueyo C. Proteomics in HepG2 hepatocarcinoma cells with stably silenced expression of PRDX1. J Proteomics. 2013;79:161–171. doi: 10.1016/j.jprot.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- 35.McGivan JD, Bungard CI. The transport of glutamine into mammalian cells. Front Biosci. 2007;12:874–882. doi: 10.2741/2109. [DOI] [PubMed] [Google Scholar]
- 36.Kondoh N, Imazeki N, Arai M, Hada A, Hatsuse K, Matsuo H, Matsubara O, Ohkura S, Yamamoto M. Activation of a system A amino acid transporter, ATA1/SLC38A1, in human hepatocellular carcinoma and preneoplastic liver tissues. Int J Oncol. 2007;31:81–87. [PubMed] [Google Scholar]
- 37.Wang K, Cao F, Fang W, Hu Y, Chen Y, Ding H, Yu G. Activation of SNAT1/SLC38A1 in human breast cancer: correlation with p-Akt overexpression. BMC Cancer. 2013;13:343. doi: 10.1186/1471-2407-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu WL, Cong WM, Zhang Y, Chen Y, Wang F, Yu G. Overexpression of ATA1/SLC38A1 predicts future recurrence and death in Chinese patients with hilar cholangiocarcinoma. J Surg Res. 2011;171:663–668. doi: 10.1016/j.jss.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 39.King N, Lin H, Suleiman MS. Oxidative stress increases SNAT1 expression and stimulates cysteine uptake in freshly isolated rat cardiomyocytes. Amino Acids. 2011;40:517–526. doi: 10.1007/s00726-010-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


