Abstract
Radiotherapy is the primary treatment for human nasopharyngeal carcinoma (NPC), yet radioresistance remains a major obstacle to successful treatment in many cases. N-acetylglucosaminyltransferase V (GnT-V), which synthesizes β1, 6-GlcNAc branched N-glycans, is closely related to the radiosensitivity of NPC cells. However, a better understanding of the functional role of GnT-V in NPC radioresistance and the related mechanisms is urgently needed. In the present study, a radioresistant NPC cell line, CNE-2R, was established by repeated γ-irradiation. We found that GnT-V levels, as well as β1, 6-GlcNAc branched N-glycans were significantly increased in the CNE-2R cells as compared with that in the parental cells. Meanwhile, knockdown of GnT-V in the CNE-2R cells enhanced cell radiosensitivity and inhibited the formation of β1, 6-branched N-glycans. In addition, the regulated expression of GnT-V in the CNE-2R cells converted the heterogeneous N-glycosylated forms of CD147. Furthermore, swainsonine, an inhibitor of N-glycan biosynthesis, was also able to reverse the radioresistance of the CNE-2R cells. Taken together, the present study revealed a novel mechanism of GnT-V as a regulator of radioresistance in NPC cells, which may be useful for fully understanding the biological role of N-glycans in NPC radioresistance.
Keywords: GnT-V, N-glycans, radioresistance, nasopharyngeal carcinoma
Introduction
Human nasopharyngeal carcinoma (NPC) is one of the most common cancers in certain areas of South- China, Southeast-Asia and North Africa [1]. Unlike other malignancies of the head and neck region, in which surgery remains the primary mode of treatment, patients with NPC are treated primarily with radiotherapy [2]. Radiotherapy can lead to permanent local disease control and improve survival for patients with NPC. Although NPC is a relatively radiosensitive disease, the therapeutic effect of radiotherapy is not satisfactory due to radioresistance [3]. In the last several years, significant progress has been made in the enhancement of radiosensitivity of NPC cells. However, more effort is needed to resolve the radioresistance of NPC.
Glycosylation is one of the most abundant and important post-translational modification of proteins [4]. There are two distinct forms of protein glycosylation, N-linked and O-linked. The N-linked glycosylation, typified by the attachment of the glycan to an asparagine side chain of the protein is by far the most common. Variations in N-linked protein glycosylation cause several effects such as, a reduced synthesis of tri- and tetraantennary glycans, accumulation of biantennary glycans, shortening of polylactosamine chains, and inhibition of sialylation reactions [5]. Abnormal N-linked glycosylation, resulting in expression of altered N-glycans, is well associated with malignant transformation of the cell. Cancer cells frequently contain N-glycans at different levels or with fundamentally different structures than those observed in normal cells [6]. Yet, the relationship between radioresistance and N-glycans has been investigated in very few studies.
An aberrant N-glycosylation induced by N-acetylglucosaminyltransferase (GnT-V) is a representative example of such a modification that is implicated in radioresistance. GnT-V catalyzes the formation of β1, 6-GlcNAc branched N-glycans on the cell surface. Upregulation of GnT-V leading to increased β1, 6-GlcNAc branching of N-linked glycans is a major hallmark of cancer progression [7]. In addition, N-glycans bearing a β-1-6-linked GlcNAc branch and GnT-V expression are also closely linked with tumor drug resistance [8]. Furthermore, it has been reported that down-regulation of GnT-V could enhance NPC cell radiosensitivity in vitro and in vivo [9]. To the best of our knowledge, the precise role of GnT-V and β1, 6-GlcNAc branched N-glycans in NPC radioresistance has not been fully unveiled.
In the present study, a radioresistant NPC cell line, CNE-2R, was established by repeated γ-ray irradiation of its parent cell line (CNE-2). Then the role of GnT-V in alteration of NPC radioresistance and the possible pathways involved were investigated by RNA interference-based approaches.
Materials and methods
Cell culture
The human NPC cell line CNE2 was purchased from the Cell Bank of the Shanghai Institute of Life Sciences (the Chinese Academy of Science, Shanghai, China). The CNE2 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (both from Gibco-BRL, Carlsbad, CA, USA) in a humidified atmosphere with 5% CO2 at 37°C.
Establishment of a radioresistant cell line
Fractionated irradiation was applied to establish a radioresistant NPC cell line from its parental cell line. Briefly, exponentially growing CNE2 cells were exposed to γ irradiation generated by a 60Co source (60Co therapeutic machine, GWXJ80 type, China) at a dose of 2 Gy (dose rate 54.3 cGy/min, 35 cm × 35 cm irradiation field, SSD 580 cm). When the irradiated cells reached an exponential growth phase, the next 2 Gy was delivered. The cells were irradiated repeatedly in the same way to a total dose of 60 Gy in 30 fractions. After the final irradiation, the radioresistant cell line was isolated and named as CNE2R.
