Abstract
Niflumic acid (NFA) was known to inhibit cell proliferation or migration in several types of cancer. However, the function of NFA in human nasopharyngeal carcinoma (NPC) cells was not clarified. The proliferation of NPC cell line CNE-2Z cells with NFA treatment was detected using the cell counting kit-8 method and transwell assay was employed to assess the effect of NFA on the CNE-2Z cell migration and invasion. The activity of MMP2 and MMP9 was detected by Gelatin Zymography. Cell cycle distribution and apoptosis were detected using flow cytometry. In vitro pull-down assay, western blot, and computational technique were applied to investigate the NFA regulating signaling pathway. Our results indicated that the growth capacity and colony formation potential of CNE-2Z cells in soft agar were significantly suppressed by treatment with NFA. NFA inhibited the proliferation of CNE-2Z cells in a concentration and time-dependent manner. NFA exerted an S phase arrest on the CNE-2Z cells in a concentration-dependent manner, while promoting apoptosis in a dose-dependent manner. Migration and invasion potential of CNE-2Z cells were decreased by NFA treatment in vitro. In vitro pull-down assay and molecular modeling indicated that NFA directly bound with early respond kinase 1 (ERK1). Finally, the anti-tumor effect of NFA was suggested to be mediated by inhibiting early respond kinases (ERK) expression and the MMP2 and MMP9 activities. NFA has proliferation-inhibiting, invasion-suppressing, cell cycle-blocking and apoptosis-promoting effects on CNE-2Z cells through regulation of ERK/MAPK and our results indicates that NFA may serve as a candidate of anticancer drug for NPC.
Keywords: Niflumic acid (NFA), nasopharyngeal carcinoma, MAPK/ERK, MMP9, MMP2, molecular modeling
Introduction
Nasopharyngeal carcinoma (NPC) is a disease in which malignant cells develop in the tissues of the nasopharynx and it poses one of the serious health problems in Southern China, especially in Guangdong province as one of the most common tumors among Chinese or Asian ancestry [1]. NPC is widely suspected to be the result of genetic susceptibility, exposure to environmental factors and Epstein-Barr virus infection [2]. Primary treatment measures for NPC include radiotherapy and chemotherapy. However, these treatments have a tendency to cause serious adverse reactions and induce multidrug resistance (MDR). Therefore, it is necessary to identify highly effective anti-NPC drugs with less serious adverse effects.
Several cellular proliferation pathways are upregulated in NPC, such as the Akt pathway, the Wnt pathway and mitogen-activated protein kinases (MAPKs) pathway [3]. The extracellular signal-regulated kinase (ERK) MAPK pathway (or Raf-MEK-ERK pathway) with each consisting of at least three components, a MAPK kinase (MAP3K), a MAPK kinase (MAP2K) and a MAPK, regulates a variety of cellular activities including proliferation, survival and differentiation [4]. Clinical outcome in cancer was improved with the use of compounds targeting components of ERK/MAPK signaling, such as Raf or MEK inhibitors [5].
Niflumic acid (NFA), which belongs to the Non-steroidal anti-inflammatory drugs (NSAIDs), is widely used in the treatment for dysmenorrhea, rheumatoid, and osteoarthritis [6]. However, NFA was found to inhibit the proliferation of several tumor cell types recently, including lung cancer, ovarian cancer, renal carcinoma, hepatoma and fibrosarcoma [6-11]. NFA induced apoptotic cell death, activated caspase-9 and caspase-3 [7]. Moreover, NFA was reported to regulate cell migration and invasion in ovarian cancer and breast cancer [9,12]. Nevertheless, the biological function of NFA in nasopharyngeal carcinoma was not clarified. In this study, we elucidated that NFA has proliferation-inhibiting, invasion-suppressing, cell cycle-blocking and apoptosis-promoting effects on CNE-2Z cells through regulation of MAPK/ERK signaling pathway and activity of MMP2 and MMP9.
Methods and materials
Reagents and materials
Niflumic acid (NFA, 98% purity) was purchased from LKT Laboratories, Inc. RPMI1640 medium, fetal bovine serum (FBS) and 0.25% trypsin was from Gibco-BRL (Grand Island, NY, USA). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories, Kumamoto (Japan). The AnnexinV-FITC Apoptosis Detection kit was obtained from Keygen Biotech (China). The agarose and CNBr-activated SepharoseTM 4B were from Sigma-Aldrich (China) Trading Co. Ltd. Transwell Boyden chamber system containing a polycarbonate filter (6.5 mm in diameter, 8 μm pore size) was from Corning Life Sciences. The polyvinylidene fluoride (PVDF) membrane was from Millipore (USA). The antibody was purchased from Cell Signaling Technology (CST).
Cell proliferation assay
Nasopharyneal carcinoma cell line CNE-2Z was friendly provided by Dr Xiaoyi Chen (Guangdong Medical University, China). Cells were grown in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and cultured at 37°C in an incubator with 5% CO2 and saturated humidity. Firstly, cells were collected and seeded into 96 well plate and incubated for 24 h, and then treated with different concentrations of NFA (50, 100, 200 and 400 μM). Cell viability was measured by the CCK-8 assay after 24, 48 and 72 h of culture. The OD values were measured by a spectrophotometric microtiter plate reader (Dynatech Laboratories, Alexandria, VA) at 450 nm. Each experiment was performed in triplicate.
