Abstract
This study aimed to detect the expression of Slit signaling protein ligand Robo protein in human bladder cancer and para-carcinoma tissue, and observe the tumor cell survival and growth by inoculating the bladder cancer cells with the blocked signaling protein into the subcutaneous tissue of nude mice. The expression of Robo protein was detected in T24 cells in human bladder uroepithelium carcinoma and cultivated human bladder uroepithelium carcinoma confirmed by pathological diagnosis. The cultivated T24 cells were coated by the protein antibody and human bladder uroepithelium carcinoma T24 tumor-bearing mice model was established. The tumor cell survival and growth were observed in the antibody coating group and non-coating group. The tumor body size was measured. The immunohistochemical detection showed that Robo protein isoforms Robo1 and Robo 4 were expressed in T24 cells of cancer tissues, paracarcinoma tissues and cultured human uroepithelium carcinoma. The expression of Robo1 was significantly higher than that of Robo4 (P<0.05). The cancer cells could be detected in nodular tumor of mice in each group. The volume of the tumor-bearing mice in the nodular tumor of the non-coating group was larger than that of anti-Robol antibody coating group and the difference was statistically significant (P<0.01). There was no significant difference in tumor volume between anti-Robo4 antibody coating group and non-coating group (P>0.05); The difference was statistically significant compared with the anti-Robo1 antibody coating group (P<0.01). In conclusion, Robo protein isoforms Robo1 and Robo4 were expressed in human bladder cancer T24 cells. To block Robo4 signal protein had little effect on the survival and growth of the transplantation tumor and to block Robo1 signal protein would seriously affect the survival and growth of the transplantation tumor, suggesting that Robo1 might play an important role in the growth and metastasis of bladder cancer, and might become a new target for the treatment of human bladder cancer and drug research.
Keywords: Bladder cancer, Slit/Robo signaling pathway, T24 cells
Introduction
Bladder cancer is one of the common malignant tumors in the urinary surgery. Its treatment mainly depends on early diagnosis and surgical resection. The special effective treatment is absent. In recent years, the biological treatment for specific proteins playing an important role in tumor genesis process and targeting anti-tumor drugs has achieved the satisfactory therapeutic effect in clinical application. The neuronal guidance factor Slit family was firstly discovered to play an important role in the process of regulating the orientated growth of neuron axon and orientated migration of neuron precursor cells. Recent studies have showed that Slit/Robo signaling pathway played an important role in regulating tumor cell migration and angiogenesis. Roundabout protein, also known as Robo receptor, is a transmembrane protein receptor. Four kinds of Robo receptors were discovered in mammals, including Robol, Robo2, Robo3 and Robo4 respectively [1,2]. Robo1 and Robo2 were expressed in most tissues and organs of mature individuals. And their expression in the developing nervous system was significant. Robo3 was only expressed in the developing central nervous system and Robo4 was mainly in the endothelial cells [3].
Previous studies showed that Robo was expressed in pancreatic cancer, ovarian cancer and oral cancer. Robo1 and Robo4 are widely expressed in ovarian cancer tissues and cytoplasm, are closely related to the invasion and metastasis of ovarian cancer [4-6]. The existing researches have confirmed that Robo gene was related with the pathologies of liver cancer, colorectal cancer and other tumors. Therefore, we hypothesized that Robo gene might also be related to the pathology of bladder cancer. There was no study on Robo gene or bladder cancer yet. Therefore, on the basis of examination of pathological specimen and study on the corresponding bladder cancer cell strains, the growth of the blocked tumor cells in vivo and in vitro was studied using biological technology. The effect of Robo gene on bladder cancer cell division and growth of bladder cancer was explored so as to seek the new method and new target.
Materials and methods
Specimen source and paraffin embedding
Eight cases of bladder uroepithelium carcinoma tissues were from the surgical specimens resected in urinary surgery of Huaihe Hospital of Henan University from January 2012 to January 2014. All cases were confirmed as bladder urothelium carcinoma by pathology. The specimens were placed on the ice within half an hour, immersed and fixed in 4% paraformaldehyde, then dehydrated, hyalinized, embedded in paraffin and sliced.