Colony formation assay
Cells in exponential growth phase were plated into a 6-well plate at 3,000 cells/well and then incubated for 24 h to allow settling. The cells were irradiated at different doses ranging from 0 to 10 Gy. These cells were incubated at 37°C for 14 days. When most cell clones had reached > 50 cells, they were stained with 0.1% crystal violet, and the surviving fraction (SF) was determined. Radiobiological parameters, including the average lethal dose(D0), quasi-threshold dose (Dq) and the extrapolation number (N) were calculated as previously described [10].
Cell apoptosis analysis
Cells in the exponential phase of growth were irradiated at a dose of 4 Gy. After 24 h of irradiation, the cells were harvested and washed twice with PBS, followed by fixation in 70% cold ethanol at 4°C overnight. Then the cells were double stained with Annexin V-FITC and PI (both from Sigma, St.Louis, MO, USA) for 30 min at room temperature in dark. Apoptotic cells were detected using flow cytometry within 1 h (Becton-Dickinson, Mountain View, CA, USA).
qRT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The cDNA was synthesized from 2 μg of total RNA using Revert AidTM first strand cDNA Synthesis kit (Fermentas, Hanover, MD, USA). Amplification reactions were conducted using the SYBR Green/ROX qPCR Master Mix (Fermentas) with an ABI PRISM 7300 system. DNA primer sequences were designed as follows: GAPDH: 5’-TGGAAGGACTCATGACCA CA-3’ (forward) and 5’-TTCAGCTCAGGGATGACCTT-3’ (reverse). GnT-V: 5’-CTTCACTCCGTG GAAGTTGTC-3’ (forward) and 5’-TGGATGGTAAAGTGCAGAAGC-3’ (reverse). PCR reactions were carried out with 30 cycles of denaturation at 95°C for 40 sec, annealing at 56°C to 60°C for 40 sec, and extension at 72°C for 1 min. The data were collected and analyzed using the comparative Ct (threshold cycle) method using GADPH as an internal control.
Western blotting
Cells were harvested with trypsinization and washed with PBS buffer. Proteins were extracted by incubation with lysis buffer [50 mM Tris-HCl (pH 7.5), 1% NP-40, 2 mM EDTA, 10 mM NaCl, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM DTT, 0.1% SDS, 1 mM PMSF]. Then 20 μg proteins were separated by 10% SDS-PAGE and transferred to PVDF membranes. The proteins were analyzed using specific antibodies as indicated. Horseradish peroxidase (HRP)-conjugated secondary antibodies and an enhanced chemiluminescence kit (Thermo Fisher Scientific, Rockford, IL, USA) were used for detection. Anti-GADPH mAb, anti-GnT-V mAb and anti-CD147 mAb as well as the second antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Lectin blotting
Cells were harvested and lysed for total protein extraction. Equal amounts of protein were separated by 10% SDS-PAGE and transferred onto PVDF membranes. After blocking with Carbo-Free Blocking Solution (Vector labs, Burlingame, CA, USA), the membrane was incubated with 2 μg/ml biotinylated L-PHA (Vector labs) for 30 min. Then the membrane was probed with HRP-conjugated streptavidin (Vector labs) for 1 h at room temperature. Immunoreactive bands were visualized by using ECL reagents as described previously [11].
Analysis of lectin labeling by flow cytometry
Cells were trypsinized and analyzed during exponential growth. Cell pellets were suspended in PBS with Triton X-100 (Sigma) for 5 min. Then FITC-labeled L-PHA (Vector labs) was added to a final concentration of 2 μg/ml and incubated at room temperature for 30 min. The fluorescence intensity of the stained cells was measured by flow cytomety and analyzed with CellQuest software (Becton-Dickinson).
RNA interference
Human GnT-V shRNA plasmid and control plasmid were purchased from Santa Cruz Biotechnology. Transfection was carried out using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol. Stable clones were selected in the presence of G418 (Invitrogen). Positive clones were obtained and screened by qRT-PCR and western blotting. The control plasmid was also transfected into cells, which was used as the control.
Treatment with swainsonine
Swainsonine (Sigma) were diluted in culture medium supplemented with 10% heat-inactivated fetal bovine serum. Cells in the exponential phase of growth were pretreated with swainsonine at concentrations of 1 or 2 μg/ml for 24 h [12]. Then the cells were harvested and prepared for subsequent analysis.
Statistical analysis
All experiments were independently repeated three times. Data are presented as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS16.0 software (SPSS, Inc., Chicago, IL, USA). Differences of P < 0.05 were considered.
Results
Biological characteristics of the CNE2R cells
To explore the mechanism responsible for radioresistance in NPC, a radioresistant cell line, CNE2R, was established by repeated γ irradiation. Then the surviving fractions of CNE2 and CNE2R cells that survived irradiation were analyzed with the colony formation assay. Compared to CNE2, CNE2R showed no change of colony formation ability when radiation was absent, but achieved enhanced colony formation and higher survival fractions when exposed to radiation (Figure 1A). In addition, CNE2R cells had higher D0, Dq and N values compared with the CNE2 cells. For the CNE2R cells, the radiosensitivity parameters were as follows: D0=2.05±0.01 Gy, Dq=1.98±0.04 Gy and N=4.37±0.08. For the CNE2 cells, D0=1.69±0.03 Gy, Dq=1.42±0.07 Gy, and N=2.85±0.05. The effect of radiation on cell apoptosis was also examined by subjecting CNE2 and CNE2R cells to 4 Gy irradiation. As shown in Figure 1B, the apoptotic rate of the CNE2R cells was lower than that of the CNE2 after radiation. Thus, the CNE2R cells were more radioresistant than the parental CNE2 cells.
