Abstract
Background
The role of repeat fine-needle aspiration (RFNA) or core needle biopsy (CNB) has not been established in nodules categorized as atypia/follicular lesion of undetermined significance (AUS/FLUS).
Objective
The purpose of this study was to retrospectively determine whether CNB is more useful for management decisions than RFNA at each subcategory of AUS/FLUS nodules.
Methods
This study included 158 AUS/FLUS nodules (≥1 cm) from 153 consecutive patients who underwent both RFNA and CNB. The AUS/FLUS nodules were subcategorized into nuclear atypia (NA) and follicular lesions with other atypia (FOA). The diagnostic results and rate of determined management by RFNA and CNB were compared at each subcategory. The diagnostic values of RFNA and CNB for malignancy were evaluated in nodules with final diagnoses.
Results
CNB showed a lower rate of AUS/FLUS diagnosis, higher rates of benign and follicular neoplasm or suspicious for a follicular neoplasm (FN/SFN) diagnoses (p ≤ 0.038), and marginally higher rates of malignant diagnosis than RFNA in the NA subcategory. CNB showed a higher rate of FN/SFN (p = 0.007) than RFNA in the FOA subcategory. CNB also demonstrated a higher rate of surgery decision than RFNA in both the NA subcategory (20.2 vs. 9.6%, p < 0.001) and FOA subcategory (20.8 vs. 5.6%, p = 0.007), and a higher rate of observation decision only in the NA subcategory (48.1 vs. 35.6%, p = 0.035). CNB demonstrated a higher diagnostic performance for malignancy overall in the nodules compared with RFNA.
Conclusion
CNB may be more useful for management decisions than RFNA in both the NA and FOA subcategories, and has the potential to be a first-line alternative diagnostic tool in initially diagnosed AUS/FLUS nodules.
Key Words: Thyroid nodule, Thyroid cancer, Core needle biopsy, Fine-needle aspiration
Introduction
The Bethesda System for Reporting Thyroid Cytopathology (BSRTC) recommends repeat fine-needle aspiration (RFNA) for thyroid nodules initially diagnosed as atypia/follicular lesion of undetermined significance (AUS/FLUS) [1,2]. However, the role of RFNA for AUS/FLUS nodules is controversial. First, RFNA also reproduce significant inconclusive results. The rate of repeated AUS/FLUS results by RFNA is estimated to approximately be up to 67% [3,4,5,6]. Second, RFNA may not provide a straightforward management decision for a nodule diagnosed as benign by RFNA because the false negative rate of a benign diagnosis by RFNA in nodules initially diagnosed as AUS/FLUS may be higher than that in a single benign diagnosis [7,8]. Thyroid nodules with AUS/FLUS diagnosis include various pathologies, and recent studies [4,9,10,11,12,13,14,15] demonstrate that subcategory nodules showing nuclear atypia (NA) have a higher malignancy risk than other subcategory nodules showing architectural or other atypia, which might require a different management strategy. Although several recent studies [16,17,18,19,20,21,22] have suggested the potential utility of core needle biopsy (CNB) in the management of AUS/FLUS or indeterminate nodules, the role of CNB has not been established and its utility has been little investigated for each subcategory of AUS/FLUS nodules [22]. This study was performed to determine whether CNB is more useful for management decisions than RFNA at each nodule subcategory in patients with AUS/FLUS nodules initially diagnosed by a prior FNA.
Materials and Methods
The institutional review board approved this retrospective study, and the requirement to obtain informed consent was waived.
Study Population
From February 2010 to June 2013, 643 (9.7%) consecutive nodules were initially diagnosed as AUS/FLUS among 6,604 thyroid nodules of 5,159 patients who underwent FNA. A total of 158 thyroid nodules of 153 consecutive patients (103 women, 50 men; mean age 51.8 ± 12.3 years) were enrolled for this study among 643 nodules diagnosed as AUS/FLUS. The inclusion criteria for enrollment were as follows: (a) patients who underwent both RFNA and CNB after an initial diagnosis of AUS/FLUS nodules by a prior FNA, and (b) thyroid nodules being equal to or larger than 10 mm. The patients who did not undergo follow-up biopsy or underwent only one of either RFNA or CNB were excluded.