Expression of Robo isoforms in tissue by immunohistochemistry
The sections were dewaxed routinely and placed in water. The antigen retrieval was conducted by microwave. The rabbit anti-Robo-1, rabbit anti-Robo-2, rabbit anti-Robo-3 and rabbit anti-Robo-4 primary antibody (Abcam, Cambridge, UK) were added, incubated overnight at 4°C refrigerator and rinsed with PBS. The HRP labeled goat anti-rabbit second antibody (Boster, Wuhan, China) was added, incubated at 37°C and rinsed with PBS. The horseradish peroxidase was added. DAB was added for coloration. The hematoxylin was used for counterstain. The gradient dehydration, hyalinization with xylene and mounting with neutral gum were performed. The immunohistochemical sections were observed under light microscope and photographed. The number of positive cells, positive cells effective target distribution and immunoreaction product integral optical density were determined using Image Pro-Plus 6.0 image analysis system software (Media Cybernetics USA). The average optical density (MOD) value was calculated.
Cell culture and cell growing on the glass slide
Human bladder cancer T24 cells were purchased from Beina Chuanglian Biotechnology Research Institute, Beijing, China. They were cultured in 1640 culture medium (Gibco, Grand Island, NY, USA) at 37°C, 5% CO2 and appropriate humidity. The sterilized coverslips were placed in the new 6-well culture plate sterilized in advance. The prepared cell suspension (cell number about 2-5 × 105/ml) was dropped in the center of the coverslip and placed in the incubator for 30 minutes. 1-2 mL 1640 culture medium was added in the incubator. The cell passage time and generations were clearly marked, set aside overnight.
Cell growing on the glass slide with immunohistochemical staining
The adherent cells were digested with the trypsin. The cell concentration was adjusted to about 1 × 105/ml and dropped on the coverslips (placed in 6-well plate). The cells were grown on the glass slide after cultured for the corresponding time. The wax circles were drawn. The sample was fixed with 95% ethanol (4% paraformaldehyde) at room temperature. 0.25% Triton was added, incubated in the thermostat at 37°C, treated with 3% H2O2 at room temperature and blocked with the confining liquid at room temperature. The appropriate concentration of primary antibody (rabbit anti-Robo-1, rabbit anti-Robo-2, rabbit anti-Robo-3 and rabbit anti-Robo-4) (Abcam, Cambridge, UK) was added and incubated in the thermostat at 37°C. The HRP labeled goat anti-rabbit second antibody (Boster, Wuhan, China) was added and incubated in the thermostat at 37°C. DAB coloration, dehydration, hyalinization and mounting were performed successively.
Cell growing on the glass slide with immunofluorescence
The slides with cell growing on were rinsed with PBS in culture plates, fixed with 4% polyformaldehyde at room temperature, perforated with 0.5% TritonX-100; The sample was immersed in PBS, blocked with 10% sheep serum, incubated overnight with the primary antibody (rabbit anti-Robo-1, rabbit anti-Robo-2, rabbit anti-Robo-3 and rabbit anti-Robo-4) (Abcam, Cambridge, UK) and incubated with the FITC-labeled goat anti-rabbit IgG second antibody (Boster, Wuhan, China) at room temperature. The nuclei were redyed; The sample was incubated with hoechest33258 away from light. The slices were sealed with mounting solution. The edges were sealed with the nail polish. The image analysis results were acquired under the fluorescence microscope.
Experimental animals and groups
24 BALB/c-nude nude mice, half male and half female, 50~60 days, weight 18-22 g, were purchased in the medical experimental animal center of Guangdong Province. The license number was SCXK (Guangdong) 2008-0002. After the animals adapted environment for one week in the individual ventilated closed sterile cages at 18-22°C and 50%-80% constant humidity, T24 cells tumor-bearing animal model was established. The nude mice were randomly divided into 3 groups, with 8 rats in each group. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA). Eighth Edition, 2010. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Henan University Huaihe Hospital.