Figure 1.
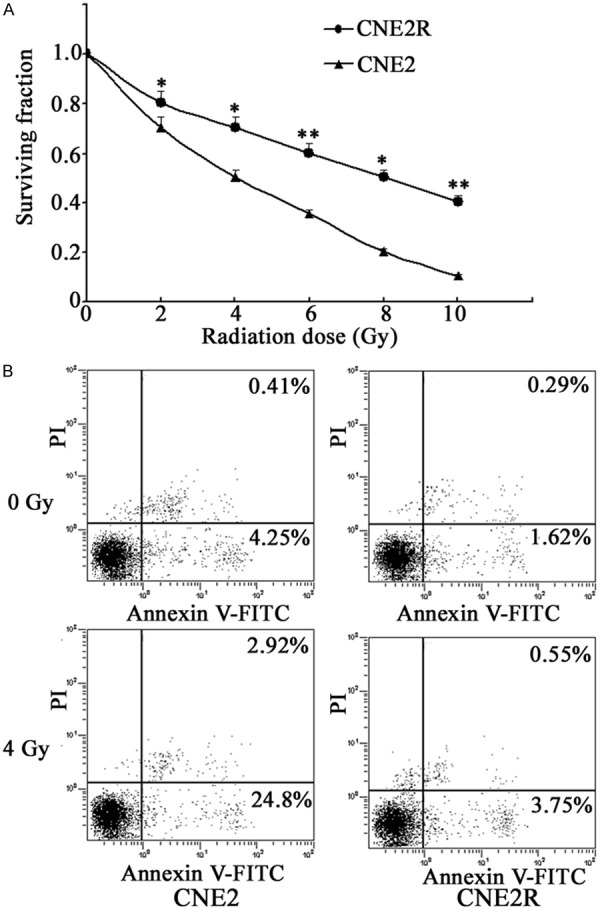
Establishment of a radioresistant cell line. A. Cells were exposed to various doses of irradiation and the surviving fractions were analyzed by colony formation assay. B. Cells were subjected to 4 Gy irradiation and the apoptotic rate was detected using flow cytometry. Results were recorded as mean ± SD of three independent experiments (*P < 0.05, **P < 0.01, compared with the CNE2 cells).
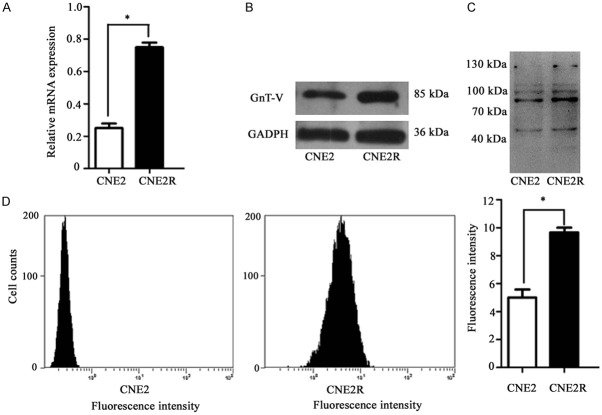
Increased expression of GnT-V and β1, 6-branched N-glycans in the CNE2R cells
To investigate the potential role of GnT-V in radioresistance, qRT-PCR was carried out to examine the mRNA expression, and western blotting was performed to examine the protein expression. As shown in Figure 2A and 2B, GnT-V expression levels were up-regulated in the CNE2R cells compared with the corresponding parental cells. β1, 6-GlcNAc branching of N-linked glycans can be specifically identified by L-PHA [11]. The altered expression of β1, 6-branched N-glycans was determined by flow cytometry and lectin blotting. As shown in Figure 2C and 2D, the intensity of L-PHA staining in the CNE2R cells was significantly increased. These results revealed that the overexpression of GnT-V, as well as the formation of β1, 6-GlcNAc branched N-glycans, may contribute to the radioresistant phenotype of NPC.
Figure 2.
Analysis of GnT-V and β1, 6-branched N-glycans expression in the CNE2R cells. The GnT-V mRNA and protein expression was determined by (A) qRT-PCR and (B) western blotting, respectively. The intensity of L-PHA staining was detected by (C) lectin blotting and (D) flow cytometry. Results are recorded as mean ± SD of three independent experiments (*P < 0.05, compared with the CNE2 cells).
Reversal of radioresistance by GnT-V knockdown in the CNE2R cells
To investigate whether GnT-V has a direct function in modulating the radioresistance in the CNE2R cells, GnT-V expression was knocked down by RNA interference. As shown in Figure 3A and 3B, the expression of GnT-V at the mRNA and protein levels were significantly inhibited (P < 0.05) in the CNE2R cells transfected with GnT-V shRNA plasmid, whereas no significant inhibitory effect was observed in the untreated cells and cells treated with the control plasmid (P > 0.05).