Cell cycle assay
Cells in the logarithmic phase were digested with 0.25% trypsin and then centrifuged to remove residual medium and trypsin. Cells were then plated at 1 × 105 cells/well in 6-well flat-bottom plates. After adherent, the cells were starved with FBS-free RPMI-1640 medium for 24 h, and then cultured in RPMI-1640 medium containing 10% FBS. Concentrations of NFA at 0, 25 and 50 μM were respectively added. Cells were collected and washed with PBS for twice, than fixed with 70% ice-cold ethanol and were maintained overnight at 4°C. Next day, the cells were washed with ice-cold PBS and stained with propidium iodide (PI) (500 μl of PBS contained 1% PI and 1% RNase A) in the dark for 30 min at room temperature. The cell cycle phase distributions were detected by flow cytometer (BD Biosciences).
Annexin V-FITC/PI double staining
Cells (1 × 105 cells/well) were seeded in 6-well plates. After incubating for 24 h, different concentrations of NFA (0, 50, 100 and 200 μM) were added. Then after culturing for another 48 h, cells were trypsinized using trypsin without EDTA. Cells were collected and washed with PBS for twice and resuspended in binding buffer. Subsequently, annexin V-FITC and PI was added. Finally, samples were incubated in the dark at room temperature for 15 min and analyzed by flow cytometry.
Foci-formation assay
For foci-formation assay, CNE-2Z cells (500 cells/well) were cultured in 6-well cluster overnight, they were treated with different concentrations of NFA (0, 25 and 50 μM). The culture medium was replaced every 3 days. After 14 days of incubation, the cells were gently washed with PBS for twice. After fixed with methanol for 15 min, the cells were stained with 0.5% of crystal violet. Colonies were manually counted.
Soft-agar colony formation assay
The soft agar colony formation assay is a technique widely used to evaluate cellular transformation in vitro [13]. Cells were plated in 0.4% semi-solid agar in growth medium (with the same volume of 1 × RPMI-1640 medium, 2 × RPMI-1640 medium and 1.2% agarose) containing NFA at different concentrations (0, 25 and 50 μM) after the lower gel which is of 0.6% semi-solid agar in growth medium were solidified. Colonies including more than 100 cells in soft agar were scored under an inverted microscope after two weeks of incubation at 37°C.
Gelatin zymography
The activities of MMP-9 in medium were measured by gelatin zymography. CNE-2Z cells to be tested were grown to approximately 80% in 6 cm dishes in complete RPMI-1640 medium. Then cells were changed to serum-free media and incubate for 24 h. NFA at different concentrations (0, 25, 50, 100 and 200 μM) were added to the dishes respectively. Collected media after 24 and 48 h of an appropriate volume were subjected to 0.1% gelatin-8% SDS-PAGE electrophoresis. After electrophoresis, gels were washed with 2.5% Triton X-100 and incubated in developing buffer (0.5 M Tris-HCl, PH 7.8, 2 M NaCl, 0.05 M CaCl2, and 0.2% Brij 35) overnight at 37°C. Then the gels were stained with Coomassie Brilliant Blue G250 (Beyotime, China) for 2 h. After that, the gels were washed with destaining solution (5% methanol and 10% acetic acid in ddH2O).
Invasion and migration assay
The ability of the cells to pass through filters was measured using a Transwell Boyden chamber system containing a polycarbonate filter (6.5 mm in diameter, 8 μm pore size). For cell invasion assay, the cell culture inserts were pre-coated with 60 μl of Matrigel which was diluted by FBS free RPMI-1640 medium at a proportion of 1:3; whereas the filters without pre-coated matrigel were used for migration assay. Firstly, cells were resuspended in serum-deprived medium. Subsequently, 200 μl of the cell suspension (1 × 105 cells/well for invasion, 3 × 104 cells/well for migration) containing NFA at different concentrations (50 and 100 μM) were added to the upper chamber, 800 μl RPMI-1640 medium containing 10% FBS served as a chemo attractant were added to the lower chamber. The system was incubated for 48 h at 37°C in an incubator with 5% CO2 and saturated humidity. Cells that did not migrate or invade after 48 h were removed with cotton swab. Then the membranes were fixed with methanol for 15 min at room temperature and stained with 0.5% crystal violet for 2 h. Finally, 5 visual fields were randomly selected from each membrane and photographed under a light microscope at 200 × magnification. The number of migrating or invading cells were then counted and analyzed to determine statistically significant differences.
Preparation of NFA-Sepharose beads
CNBr-Sepharose 4B beads were washed with 1 mM HCl medium for 3-5 times and mixed with NFA or DMSO (DMSO as a control) in coupling buffer (0.1 M NaHCO3, pH 8.3, 0.5 M NaCl) and then slowly rotated overnight at 4°C. Excess NFA was washed away with coupling buffer, followed by blocking any remaining active groups with 0.1 M Tris-HCl buffer (pH 8.0) for 2 h. The medium was then washed with 0.1 Macetate buffer (pH 4.0) containing 0.5 M NaCl and 0.1 M Tris-HCl containing 0.5 M NaCl (pH 8.0) for three times. Till then the NFA Sepharose 4B beads were now ready for use in the pull-down assay.
In vitro pull down assay
Either NFA-Sepharose 4B or DMSO (the solvent of NFA) Sepharose 4B was combined with CNE-2Z cellular supernatant fraction (600 μg) in reaction buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP-40, 2 μg/mL of BSA, 0.02 mM PMSF, 1 × protease inhibitor cocktail). After incubating with gentle rocking overnight at 4°C, the beads were washed five times with washing buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP-40, and 0.02 mM PMSF) and proteins bound to the beads were analyzed by western blot (WB).