The final diagnoses of malignant tumor and neoplasm were determined by the pathological results from surgical resections. Final diagnoses of benign nodules were determined by: (a) the pathological results of surgical resections, (b) with benign results of FNA or CNB repeated at least twice, or (c) with an initial benign result of FNA or CNB and a significant decrease of nodule size at ultrasound (US) follow-up.
US-Guided FNA and CNB Procedures
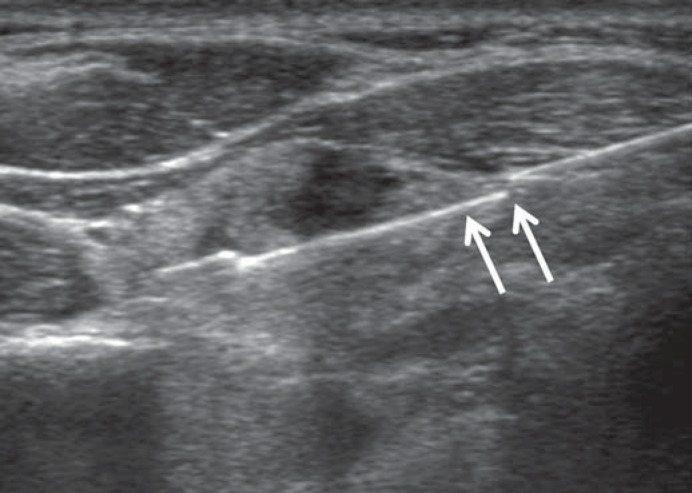
The FNA and CNB procedures were performed under high-resolution color-Doppler US guidance using a 10- to 12-MHz linear transducer (AplioXG, Toshiba, Otawarashi, Japan; iU22, Philips Medical Systems, Bothell, Wash., USA) and by two experienced radiologists (D.G.N. and H.S.) with 15 and 7 years of experience of thyroid US imaging and intervention, respectively. FNA was performed with a conventional method and at least two samplings were performed for each nodule [23]. CNB was performed using a disposable 18-gauge, single- or double-action spring-activated needle (approx. 1- or 2-cm excursion; TSK Acecut or Stericut, Create Medic, Yokohama, Japan) as described elsewhere [17]. The needle notch of CNB was positioned to cut a portion of normal parenchyma (about 2 mm in length) at the nodule margin if technically feasible (fig. 1). After patients underwent biopsy, we immediately compressed the biopsy site and they were observed with self manual compression of the biopsy site for 20-30 min. We made an effort to obtain the qualified cytology and histology specimens at each FNA and CNB procedure.
Fig. 1.
The needle notch of CNB is positioned to include a portion of normal parenchyma (about 2 mm in length; arrows) at the margin of a nodule.
Cytology and Histology Analysis
All FNA cytology specimens were interpreted according to 6 categories of the BSRTC [1]. Two endocrine pathologists (H.S.M. and H.L.) retrospectively subcategorized the AUS/FLUS category into 2 subcategories of NA (n = 104) and follicular lesions with other atypia (FOA; n = 54). The NA subcategory included nodules with NA such as the presence of occasional nuclear grooves and irregularity, enlarged nuclei with a pale chromatin pattern, and nuclear overlapping or crowding, but which were not enough to be considered suspicious for malignancy. The FOA subcategory included nodules with architectural atypia such as the presence of a prominent population of microfollicles or Hurthle cells in sparsely cellular aspirates with scant colloid, but not enough to be diagnosed as a follicular neoplasm or suspicious for a follicular neoplasm (FN/SFN), and also included other undetermined atypical follicular lesions that were not enough to be included in the NA subcategory. A thyroid nodule showing both nuclear and architectural atypia was included in the NA subcategory.