Figure 3.
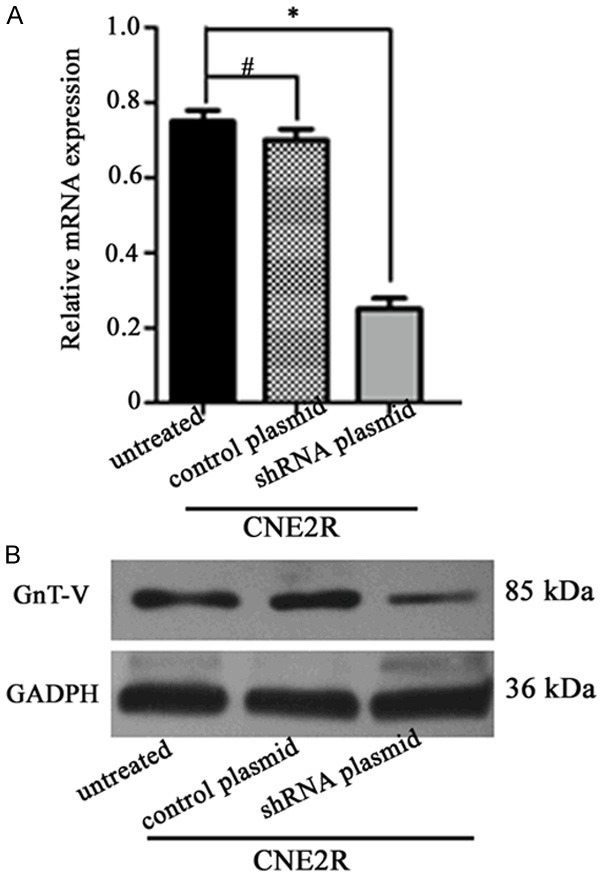
Expression of GnT-V in the different groups. CNE2R cells were stably transfected with the GnT-V shRNA plasmid or the control plasmid. The GnT-V mRNA and protein expression was determined by (A) qRT-PCR and (B) western blotting, respectively. Results are recorded as mean ± SD of three independent experiments (*P < 0.05, #P > 0.05, compared with the untreated CNE2R cells).
Next, cell radiosensitivity was monitored by colony formation assay (Figure 4A). The survival fractions in the GnT-V knockdown cells were markedly decreased compared with that of the untreated cells, and the cells treated with the control plasmid (P < 0.05). Then flow cytometry assay was performed to evaluate potential effects of GnT-V knockdown on cell apoptosis after 4 Gy radiation. As shown in Figure 4B, no significant difference in the proportion of apoptotic cell was observed between the untreated cells and the cells treated the control plasmid (P > 0.05). However, notable apoptosis was found in the CNE2R cells, when GnT-V was knocked down, suggesting that inhibition of GnT-V reverses the radioresistance of NPC cells.
Figure 4.
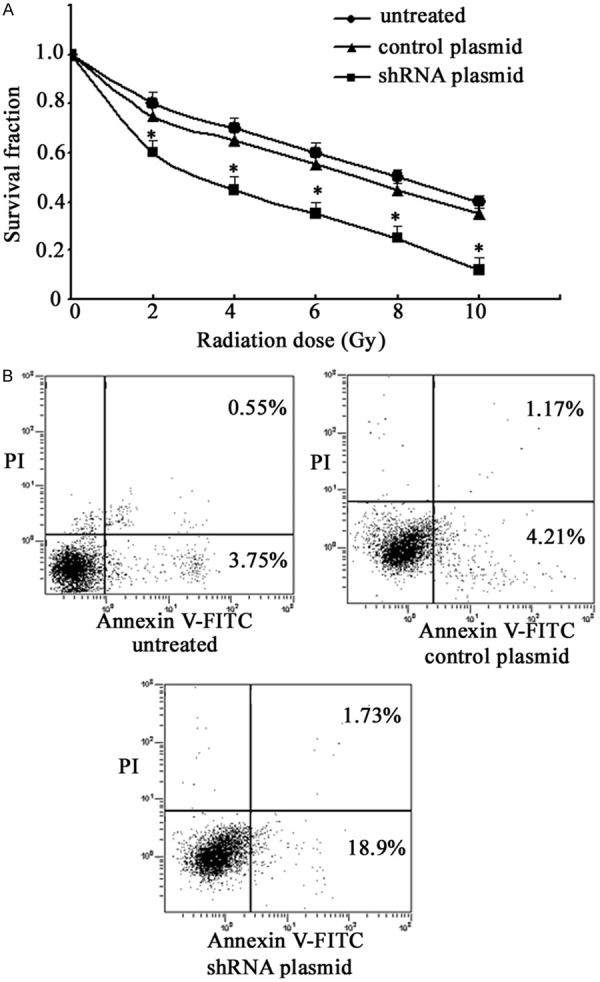
Effects of GnT-V knockdown on cell radiosensitivity and apoptosis. CNE2R cells were stably transfected with the GnT-V shRNA plasmid or the control plasmid. A. The surviving fractions were analyzed by colony formation assay. B. The apoptotic rate was detected using flow cytometry. Results are recorded as mean ± SD of three independent experiments (*P < 0.05, compared with the untreated CNE2R cells).