Western blot assay
In accordance with our previous study [14], similar procedures of western blot assay were conducted to determine whether NFA might have effect on MAPK signaling pathway. Cells were cultured in 6 cm dishes. After being incubated for 24 h, cells were directly treated with different concentrations (0, 25, 50, 100 and 200 μM) of NFA for 48 h. The cells were then disrupted in 150 μl lyses buffer (RIPA buffer) supplemented with 1% protease inhibitor (Millipore) and 1% phosphates inhibitor (Millipore). Protein concentration was measured by the PierceTM BCA protein assay kit (Thermo Scientific) and equalized with the extraction reagent. Equal amount of the extracts were loaded and separated in a 8% or 10% polyacrylamide gel, then proteins were transferred onto a PVDF membrane, blocking by 5% non-fat milk in TBST for 2 hours. After then membranes were incubated with the primary antibody 1:500 or 1:1000 diluted in primary antibody diluents overnight at 4°C. Next day, the membranes were washed with TBST at room temperature thrice, 10 min every time. Membranes were then incubated with secondary antibody for 1-2 h. Protein expression level was analyzed by LI-COR Odyssey Infrared Imaging System. In this experiment, β-actin was used as internal control.
Flexible protein docking between compound NFA and ERK1
The crystal structure of ERK1 (Protein Data Bank code 4QTB [15] was used as the receptor model structure and the 3D coordinates of NFA from PubChem compound database (http://pubchem.ncbi.nlm.nih.gov/) was used as the ligand structure. Protein-ligand docking was run using Schrödinger Glide [16,17] in XP (Extra Precision) mode [18]. The final binding model structure of compound NFA-ERK1 was generated from Induced Fit Docking module [19], which combines the predictive force of Prime with the docking and scoring abilities of Glide for accommodating the possible protein structural change upon ligand binding. During induced fit docking, the grid box and center were settled default by using the co-crystal ligand in its active binding site. The binding pose with the lowest XP GScore was considered as the correct binding structure and used as the representative docking structure for NFA and ERK1.
Statistical analysis
All values in this article are expressed as the mean ± standard deviation (SD). The Graphpad Prism (GraphPad Software, Inc. USA) was used for all statistical analyses and graph drawing. Twotailed Student’s t test was conducted to compare measurements of pairs of samples if appropriate.
Results
NFA inhibits the proliferation of CNE-2Z cells
The cell proliferation assay showed a significant inhibition of CNE-2Z cells proliferation by NFA in a time and concentration-dependent manners (Figure 1). At a concentration of 50 μM for 24 h, NFA had slight inhibition (P=0.4112) on the proliferation of CNE-2Z cells. At 48 h, the inhibitory effect became significant (*P<0.05). At the concentration of 200 μM, the inhibitory effect became significant (*P<0.05) at 24 h. As the time gone on and drug concentration increased, the inhibitory effects became obvious. When the drug concentration was added up to 400 μM, cell proliferation was completely inhibited. The NFA showed the effect of cell-growth inhibition in CNE-2Z with the IC50 of 377.7, 133.7 and 83.7 μM at 24 h, 48 h and 72 h respectively.
Figure 1.
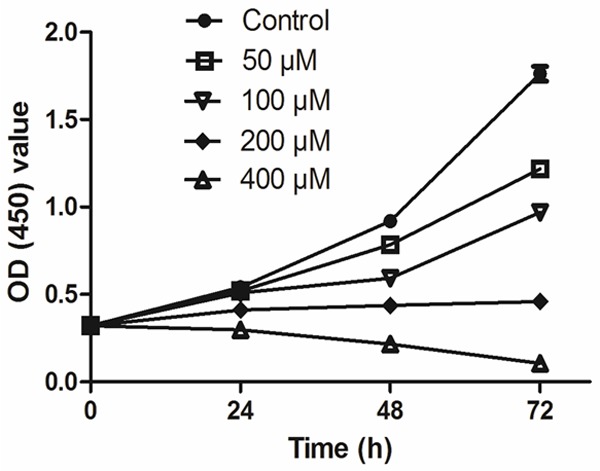
Effects of NFA with different concentrations on the proliferation of CNE-2Z cells at different time points.
Effects of NFA on the cell cycle and apoptosis of CNE-2Z cells
NFA exerted an S phase cell cycle arrest on the CNE-2Z cells in a concentration-dependent manner (Figure 2A). The percentage of S phase cells increased slightly (P=0.0765) with the NFA treatment at a low concentration (25 μM) compared to the control. Whereas the percentage of S phase cells increased significantly (**P<0.01) with the NFA treatment at a high dose (50 μM).
Figure 2.