T24 cell culture and establishment of subcutaneous transplantation tumor
When T24 cells arrived to a certain amount and were in the logarithmic growth phase, they were digested with 0.25% trypsin. The cells were collected for cell counting and centrifuged at 1000 r/min for 3 min. The supernatant was discarded. The sterile PBS was added and the cells were dissociated into single cell suspension. The cell concentration was adjusted into 1.5 × 107 cells/ml with serum-free 1640 culture media. The inoculated puncture point was the subcutaneous tissue about 1 cm from the axilla. The needle tip was placed with the slope upward. The puncture angle was less than 5°. After the needle tip was penetrated in the subcutaneous tissue, it traveled into the axilla at 0°. The syringe needle was rotated to make the slope inward. The needle handle was fixed. 0.2 ml cell suspension was inoculated. The tumor module was observed in a week and measured regularly. The tumor formation rate was 100%.
Growth of tumor-bearing mice
The nude mice were randomly divided into 3 groups, with 8 mice in each group. Group I: control group; group II: Robo1 blocking group; group III: Robo4 blocking group; The antibody was locally injected every 3 days from the 7th day, a total of 10 times. The tumor sizes (mm) were measured every 7 days after injection. The tumor volume was calculated according to the formula: volume =0.5 × a × b (a: long diameter, b: short diameter).
Tissue morphology detection
The experimental mice were killed. The bladder cancer and paracarcinoma tissue were fixed with 4% paraformaldehyde, dehydrated, hyalinized and dewaxed. The morphological changes were observed by routine HE staining.
Statistical analysis
All data were analyzed using SPSS 16.0 statistical software (SPSS Inc, Chicago, IL, USA). The data were analyzed with single factor analysis of variance. P<0.05 showed the difference was statistically significant.
Results
Immunohistochemistry of bladder cancer tissues and paracarcinoma tissues
The immunohistochemistry result showed that expression of Robo protein isoforms Robo1 and Robo4 were expressed in bladder cancer tissues and para-carcinoma tissues, but Robo3 and Robo2 were not expressed. The expression of Robo1 in bladder cancer tissues and paracarcinoma tissues was higher than that of Robo4. Compared with the expressions of Robo2 and Robo3, the expressions of Robo1 and Robo4 were significantly different (Figures 1 and 2).
Figure 1.
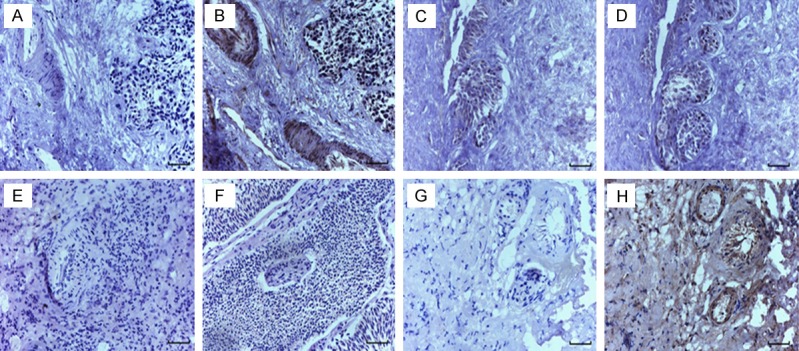
Immunohistochemical staining of Robo protein in human bladder cancer tissues. A: PBS negative control, B: Robo1 protein, C: PBS negative control, D: Robo2 protein, E: PBS negative control, F: Robo3 protein, G: PBS negative control, H: Robo4 protein. (scale bar =50 μm).
Figure 2.