Knockdown of GnT-V inhibits the formation of β1, 6-branched N-Glycans in the CNE2R cells
To explore whether GnT-V knockdown could modify the formation of β1, 6-branched N-Glycans, each cell group was bound to L-PHA. As shown in Figure 5A, knockdown of GnT-V by RNA interference showed a marked suppression of β1, 6-GlcNAc branched N-glycans. In addition, GnT-V knockdown resulted in a decrease of fluorescence intensity compared with the untreated cells and the cells treated with the control plasmid (P < 0.05) (Figure 5B). Taken together, these results confirmed that the effects of GnT-V on radioresistance of NPC cells may occur primarily by affecting the biosynthesis of β1, 6-GlcNAc branched N-glycans.
Figure 5.
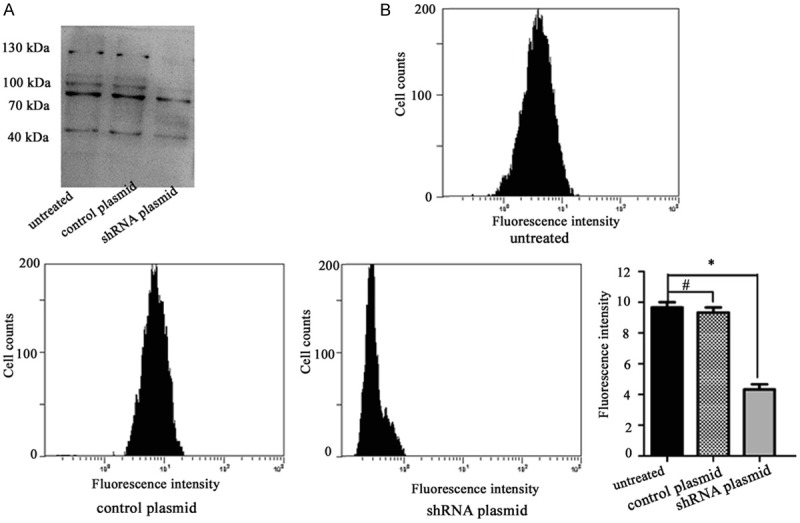
Effects of GnT-V knockdown on the formation of β1, 6-branched N-Glycans. CNE2R cells were stably transfected with the GnT-V shRNA plasmid or the control plasmid. The intensity of L-PHA staining was detected by (A) lectin blotting and (B) flow cytometry. Results are recorded as mean ± SD of three independent experiments (*P < 0.05, #P > 0.05, compared with untreated CNE2R cells).
Effects of GnT-V on glycosylation of CD147
CD147 is a major carrier of β1, 6-branched N-glycans on tumor cells [13]. Western blotting analysis revealed that almost no HG-CD147 expression in the CNE2 cells, but greater amounts of HG forms of CD147 were observed in the CNE2R cells. Furthermore, HG-CD147 was reduced following silencing of GnT-V expression in the CNE2R cells compared with untreated cells and cells treated with control plasmid (Figure 6). Therefore, the variation of N-glycans on CD147 indicated that CD147 is a target glycoprotein affected by GnT-V in NPC cells.
Figure 6.
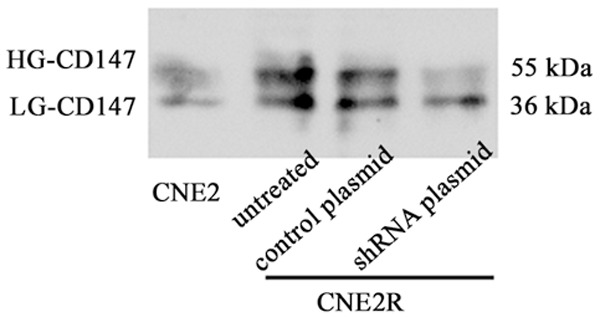
Effects of GnT-V knockdown on glycosylation of CD147. CNE2R cells were stably transfected with the GnT-V shRNA plasmid or the control plasmid. The glycosylation of CD147 was detected by western blotting.
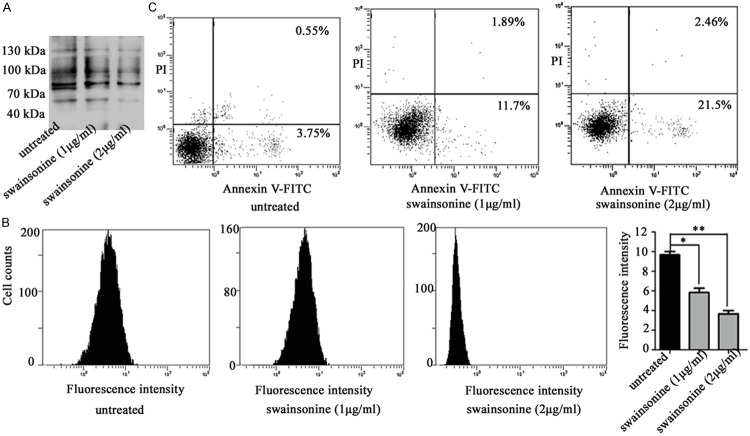
Effects of swainsonine on apoptosis of the CNE2R cells
Swainsonine inhibited Golgi α-mannosidase II and ultimately caused the inhibition of β1, 6-GlcNAc branched N-glycan formation [14]. The CNE2R cells were pretreated with different concentrations of swainsonine for 24 h. As shown in Figure 7A and 7B, swainsonine treatment resulted in a dramatically reduced binding with L-PHA as determined by lectin blotting and flow cytometry. Then the potential effects of swainsonine on cell apoptosis after 4 Gy radiation were investigated. As shown in Figure 7C, the apoptotic rate was significantly increased after radiation when the CNE2R cells were pretreated with swainsonine. These results further confirmed that the inhibition of β1, 6-GlcNAc branched N-glycans was able to enhance cell radiosensitivity.