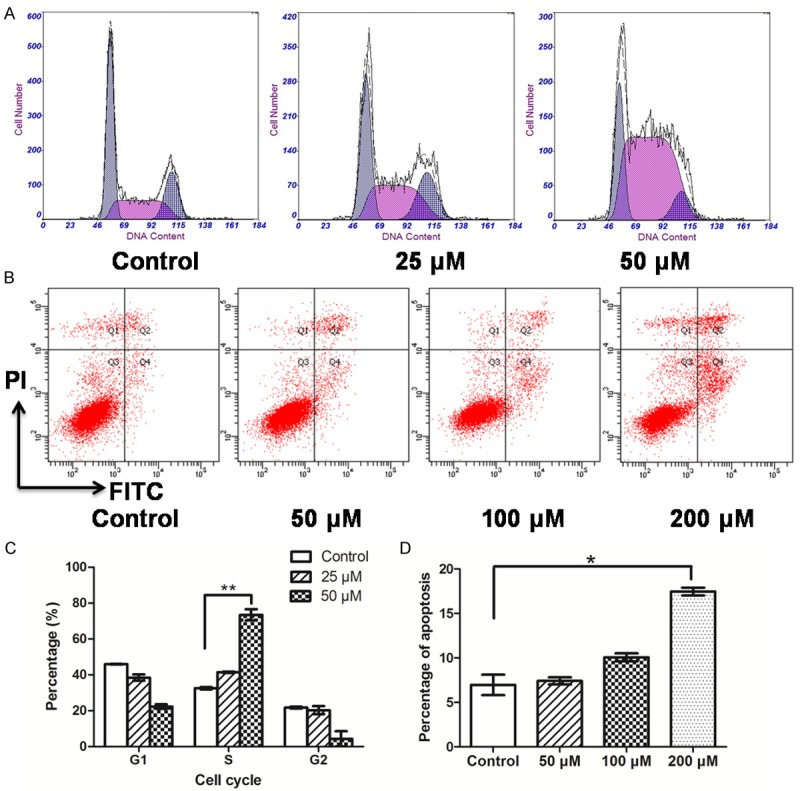
Effects of NFA with different concentrations on cell cycle distribution and apoptosis of CNE-2Z. A. The cell cycle profile of CNE-2Z cells that were treated with 0, 25 and 50 μM of NFA in 1640 medium for 24 h were detected using flow cytometry. B. The apoptosis of CNE-2Z cells that were treated with 0, 50 and 100 μM of NFA in 1640 medium for 48 h were detected using flow cytometry. C. Comparisons between the percentages of cell cycle distribution of CNE-2Z with the different concentrations of NFA. D. Comparisons between the apoptosis percentage of CNE-2Z with the different concentrations of NFA on CNE-2Z cell.
The apoptosis assays indicated that NFA induced CNE-2Z cells apoptosis at a high concentration (Figure 2B). In the low concentration group (50 μM), the effect of NFA on CNE-2Z cells apoptosis was insignificant. As the concentration increased (100 and 200 μM), the effect became obvious. At the concentration of 200 μM, the percentage of apoptosis cells increased greatly comparing to the control (*P<0.05).
Effects of NFA on the colony formation of CNE-2Z cells
To test the effects of NFA on the transformation ability of nasopharyngeal carcinoma cell line CNE-2Z, the foci-formation assay was performed with different concentrations of NFA treated on CNE-2Z cells. Foci-formation assay showed that NFA significantly inhibited colony formation at the concentrations of both 25 and 50 μM in a dose-dependent approach (*P<0.05, Figure 3A). Furthermore, the soft agar colony formation assay indicated that NFA inhibited the anchorage independent colony formation of CNE-2Z at higher concentrations (50 and 100 μM) in a concentration-dependent approach (Figure 3B).
Figure 3.
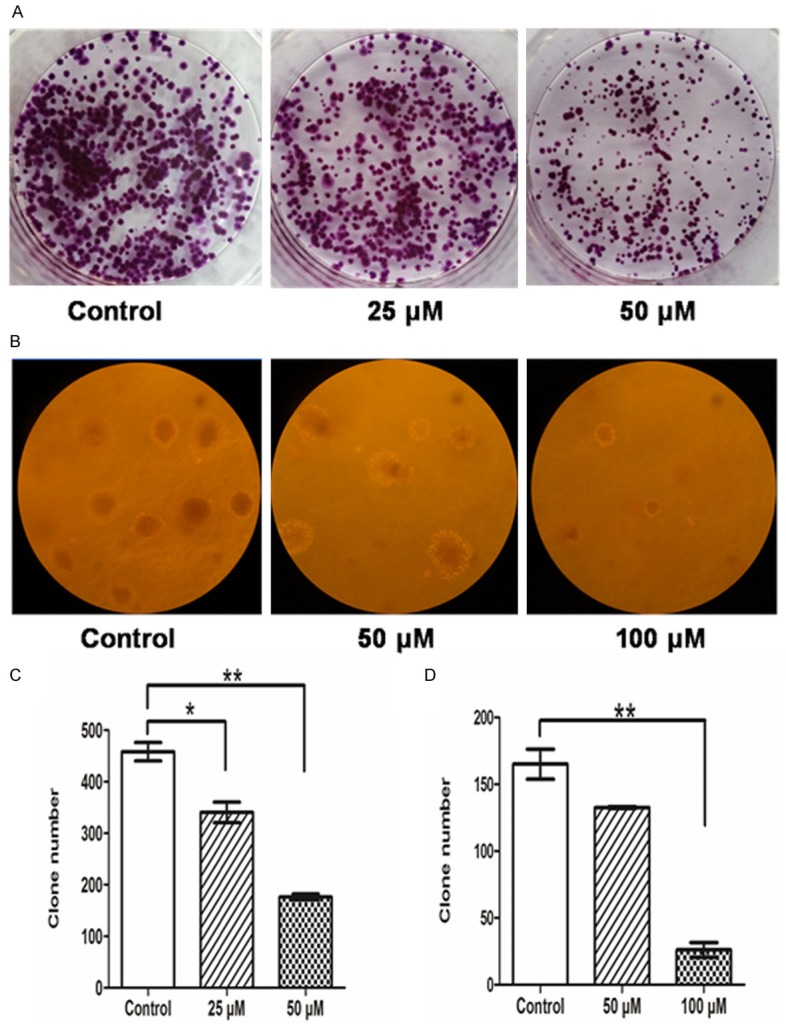
Effects of NFA on colony-forming ability of CNE-2Z cells. A. Foci-formation assay. B. Colony-forming ability of CNE-2Z cells in soft agar. C. Data are presented as the means ± SD for foci-formation experiments, *P<0.05; **P<0.001. D. Data are presented as the means ± SD for colony-forming ability of CNE-2Z cells in soft agar experiments, **P<0.001.