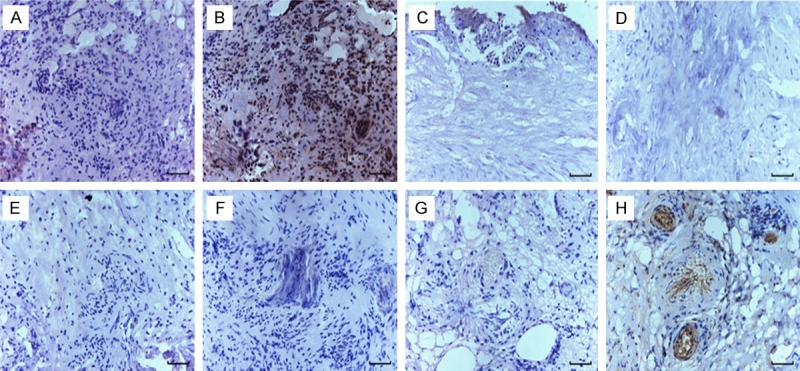
Immunohistochemical staining of Robo protein in human bladder paracarcinoma tissues. A: PBS negative control, B: Robo1 protein, C: PBS negative control, D: Robo2 protein, E: PBS negative control, F: Robo3 protein, G: PBS negative control, H: Robo4 protein. (scale bar =50 μm).
Immunocytoochemistry
The human bladder urothelium carcinoma T24 cells were cultured in vitro and cell growing on the slide was prepared. The expression of Slit/Robo in human bladder urothelium carcinoma T24 cells was detected by immunohistochemical S-ABC method. As shown in Figures 3 and 4, positive brown yellow particles in the cytoplasm and nuclei in Robo1 and Robo4 groups were shown with the black arrow. Robo1 and Robo4 were expressed in T24 cells. And Compared with Robo4, the expression of Robo1 protein was higher. Immunohistochemical result was similar with that of clinical pathological examination. Robo3 and Robo2 were not significantly expressed. Compared with the expressions of Robo3 and Robo1, the expressions of Robo2 and Robo4 were significantly different.
Figure 3.
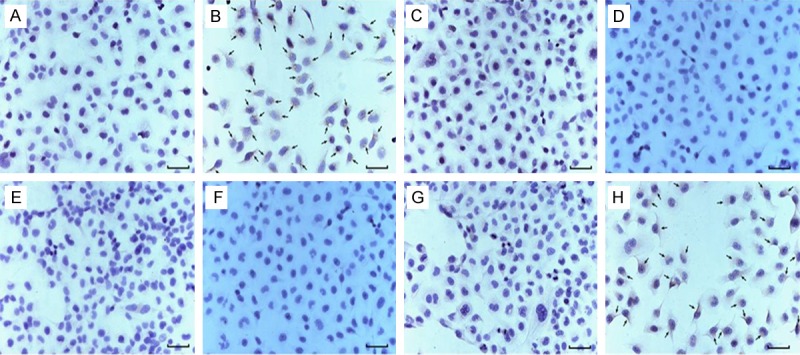
Immunohistochemical staining of Robo protein in T24 cells. A: PBS negative control, B: Robo1 protein, C: PBS negative control, D: Robo2 protein, E: PBS negative control, F: Robo3 protein, G: PBS negative control, H: Robo4 protein. The black arrow showed the positive brown yellow particles in Robo1 and Robo4 cytoplasm and nuclei. (scale bar =50 μm).
Figure 4.

Quantitative analysis of Robo protein by immunohistochemical staining. Note: A: Human bladder cancer tissues; B: Human bladder paracarcinoma tissues; C: T24 cells. **P<0.01 vs. Robo2, ***P<0.005 vs. Robo2, ##P<0.01 vs. Robo3, ###P<0.005 vs. Robo3.
Cell immunofluorescence
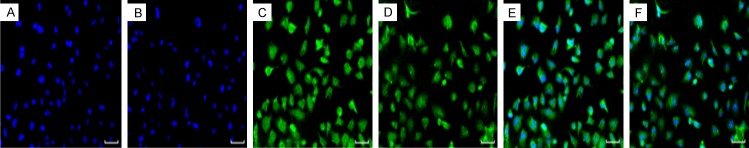
The immunofluorescence result showed that Robo1 and Robo4 were expressed in human bladder urothelium carcinoma T24 cells. Robo1 and Robo4 were highly expressed in the cytoplasm of T24 cells, especially in the nucleus. The results showed that Robo1 and Robo4 were expressed in human bladder urothelium carcinoma T24 cells (Figure 5).