Figure 7.
Effects of swainsonine on the synthesis of β1, 6-branched N-glycans and cell apoptosis. CNE2R cells were treated with different concentrations of swainsonine. The intensity of L-PHA staining was detected by (A) lectin blotting and (B) flow cytometry. (C) The apoptotic rate was detected using flow cytometry. Results are recorded as mean ± SD of three independent experiments (*P < 0.05, **P < 0.01, compared with the untreated CNE2R cells).
Discussion
High doses of radiotherapy in NPC are necessary to obtain locoregional control of the disease. Due to the close anatomic proximity to critical structures, NPC is one of the most difficult and challenging cancer sites for radiation oncologists. However, radioresistance limits the therapeutic efficacy of radiotherapy. Therefore, elucidating the underlying molecular mechanisms responsible for radioresistance is crucial for the development of novel target agents for radiation sensitization, and for exploring optimal radiotherapeutic regimens. In the present study, we found that GnT-V expression and its products β1, 6-GlcNAc modification on the cell surface were correlated with radioresistance of NPC cells. We showed for the first time, that GnT-V is an important mediator in control of radioresistance.
Radioresistance arises from tolerance of stimuli, decreased transducer activity, inactivation of normal cell death, decreased pro-apoptotic factors, altered cell cycling factors and proliferation signals [15,16]. There are numerous identified and potential modulators that contribute to the radioresistance of NPC. For example, fatty acid synthase, an adverse prognostic marker in NPC, was found to confer cell growth advantage and resistance to radiation-induced apoptosis [17]. In addition, aberrant DNA methylation was also found to be involve in the NPC response to radiotherapy, which was linked to inactivation of microRNA-24 [18]. Recently, research has shown that GnT-V was positively correlated with NPC radioresistance [9]. This may be linked to cell cycle G2-M arrest and the reduction in the Bcl-2/Bax ratio. But the precise role of GnT-V and β1, 6-GlcNAc branched N-glycans in NPC radioresistance has not been fully elucidated. In this study, CNE2R, a radioresistant cell line, was obtained by exposing the parental CNE2 cells to repeated fractions of a total dose of 60 Gy. We found that GnT-V expression levels were significantly increased in the CNE-2R cells. Lectins are sugar-binding proteins which are highly specific for their sugar moieties. L-PHA is used for glycan profiling to identify β1, 6-GlcNAc branched N-glycans in the cell surface. The results of this study clearly showed that L-PHA signal was significantly upregulated in the CNE-2R cells. To the best of our knowledge, this study is the first revealing the expression patterns of GnT-V and its products in radioresistant cancer cells.
N-glycans are implicated in the pathogenesis and prognosis of human types of cancers [19]. The importance of N-glycans as potential prognostic indicators or therapeutic targets for cancers are underscored by their functions in regulating fundamental cellular processes, such as cell proliferation, differentiation, invasion and apoptosis [20]. N-glycans also modulate responses to anticancer therapy. For example, the N-glycan regulation by way of tunicamycin application or PNGase F treatment in K562/ADR cells was found to result in increased chemosensitivity to anti-tumor drugs [21]. Inhibition of the formation of N-Glycans could reverse 5-fluorouracil resistance in colorectal cancer cells [22]. It is well known that N-glycan alterations are associated with differential expression of enzymes such as glycosyltransferase and glycosidases. In addition, the most important glycosyltransferase responsible for regulating responsiveness to anticancer therapy is GnT-V. Kudo et al identified that GnT-V was altered as cells acquired tolerance [23]. In another study, Ma et al found that the down-regulation of GnT-V enhanced the chemosensitivity to antitumor drugs [8]. In the present study, we confirmed that knockdown of GnT-V in the CNE-2R cells enhanced radiosensitivity, and inhibited the formation of β1, 6-branched N-Glycans. To a certain extent, the results of the present study were consistent with the effects of GnT-V and its products on anticancer therapy as described previously.