The diagnostic categories of CNB for thyroid nodules have not yet been standardized. For this study, CNB histology diagnoses were also categorized into the same 6 categories of the Bethesda system according to the histopathology result of CNB [17,24]. Category I of CNB included the absence of any identifiable follicular thyroid tissue, the presence of only a normal thyroid gland, and tissue containing only a few follicular cells that were insufficient for diagnosis. Category III of CNB included nodules with some atypical cells, but which were not diagnostic of suspected malignancy or malignancy, and included cellular follicular nodules in which the distinction between a follicular neoplasm and a hypercellular hyperplastic nodule was not possible. Category IV included nodules with histology features favoring follicular neoplasm and nodule capsules. The finding of the immunohistochemistry study was not considered when determining the categories of the CNB histopathology results.
Data Analysis and Statistics
McNemar's test was used to compare each diagnostic result of RFNA and CNB in the overall and subcategories of the nodules, to compare the rate of management decision, and to compare the sensitivity and specificity for the diagnosis of thyroid malignancy between RFNA and CNB. The χ2 test or Fisher's exact test was used to compare the diagnostic results of RFNA or CNB between the subcategories of NA and FOA nodules. We compared the diagnostic performance for malignancy between RFNA and CNB by receiver operating characteristic (ROC) analysis.
For the assessment of diagnostic values we used two criteria of RFNA or CNB for the diagnosis of malignancy. Criteria 1 indicated diagnostic results of malignancy (BSRTC category VI) and criteria 2 indicated diagnostic results of FN/SFN or suspicious for malignancy or malignancy (BSRTC category IV/V/VI). Statistical analysis was performed with the SPSS software package (version 18.0 for Windows; SPSS Inc., Chicago, Ill., USA), and a p value <0.05 was indicative of a statistically significant difference.
Results
Demographic Data
The size (maximal diameter) of 158 thyroid nodules ranged from 10 to 64 mm (mean ± standard deviation 16.8 ± 8 mm). The RFNA and CNB were performed simultaneously in 146 (92.4%) of the thyroid nodules. The final diagnoses of 91 nodules included 73 (80.2%) benign nodules, including 4 follicular adenomas diagnosed by surgery and 18 (19.8%) malignant nodules. Malignant tumors were found in 13 (22.4%) of 58 NA subcategory nodules (8 conventional papillary carcinomas, 3 follicular variant papillary carcinomas and 2 follicular carcinomas) and in 5 (15.2%) of 33 FOA subcategory nodules (2 conventional papillary carcinomas, 1 follicular variant papillary carcinoma, 1 oncocytic variant papillary carcinoma and 1 follicular carcinoma). The final diagnoses of 73 benign nodules were determined by: (a) the pathological results of surgical resections (n = 11), (b) with benign results of FNA or CNB repeated at least twice (n = 49), or (c) with an initial benign result of FNA or CNB and a decrease of nodule size (n = 13). There were no major complications such as serious hemorrhage in any of the patients and none required hospital admission or intervention. There were no cases of infection or needle track seeding during the follow-up period.
Comparison of RFNA and CNB Diagnoses in AUS/FLUS Thyroid Nodules
Table 1 displays the comparison of diagnostic results between RFNA and CNB in overall AUS/FLUS and each nodule subcategory. The overall nondiagnostic rate of RFNA and CNB was 8.2 and 0% in the nodules, respectively. CNB showed a significantly lower rate of AUS/FLUS (p = 0.038), higher rates of benign and FN/SFN diagnoses (p = 0.035 and 0.006, respectively), and a marginally higher rate of malignant diagnosis (p = 0.063) than RFNA in the NA subcategory. CNB showed a significantly higher rate of FN/SFN (p = 0.007) than RFNA; however, there was no significant difference of other BSRTC category results in the FOA subcategory.
Table 1.