Figure 5.
Robo protein in T24 cells by immunofluorescence staining. (A) Hoechst staining, (B) Hoechst staining, (C) Immunofluorescent staining of Robo1 protein in T24 cells, (D) Immunofluorescent staining of Robo4 protein in T24 cells, (E) Synthesized by (A) and (C), (F) Synthesized by (B) and (D). (scale bar =50 μm).
Growth of tumor-bearing nude mice
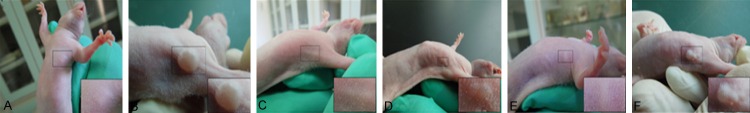
T24 cells were inoculated in the subcutaneous tissue of the axilla of nude mice. The small skin hill would not completely subside after inoculated for 12-24 h. The tumor nodule appeared in the axillary subcutaneous tissue after inoculated for a week. The tumor nodule grew in 15-20 days by measurement; The nodules were differently shaped, mostly round, oval or irregular. There was no obvious ulcer on the nodular surface. The nodular activity was poor. The tumor formation rate was 100%. The long and short diameters of the tumor were measured every 7 days and the volume was calculated. The result showed that the transplantation tumor volumes of nude mice in Robo1 blocking group and Robo4 blocking group were significantly lower than that of the blank control group. And the transplanted tumor volumes of nude mice in Robo1 blocking group were significantly smaller than that of Robo4 blocking group (Figure 6; Table 1).
Figure 6.
Growth of nude mice. A: Inoculated for 2 w in the control group; B: Inoculated for 5 w in the control group; C: Inoculated by closing Robo1 for 2 w; D: Inoculated by closing Robo1 for 5 w; E: Inoculated by closing Robo4 for 2 w; F: Inoculated by closing Robo4 for 5 w.
Table 1.
Tumor volumes of nude mice at different stages (x̅ ± s)
| Groups | Two weeks | Three weeks | Four weeks |
|---|---|---|---|
|
| |||
| Tumor volume/mm3 | Tumor volume/mm3 | Tumor volume/mm3 | |
| Control group | 2.18±0.25 | 3.44±0.13 | 4.02±0.31 |
| Robo1 blocking group | 0.96±0.23 | 1.31±0.17 | 2.06±0.30 |
| Robo4 blocking group | 1.01±0.24 | 2.20±0.52 | 3.52±0.12 |
Note: The volumes of the transplantation tumor in Robo1 blocking group and Robo4 blocking group were significantly lower than that of the control group, and the volume of the transplantation tumor in Robo1 closing group was significantly less than that of Robo4 blocking group.
Histopathological examination of tumor-bearing tissues
The tumor-bearing sections were used to observe the morphological changes of nude mice by routine HE staining. The results showed that tumor cells were heterologous, the cell sizes were different, the shapes were irregular and the mitosis was observed (Figure 7). Thus the tumor tissues were confirmed as the cancer tissues.
Figure 7.
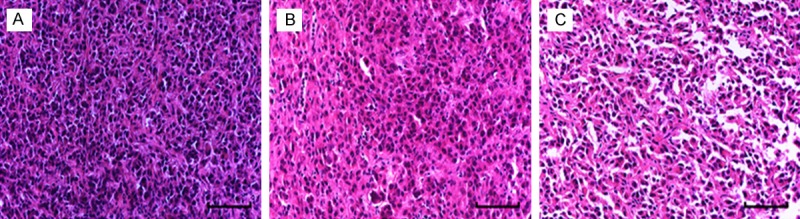
HE staining of tumor tissue in nude mice. A: Control group; B: Robo1 blocking group; C: Robo4 blocking group. (scale bar =50 μm).