CD147 is a plasma transmembrane glycoprotein widely expressed on many cells [24]. CD147 is a highly N-glycosylated protein that is composed of two extracellular Ig domains, which contribute to a highly N-glycosylated HG-CD147 (~40-60 kDa) and a low glycosylated form, LG-CD147 (~33 kDa) [25]. Recently, CD147 has garnered attention because of its high expression in many malignant tumor cells, and its key role is in the radioresistance. For example, CD147 promoted radioresistance in hepatocellular carcinoma cells. The knockout and antibody blockade of CD147 decreased the tumor growth and metastatic potentials of cells under radiation [26]. Patients with high expression of CD147 also showed greater resistance to radiotherapy than those with low expression [27]. A previous study showed that overexpression of GnT-V resulted in an elevated level of CD147 N-glycosylation at the plasma membrane [28]. Here, we found almost no HG-CD147 expression in the CNE2 cells, but greater amounts of HG forms of CD147 were observed in the CNE2R cells. Furthermore, the regulated expression of GnT-V converted the heterogeneous N-glycosylated forms of CD147. Therefore, the variation of N-glycans on CD147 indicated that CD147 is a target glycoprotein affected by GnT-V in NPC radioresistance.
Swainsonine, a known glycosylation inhibitor, inhibits α-mannosidase II activity in the N-glycan biosynthesis pathway and blocks production of complex-type oligosaccharides. Swainsonine causes the inhibition of β1, 6-GlcNAc branched N-glycan formation. Swainsonine has been reported to exhibit anticancer activity on several human cancers [29]. Swainsonine was also able to reverse 5-fluorouracil tolerance in the multistage resistance of colorectal cancer cells [14]. In the present study, we confirmed that swainsonine treatment resulted in a markedly reduction in the amount of β1, 6-GlcNAc branched N-glycans. We also found that the apoptotic rate was significantly increased after radiation when CNE2R cells were pretreated with swainsonine. These results further confirmed that the inhibition of β1, 6-GlcNAc branched N-glycans was able to enhance cell radiosensitivity. It was suggested that swainsonine has further applications in anticancer therapy.
In conclusion, we confirmed that GnT-V expression was upregulated in radioresistant cancer cells and that the knockdown of GnT-V reversed the radioresistance at least partly, through suppression of the biosynthesis of β1, 6-branched N-Glycans. Therefore, GnT-V is a potential molecular target to overcome NPC radioresistance. These findings contribute to the clinical investigation of prediction markers, prognostic factors, as well the development of radiosensitizing molecules for therapeutic use.
Acknowledgements
This study was supported by grants from the Foundation for Innovative Research Team of Hubei University of Medicine (2014CXG02), the Key Discipline Project of Hubei Province (2014XKJSXJ12) and the Natural Science Foundation of Hubei Province (2015CFA076).
Disclosure of conflict of interest
None.
References
- 1.Lo KW, Chung GT, To KF. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin Cancer Biol. 2012;22:79–86. doi: 10.1016/j.semcancer.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Qu C, Liang Z, Huang J, Zhao R, Su C, Wang S, Wang X, Zhang R, Lee MH, Yang H. MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle. 2012;11:785–796. doi: 10.4161/cc.11.4.19228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan Y, Wang M, Bu X, Zuo Y, Wang S, Wang D, Liu Q, Su B, Xu T, Wang C, Claret FX, Yang H. Curcumin analogue T83 exhibits potent antitumor activity and induces radiosensitivity through inactivation of Jab1 in nasopharyngeal carcinoma. BMC Cancer. 2013;13:323. doi: 10.1186/1471-2407-13-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan JS, Rao A, Raghava GP. In silico platform for prediction of N-, O- and C-glycosites in eukaryotic protein sequences. PLoS One. 2013;8:e67008. doi: 10.1371/journal.pone.0067008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lizzi AR, D’Alessandro AM, Bozzi A, Cinque B, Oratore A, D’Andrea G. Pattern expression of glycan residues in AZT-treated K562 cells analyzed by lectin cytochemistry. Mol Cell Biochem. 2007;300:29–37. doi: 10.1007/s11010-006-9343-z. [DOI] [PubMed] [Google Scholar]
- 6.Varelas X, Bouchie MP, Kukuruzinska MA. Protein N-glycosylation in oral cancer: dysregulated cellular networks among DPAGT1, E-cadherin adhesion and canonical Wnt signaling. Glycobiology. 2014;24:579–591. doi: 10.1093/glycob/cwu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Akinaga A, Yamanaka K, Nakagawa T, Kondo A, Dickson RB, Lin CY, Miyauchi A, Taniguchi N, Miyoshi E. Co-expression of matriptase and N-acetylglucosaminyltransfe- rase V in thyroid cancer tissues--its possible role in prolonged stability in vivo by aberrant glycosylation. Glycobiology. 2006;16:368–374. doi: 10.1093/glycob/cwj084. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Miao X, Ma Q, Zheng W, Zhou H, Jia L. Functional roles of glycogene and N-glycan in multidrug resistance of human breast cancer cells. IUBMB Life. 2013;65:409–422. doi: 10.1002/iub.1133. [DOI] [PubMed] [Google Scholar]