NFA has a decreased migration and invasion potential by affecting MMP9 and MMP2 activity
We investigated the migration and invasion effects of the NFA on CNE-2Z cells by Transwell Boyden chamber assays and Gelatin Zymography. The migration and invasion potential of the CNE-2Z cells were notably decreased by NFA (Figure 4). At 50 μM, the cell number of migration were decreased (*P<0.05), while the cell number of invasion decreased significantly (**P<0.01). When the concentration was up to 100 μM, the cell numbers of both were more significantly decreased (**P<0.01).
Figure 4.
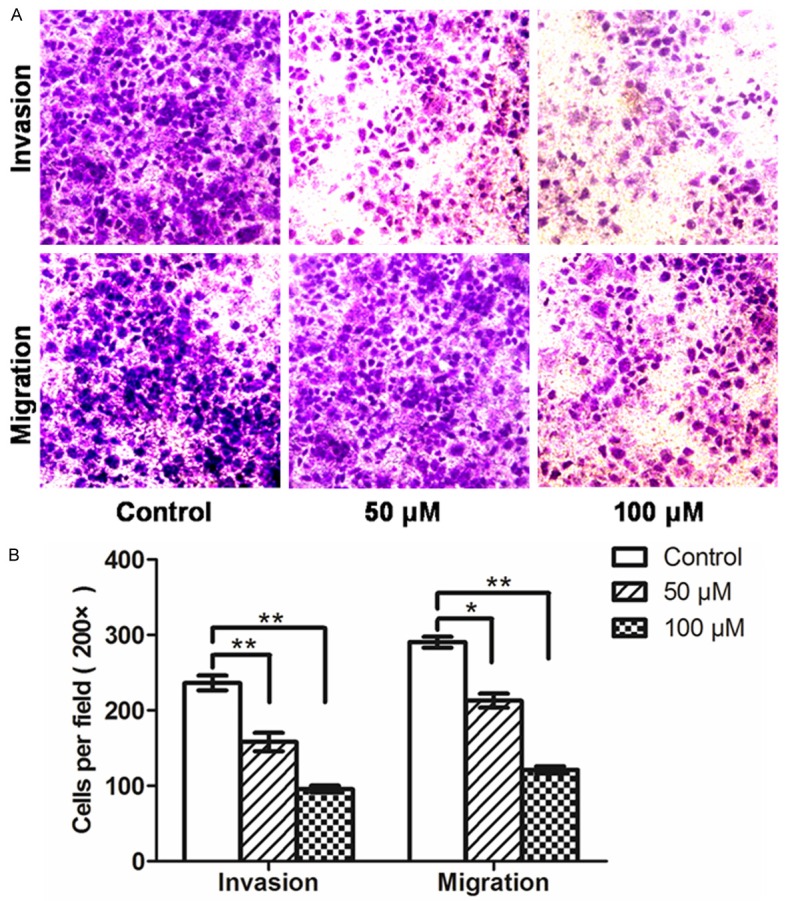
NFA inhibited the invasion and migration ability of CNE-2Z cells. A. Transwell Boyden chamber assays were performed to compare the invasion and migration potential of NFA at different concentrations. Cells after 48 h incubation at 200 × magnification. B. Quantitative results were shown as invasion and migration cells, which were calculated by 5 visual fields selected in the transwell migration and invasion assays (means ± SD, n=3, *P<0.05; **P<0.001).
We detected the activity of MMP9 and MMP2 by Gelatin Zymography. NFA significantly inhibited MMP9 and MMP2 enzyme activity (Figure 6B) in a dose-dependent manner. At 25 μM, the activity of MMP9 and MMP2 was slightly attenuated. Moreover, with the increase of concentration of NFA, the activity of MMP9 and MMP2 was gradually diminished. The results showed that NFA induced remarkable reductions in the migration and invasion abilities of CNE-2Z cells in a dose-dependent approach.
Figure 6.
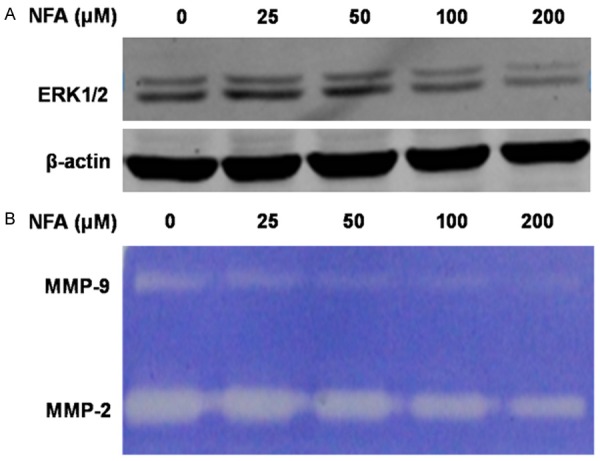
NFA influenced the proteins expression of ERK1/2 and the activity of MMP9 and MMP2 in a dose-dependent manner. A. NFA at different concentrations (0, 25, 50, 100 and 200 μM) acted on CNE-2Z cells for 48 h, the expression of ERK1/2 was detected. B. CNE-2Z cells were treated with NFA (0, 25, 50, 100 and 200 μM) for 24 h in serum-free medium and then subjected to gelatin zymography to analyze the activity of MMP9 and MMP2.