Discussion
Slit gene was firstly discovered in Drosophila melanogaster embryo by Jürgens et al in 1984 [7]. And it was showed that it played a role in Drosophila melanogaster larva luster. Rothberg et al. [8] reported that Slit protein could be synthesized in neurogliocyte of central nervous system in Drosophila melanogaster in 1988, the decrease of Slit protein could lead to the abnormal aggregation of internuncial neuron axons and longitudinal conduction pathway in the midline [9,10]. In 1998, Robo was firstly discovered as a receptor of Slit, which laid a foundation for further study of Slit protein and its function. Robo4 was a recently discovered member of Robo family, which was considered to be a specific gene of endothelial cell. It was reported that Robo4 could be detected in endothelial tissue by immunohistochemistry, in situ hybridization and RT-PCR. Meanwhile it was highly expressed in pathological new vessels, such as tumor new vessels, but it was almost not expressed in the human adult tissue [11,12]. The expressions of Robo in human bladder urothelial carcinoma and paracancerous tissues were detected by immunohistochemistry method in this experiment. The study showed that Robo protein isoforms Robo1 and Robo4 were expressed in the bladder cancer tissue and paracancerous tissues, Robo2 and Robo3 were almost not expressed, and the expressions of Robo1 in bladder cancer tissue and paracancerous tissues were higher than that of Robo4. The expressions of Robo protein isoforms may be related to the tissue origin and severity of bladder cancer, and the cell types had different effects on the expression of Robo.
Human bladder cancer cell strain T24 cells were derived from the bladder urothelium. The differentiation of T24 cells was poor and the malignant degree was high [13]. In this study, the expression of Robo in T24 cells was detected by using immunofluorescence technique, the T24 cell strain of human bladder cancer was selected as the object. Study showed that Robo1 and Robo4 protein were expressed in human bladder cancer cell strain T24 cells in Robo family, but Robo2 and Robo3 were not expressed. The experimental results in vitro were consistent with that of immunohistochemical experiments. The expression of Robo1 in human bladder cancer cell strain T24 cells was higher than that of Robo4, indicating that the effect of Robo1 on the regulation of bladder cancer was more obvious. Robo1 had a regulatory role in tumor occurrence and metastasis. Wang Jing et a. [14] detected the expressions of Robo1 protein in breast infiltrating ductal carcinoma with cerebral metastasis, breast infiltrating ductal carcinoma without brain metastasis, breast intraductal carcinoma breast cancer and breast fibroadenoma. The result showed that the expression of Robo1 in breast infiltrating ductal carcinoma was negatively correlated with brain metastases, positively correlated with patient age and prognosis, which could be a molecular marker for evaluating the prognosis of breast cancer and brain metastases. Chen et al. [15] reported that Slit2/Robo1 protein played an important role in colorectal tumor angiogenesis, Slit2/Robo1 protein and microvessel density were closely related to the growth, invasion and metastasis of colorectal cancer.
Then the effect of blocking Robo on the transplantation tumor of human urinary bladder urothelium carcinoma in nude mice was investigated in this experiment. Through the construction of the transplantation tumor model of human bladder urothelium carcinoma T24 cell strain in nude mice bladder, the tumor-bearing nude mice were randomly divided into Robo1 blocking group, Robo4 closed group and blank control group. Robo1 antibody, Robo4 antibody and sterile saline were injected in paracarcinoma tissues respectively in each group. The tumor growth was monitored. The tumor sizes in nude mice were compared. The pathological changes of tumor tissues in nude mice were detected. The results showed that the volumes of the transplantation tumor in Robo1 group and Robo4 group were significantly smaller than that of the control group, and the volume of the transplantation tumor in Robo1 group was significantly smaller than that of Robo4 group. We thought that Robo1 and robo4 played an important role in subcutaneous transplantation tumor growth process of human bladder cancer T24 cells in nude mice. The intratumoral injection of Robo1 and Robo4 especially Robo1 could effectively and safely inhibit the growth of subcutaneous transplantation tumor in bladder cancer T24 cells nude mice, suggesting that the process might be related to Robo4 inhibiting the formation of bladder cancer by inhibiting the vascularization of bladder cancer, and then inhibiting the growth of tumor cells.