- 9.Zhuo E, He J, Wei T, Zhu W, Meng H, Li Y, Guo L, Zhang J. Down-regulation of GnT-V enhances nasopharyngeal carcinoma cell CNE-2 radiosensitivity in vitro and in vivo. Biochem Biophys Res Commun. 2012;424:554–562. doi: 10.1016/j.bbrc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Peng G, Cao RB, Li YH, Zou ZW, Huang J, Ding Q. Alterations of cell cycle control proteins SHP1/2, p16, CDK4 and cyclin D1 in radioresistant nasopharyngeal carcinoma cells. Mol Med Rep. 2014;10:1709–1716. doi: 10.3892/mmr.2014.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Wang S, Yu S, He J, Zheng W, Zhang J. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconj J. 2012;29:323–334. doi: 10.1007/s10719-012-9414-1. [DOI] [PubMed] [Google Scholar]
- 12.de Freitas Junior JC, Silva Bdu R, de Souza WF, de Araujo WM, Abdelhay ES, Morgado-Diaz JA. Inhibition of N-linked glycosylation by tunicamycin induces E-cadherin-mediated cell-cell adhesion and inhibits cell proliferation in undifferentiated human colon cancer cells. Cancer Chemother Pharmacol. 2011;68:227–238. doi: 10.1007/s00280-010-1477-8. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Huang W, Ma LT, Jiang JL, Chen ZN. Importance of N-glycosylation on CD147 for its biological functions. Int J Mol Sci. 2014;15:6356–6377. doi: 10.3390/ijms15046356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamaguchi J, Nakagawa H, Takahashi M, Kudo T, Kamiyama N, Sun B, Oshima T, Sato Y, Deguchi K, Todo S, Nishimura S. Swainsonine reduces 5-fluorouracil tolerance in the multistage resistance of colorectal cancer cell lines. Mol Cancer. 2007;6:58. doi: 10.1186/1476-4598-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK, Chao KS. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys. 2011;80:851–859. doi: 10.1016/j.ijrobp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol. 2014;90:615–621. doi: 10.3109/09553002.2014.892227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao YC, Lee SW, Lin LC, Chen LT, Hsing CH, Hsu HP, Huang HY, Shiue YL, Chen TJ, Li CF. Fatty acid synthase overexpression confers an independent prognosticator and associates with radiation resistance in nasopharyngeal carcinoma. Tumour Biol. 2013;34:759–768. doi: 10.1007/s13277-012-0605-y. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Zhang R, Claret FX, Yang H. Involvement of microRNA-24 and DNA methylation in resistance of nasopharyngeal carcinoma to ionizing radiation. Mol Cancer Ther. 2014;13:3163–3174. doi: 10.1158/1535-7163.MCT-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18:750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv Cancer Res. 2015;126:11–51. doi: 10.1016/bs.acr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhao Y, Jiang L, Miao X, Zhou H, Jia L. Glycomic alterations are associated with multidrug resistance in human leukemia. Int J Biochem Cell Biol. 2012;44:1244–1253. doi: 10.1016/j.biocel.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Shen L, Yu M, Xu X, Gao L, Ni J, Luo Z, Wu S. Knockdown of beta3GnT8 reverses 5-fluorouracil resistance in human colorectal cancer cells via inhibition the biosynthesis of polylactosamine-type N-glycans. Int J Oncol. 2014;45:2560–2568. doi: 10.3892/ijo.2014.2672. [DOI] [PubMed] [Google Scholar]
- 23.Kudo T, Nakagawa H, Takahashi M, Hamaguchi J, Kamiyama N, Yokoo H, Nakanishi K, Nakagawa T, Kamiyama T, Deguchi K, Nishimura S, Todo S. N-glycan alterations are associated with drug resistance in human hepatocellular carcinoma. Mol Cancer. 2007;6:32. doi: 10.1186/1476-4598-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu D, Zhu S, Li J, Ji G, Wang W, Wu G, Zheng J. CD147 expression in human gastric cancer is associated with tumor recurrence and prognosis. PLoS One. 2014;9:e101027. doi: 10.1371/journal.pone.0101027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004;15:4043–4050. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Li Y, Dang YZ, Gao HX, Jiang JL, Chen ZN. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: a potential role for integrin beta1 signaling. Mol Cancer Ther. 2015;14:553–563. doi: 10.1158/1535-7163.MCT-14-0618. [DOI] [PubMed] [Google Scholar]
- 27.Huang XQ, Chen X, Xie XX, Zhou Q, Li K, Li S, Shen LF, Su J. Co-expression of CD147 and GLUT-1 indicates radiation resistance and poor prognosis in cervical squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:1651–1666. [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W, Luo WJ, Zhu P, Tang J, Yu XL, Cui HY, Wang B, Zhang Y, Jiang JL, Chen ZN. Modulation of CD147-induced matrix metalloproteinase activity: role of CD147 N-glycosylation. Biochem J. 2013;449:437–448. doi: 10.1042/BJ20120343. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Xu X, Huang Y, Ding L, Wang Z, Yu G, Xu D, Li W, Tong D. Swainsonine activates mitochondria-mediated apoptotic pathway in human lung cancer A549 cells and retards the growth of lung cancer xenografts. Int J Biol Sci. 2012;8:394–405. doi: 10.7150/ijbs.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]