NFA regulates and binds directly with ERK
ERK is a key component of the MAPK signaling. The NFA in-vitro pull-down assay was applied to demonstrate that ERK1 bind directly with the NFA (Figure 5A, lane 3). The total protein was loaded as a control (Figure 5A, lane 1) and no binding was found in the solvent (Figure 5A, lane 2), whereas ERK2 was not found to be bounded with NFA. Furthermore, the western blot showed that NFA down-regulated the protein level of ERK1/2 (Figure 6A).
Figure 5.
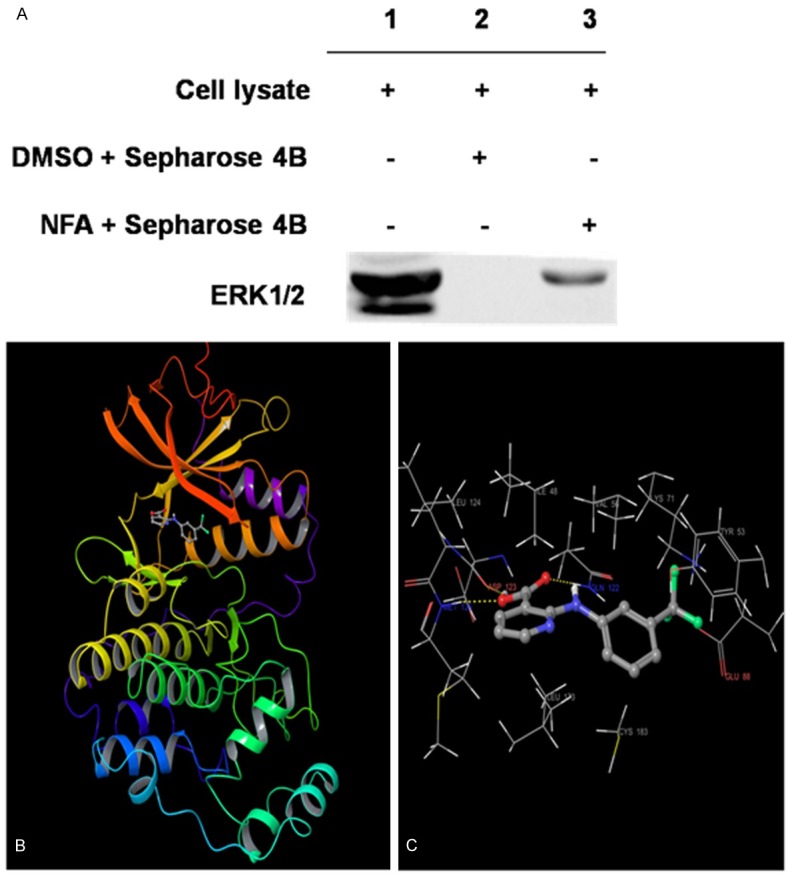
The correlation between NFA and ERK1. A. NFA was bound with ERK1. Lanes 1 show on total protein, lanes 2 and lanes 3 respectively show the solvent group and NFA group. B. The binding pose between NFA and ERK1. C. Hydrogen bonds between NFA and three residues of ASP123, MET125, and GLN122 in the ATP binding site of ERK1 (for clarity, the backbone atoms of 8 protein residues: ILE48, TYR53, VAL56, LYS71, GLU88, GLN122, LEU173 and CYS183 to form the hydrophobic interactions with NFA are not shown). Note: the α-helices are drawn as cylinders and the β-strands as arrows. NFA is shown in stick model and protein residues are shown in line model.
Molecular modeling of the binding of NFA inside ERK1 binding site
To assess the possible binding mode between NFA and ERK1, the hierarchical docking algorithm Glide [16,17] in the Schrödinger package was used for docking experiments. To capture the ligand-induced conformational changes in the receptor binding site, we performed flexible-ligand flexible-protein docking by using the induced fit docking module [19]. The binding pose of compound NFA-ERK1 obtained from the induced fit docking results suggested that compound NFA bound inside the ATP binding pocket of ERK1 (Figure 5B and 5C). NFA formed three hydrogen-bonds with ERK1: Two involved in the backbone atoms of two hinge loop residues (ASP123 and MET125) and the other one engaged with the side-chain atom of GLN122. In addition, 9 residues around the binding pocket, including ILE48, TYR53, VAL56, LYS71, CYS183, GLU88, GLN122, LEU124 and LEU173 formed hydrophobic interactions with the carbons of compound NFA (Figure 5C). The computational results indicate that NFA could show ATP-competitive inhibitory effects to ERK1. The figures were generated by using Maestro [20].
Discussion
There were an estimated 80,000 new cases of NPC and 50,000 deaths in 2008 worldwide [21]. Although it may be regarded as a rare cancer globally, it is notable for its high incident rate in special geographic and ethnic populations. With the improvement and extensive application of the intensity modulated radiotherapy, image guided radiotherapy techniques and other new therapeutic strategies, this survival rate is increasing [22-24]. However, recurrence or metastasis is still a problem for NPC patients within five years. Radiation tolerance of tumors [25-28] and MDR [29-31] are the chief culprits for treatment failure. Therefore, the identification of highly effective and low toxicity drugs is currently a research focus.