Therefore, to accelerate the study on the molecular mechanism of Robol and Robo4 in bladder cancer occurrence, development and metastasis processes, to clarify its important signaling pathway involved as soon as possible, have the significance to the targeted therapy of bladder cancer and improving bladder cancer patients’ prognosis.
Acknowledgements
This work was supported by the Science and Technology Project of Henan Province (No. 152102310076).
Disclosure of conflict of interest
None.
References
- 1.Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139:697–704. doi: 10.1530/REP-10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard MS, Hinck L. A roundabout way to cancer. Adv Cancer Res. 2012;114:187–235. doi: 10.1016/B978-0-12-386503-8.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews WD, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211:188–198. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He H, Di Y, Liang M, Yang F, Yao L, Hao S, Li J, Jiang Y, Jin C, Fu D. The microRNA-218 and ROBO-1 signaling axis correlates with the lymphatic metastasis of pancreatic cancer. Oncol Rep. 2013;30:651–658. doi: 10.3892/or.2013.2516. [DOI] [PubMed] [Google Scholar]
- 5.Dai CF, Jiang YZ, Li Y, Wang K, Liu PS, Patankar MS, Zheng J. Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol. 2011;135:475–485. doi: 10.1007/s00418-011-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma YG, Wang LJ, Han B, Zhang J. Effect of Slit-Robo signal on apoptosis of oral cancer cell line Tb. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41:232–235. [PubMed] [Google Scholar]
- 7.Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophilamelanogaster II. Roux’s Archives of Developmental Biology. 1984;5:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- 8.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Fontelonga T, Roesener AP, Lee H, Gurung S, Mendonca PR, Mastick GS. Motor neuron cell bodies are actively positioned by Slit/Robo repulsion and Netrin/DCC attraction. Dev Biol. 2015;399:68–79. doi: 10.1016/j.ydbio.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todenhöfer T, Hennenlotter J, Guttenberg P, Mohrhardt S, Kuehs U, Esser M, Aufderklamm S, Bier S, Harland N, Rausch S, Gakis G, Stenzl A, Schwentner C. Prognostic relevance of positive urine markers in patients with negative cystoscopy during surveillance of bladder cancer. BMC Cancer. 2015;15:155. doi: 10.1186/s12885-015-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London NR, Li DY. Robo4-dependent Slit signaling stabilizes the vasculature during pathologic angiogenesis and cytokine storm. Curr Opin Hematol. 2011;18:186–190. doi: 10.1097/MOH.0b013e328345a4b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Görn M, Anige M, Burkholder I, Müller B, Scheffler A, Edler L, Boeters I, Panse J, Schuch G, Hossfeld DK, Laack E. Serum levels of Magic Roundabout protein in patients with advanced non-small cell lung cancer (NSCLC) Lung Cancer. 2005;49:71–76. doi: 10.1016/j.lungcan.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Fujiyama C, Jones A, Fuggle S, Bicknell R, Cranston D, Harris AL. Human bladder cancer invasion model using rat bladder in vitro and its use to test mechanisms and therapeutic inhibitors of invasion. Br J Cancer. 2001;84:558–564. doi: 10.1054/bjoc.2000.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Wang L, Liu FF, Ma YJ, Fu L, Li WL, Gu F. Robo1 expression in breast cancer and its relationship to brain metastasis. Chinese Journal of Oncology. 2011;33:447–451. In Chinese. [PubMed] [Google Scholar]
- 15.Chen J, Yu DF, Liu Y, Yang JL. Slit2/Robo1 proteins expression and their correlation with microvascular density in rectal cancers. Anhui Medical and Pharmaceutical Journal. 2011;15:735–737. [Google Scholar]