NFA is widely used in the treatment for dysmenorrhea, rheumatoid, and osteoarthritis [6]. In the management of rheumatoid and osteoarthritis, NFA was devoid of side effects and with excellent gastric tolerance [32]. Long-term use of NSAIDs is reported to be associated with a lower incidence risk of tumors, such as the colorectal, breast, lung, prostate, bladder, ovary, esophagus, and stomach [33]. NSAIDs have been shown to induce apoptosis and inhibit metastasis in various cancer cell lines [33]. As a non-steroidal anti-inflammatory drug, NFA was found to inhibit the proliferation of several tumor cell types recently [7-12]. In our study, the results showed that NFA markedly inhibited the proliferation and colony formation of NPC CNE-2Z cells, arrested the cells in the S phase and promoted apoptosis. Metastasis is the major cause of cancer-associated mortality. The median survival time for NPC patients with distant metastasis is around 9 months [34]. NFA was reported to inhibit invasion in breast cancer cell and ovarian cancer cell [9,12]. Our results indicated that NFA decreased migration and invasion potential of NPC CNE-2Z cells by attenuating the activity of MMP9 and MMP2. These data suggested that NFA could be a candidate for chemotherapy or adjuvant therapy of NPC.
Most of NSAIDs exhibit strong inhibitory effects of cyclooxygenase COX-1 or COX-2. Inhibition of COX-2 is demonstrated to modulate tumorigenic, angiogenic and apoptotic events of cancer [35]. However, the anti-tumor effect of NSAIDs may not solely be mediated by the inhibition of COX-2 activity based on the following observations. First, the concentrations of NSAIDs to induce apoptosis were significantly higher than those required for COX-2 inhibition [36]. Second, NSAIDs inhibited cell growth not only in COX-2-expressing cells, but also in COX-2-deficient cell lines [37]. NFA is a selective inhibitor of COX-2. The NFA inducing apoptosis is independent of COX-2 inhibition. The inhibitory effect of store-operated channels was found to play a major role in NFA-induced apoptosis in human erythroleukemic K562 cell line [6]. NFA-induced apoptosis in human lung cancer cells was through the activation of caspase-9, caspase-3 and caspase-3-mediated PARP cleavage [7]. Our study indicated that the anti-tumor effect of NFA was associated with MAPK/ERK signaling pathway. The upregulation of MAPK pathway was notable in NPC [3]. ERK1, a key component of the MAPK signaling, was found to bind directly with the NFA in our study. The signaling of ERK1/2 was thereby down-regulated by the NFA in NPC. Our study indicated that NFA might affect MMP2 and MMP9 activities. Suppression of the MAPK/ERK signaling pathway causes reductions in MMP9 activities [38]. Our results indicated that NFA might affect MMP2 and MMP9 activities by regulating the expression of ERK1/2 in NPC.
In conclusion, our study suggested that NFA inhibited the proliferation, colony formation ability, migration and invasion potential of NPC CNE-2Z cells, arrested the cells in the S phase and promoted apoptosis by affecting the MAPK/ERK signaling pathway and activity of MMP2 and MMP9. These results suggested that NFA may serve as a candidate for chemotherapy or adjuvant therapy of NPC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81372137 and 31170676). We thank the Schrödinger for providing an evaluation license.
Disclosure of conflict of interest
None.
References
- 1.Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol Cancer. 2007;6:1. doi: 10.1186/1476-4598-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeyakumar A, Brickman TM, Doerr T. Review of nasopharyngeal carcinoma. Ear Nose Throat J. 2006;85:168–170. 172–163, 184. [PubMed] [Google Scholar]
- 3.Chou J, Lin YC, Kim J, You L, Xu Z, He B, Jablons DM. Nasopharyngeal carcinoma--review of the molecular mechanisms of tumorigenesis. Head Neck. 2008;30:946–963. doi: 10.1002/hed.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 6.Kucherenko YV, Lang F. Niflumic acid affects store-operated Ca(2+)-permeable (SOC) and Ca (2+)-dependent K (+) and Cl (-) ion channels and induces apoptosis in K562 cells. J Membr Biol. 2014;247:627–638. doi: 10.1007/s00232-014-9680-x. [DOI] [PubMed] [Google Scholar]
- 7.Kim BM, Maeng K, Lee KH, Hong SH. Combined treatment with the Cox-2 inhibitor niflumic acid and PPARgamma ligand ciglitazone induces ER stress/caspase-8-mediated apoptosis in human lung cancer cells. Cancer Lett. 2011;300:134–144. doi: 10.1016/j.canlet.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Nejime N, Kagota S, Tada Y, Nakamura K, Hashimoto M, Kunitomo M, Shinozuka K. Possible participation of chloride ion channels in ATP release from cancer cells in suspension. Clin Exp Pharmacol Physiol. 2009;36:278–282. doi: 10.1111/j.1440-1681.2008.05060.x. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Wang Q, Lin W, Wang B. Regulation of ovarian cancer cell adhesion and invasion by chloride channels. Int J Gynecol Cancer. 2009;19:526–530. doi: 10.1111/IGC.0b013e3181a3d6d2. [DOI] [PubMed] [Google Scholar]
- 10.Lock EA, Reed CJ, Kinsey GR, Schnellmann RG. Caspase-dependent and -independent induction of phosphatidylserine externalization during apoptosis in human renal carcinoma Cak(1)-1 and A-498 cells. Toxicology. 2007;229:79–90. doi: 10.1016/j.tox.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Tao L, Cao YX, Dong L, Hu YZ, Yang AG, Zhou SS. [Effects of niflumic acid on the proliferation of human hepatoma cells] . Sheng Li Xue Bao. 2003;55:160–164. [PubMed] [Google Scholar]
- 12.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK, Winn RA. The soft agar colony formation assay. J Vis Exp. 2014:e51998. doi: 10.3791/51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Z, Tang F, Ermakova S, Li M, Zhao Q, Cho YY, Ma WY, Choi HS, Bode AM, Yang CS, Dong Z. Fyn is a novel target of (-)-epigallocatechin gallate in the inhibition of JB6 Cl41 cell transformation. Mol Carcinog. 2008;47:172–183. doi: 10.1002/mc.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaikuad A, Tacconi EM, Zimmer J, Liang Y, Gray NS, Tarsounas M, Knapp S. A unique inhibitor binding site in ERK1/2 is associated with slow binding kinetics. Nat Chem Biol. 2014;10:853–860. doi: 10.1038/nchembio.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 17.Schrödinger Release 2014-3: Glide version 6.4. New York, NY: Schrödinger. LLC; 2014. [Google Scholar]
- 18.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 19.Schrödinger Release 2014-3: Schrödinger Suite 2014 Induced Fit Docking protocol. Glide version 6.4. New York, NY: Schrödinger. LLC; 2014. Prime version 3.7. [Google Scholar]
- 20.Schrödinger Release 2014-3: Maestro version 9.9. New York, NY: Schrödinger. LLC; 2014. [Google Scholar]
- 21.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Fee W, Chen J, Chan C, Khong B, Hara W, Goffinet D, Li D, Le QT. Salvage treatment for locally recurrent nasopharyngeal carcinoma (NPC) Am J Clin Oncol. 2014;37:327–331. doi: 10.1097/COC.0b013e318277d804. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Zhu G, He X, Ying H, Hu C. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral Oncol. 2014;50:71–76. doi: 10.1016/j.oraloncology.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhao C, Chen J, Yu B, Chen X. Improvement in quality of life in patients with nasopharyngeal carcinoma treated with non-invasive extracorporeal radiofrequency in combination with chemoradiotherapy. Int J Radiat Biol. 2014;90:853–858. doi: 10.3109/09553002.2014.916579. [DOI] [PubMed] [Google Scholar]
- 25.Han L, Lin SJ, Pan JJ, Chen CB, Zhang Y, Zhang XC, Liao XY, Chen QS. Prognostic factors of 305 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Chin J Cancer. 2010;29:145–150. doi: 10.5732/cjc.009.10332. [DOI] [PubMed] [Google Scholar]
- 26.Hornhardt S, Rossler U, Sauter W, Rosenberger A, Illig T, Bickeboller H, Wichmann HE, Gomolka M. Genetic factors in individual radiation sensitivity. DNA Repair (Amst) 2014;16:54–65. doi: 10.1016/j.dnarep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Wen ZS, Lin P, Wang X, Xie FY. The results and prognosis of different treatment modalities for solitary metastatic lung tumor from nasopharyngeal carcinoma: a retrospective study of 105 cases. Chin J Cancer. 2010;29:787–795. doi: 10.5732/cjc.010.10098. [DOI] [PubMed] [Google Scholar]
- 28.Ruan L, Wang GL, Yi H, Chen Y, Tang CE, Zhang PF, Li MY, Li C, Peng F, Li JL, Chen ZC, Xiao ZQ. Raf kinase inhibitor protein correlates with sensitivity of nasopharyngeal carcinoma to radiotherapy. J Cell Biochem. 2010;110:975–981. doi: 10.1002/jcb.22611. [DOI] [PubMed] [Google Scholar]
- 29.Ji XN, Yang F, Sui XM, Wang FG, Ge RG, Quan XL, Zhao T, Gao BW, Wang RY. Effect of fractionated irradiation on the expression of multidrug resistance genes in the CNE1 human nasopharyngeal carcinoma cell line. Mol Med Rep. 2013;7:187–194. doi: 10.3892/mmr.2012.1148. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Chen L, Feng B, Liu G. Reversing effect of sorcin in the drug resistance of human nasopharyngeal carcinoma. Anat Rec (Hoboken) 2014;297:215–221. doi: 10.1002/ar.22832. [DOI] [PubMed] [Google Scholar]
- 31.Tao ZQ, Liu SC, Si YF, Zhang Z, Zhou XZ, Deng ZX, Zhou RJ, Huang B. [Relationship between expression of multidrug-resistant genes in nasopharyngeal carcinoma tissue and sensitivity to chemotherapy] . Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005;40:203–207. [PubMed] [Google Scholar]
- 32.Tilve GH, Lengade JK, Bavadekar AV, Nair KG. Niflumic acid in the management of rheumatoid and osteoarthritis. J Postgrad Med. 1976;22:124–129. [PubMed] [Google Scholar]
- 33.Gurpinar E, Grizzle WE, Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res. 2014;20:1104–1113. doi: 10.1158/1078-0432.CCR-13-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13:1007–1015. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279–286. doi: 10.1002/jcp.10068. [DOI] [PubMed] [Google Scholar]
- 36.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 37.Totzke G, Schulze-Osthoff K, Janicke RU. Cyclooxygenase-2 (COX-2) inhibitors sensitize tumor cells specifically to death receptor-induced apoptosis independently of COX-2 inhibition. Oncogene. 2003;22:8021–8030. doi: 10.1038/sj.onc.1206837. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Kim JH, Kim SC, Yi YS, Yang WS, Yang Y, Kim HG, Lee JY, Kim KH, Yoo BC, Hong S, Cho JY. Adenosine dialdehyde suppresses MMP-9-mediated invasion of cancer cells by blocking the Ras/Raf-1/ERK/AP-1 signaling pathway. Biochem Pharmacol. 2013;86:1285–1300. doi: 10.1016/j.bcp.2013.08.022. [DOI] [PubMed] [Google Scholar]