Comparison of RFNA and CNB diagnoses in AUS/FLUS thyroid nodules
| Diagnosis1(category) | All (n = 158) |
NA (n = 104) |
FOA (n = 54) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RFNA | CNB | p value | RFNA | CNB | p value | RFNA | CNB | p value | |
| Nondiagnostic (I) | 13 (8.2) | 0 (0) | – | 10 (9.6) | 0 (0) | – | 3 (5.6) | 0 (0) | – |
| Benign (II) | 61 (38.6) | 74 (46.8) | 0.079 | 37 (35.6) | 50 (48.1) | 0.035 | 24 (44.4) | 24 (44.4) | 1 |
| AUS/FLUS (III) | 70 (44.3) | 48 (30.4) | 0.01 | 47 (45.2) | 33 (31.7) | 0.038 | 23 (42.6) | 15 (27.8) | 0.185 |
| FN/SFN (IV) | 6 (3.8) | 27 (17.1) | <0.001 | 3 (2.9) | 13 (12.5) | 0.006 | 3 (5.6) | 14 (25.9) | 0.007 |
| Suspicious for malignancy (V) | 4 (2.5) | 0 (0) | – | 4 (3.8) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Malignant (VI) | 4 (2.5) | 9 (5.7) | 0.063 | 3 (2.9) | 8 (7.7) | 0.063 | 1 (1.9) | 1 (1.9) | 1 |
Data in parentheses are percentages.
Diagnoses according to the 6 categories of the BSRTC.
Comparison of Diagnostic Results between the NA and FOA Subcategory
When the results of RFNA were compared between the NA and FOA subcategories, there was no significant difference in the results of the 6 BSRTC categories (p ≥ 0.185). However, when the CNB results were compared, the FN/SFN rate was significantly higher in the FOA subcategory than that in the NA subcategory (25.9 vs. 12.5%, p = 0.033). There was no significant difference in other BSRTC category results between the NA and FOA subcategories at CNB (p ≥ 0.133).
Management Decision by RFNA and CNB in AUS/FLUS Thyroid Nodules
CNB demonstrated significantly higher rates of determined management for observation (BSRTC category II) or surgery (BSRTC category IV, V and VI) than RFNA in the overall nodules and the NA subcategory (p < 0.001), and a marginally higher rate of determined management in the FOA subcategory (p = 0.061; table 2). CNB demonstrated significantly higher rates of surgery decision than RFNA in both the NA subcategory (20.2 vs. 9.6%, p < 0.001) and the FOA subcategory (20.8 vs. 5.6%, p = 0.007), and a higher rate of observation decision only in the NA subcategory (48.1 vs. 35.6%, p = 0.035).
Table 2.
Management decision by RFNA and CNB diagnoses in AUS/FLUS thyroid nodules
| Determined management | All (n = 158) |
NA (n = 104) |
FOA (n = 54) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RFNA | CNB | p value | RFNA | CNB | p value | RFNA CNB | p value | ||
| Overall | 75 (47.5) | 110 (69.6) | <0.001 | 47 (45.2) | 71 (68.3) | <0.001 | 28 (38.9) 39 (54.2) | 0.061 | |
| Observation | 61 (36.9) | 74 (46.8) | 0.079 | 37 (35.6) | 50 (48.1) | 0.035 | 24 (33.3) 24 (33.3) | 1 | |
| Surgery | 14 (10.6) | 36 (22.8) | <0.001 | 10 (9.6) | 21 (20.2) | <0.001 | 4 (5.6) 15 (20.8) | 0.007 | |
Data in parentheses are percentages. Observation indicates a management decision by benign diagnosis. Surgery indicates a management decision by one of FN/SFN, suspicious malignancy or malignant diagnoses.
Diagnostic Values of RFNA and CNB for Thyroid Malignancy
Table 3 depicts the diagnostic values of RFNA and CNB in 91 nodules with final diagnoses. In the overall AUS/FLUS nodules, the sensitivity of CNB for malignancy was higher than that of RFNA with criteria 1 (BSRTC category VI) or criteria 2 (BSRTC category IV/V/VI), but was statistically significant only for criteria 2 (p = 0.021). CNB showed a tendency of higher sensitivity for malignancy than RFNA with criteria 2 in each NA and FOA subcategory, but this was statistically insignificant. The false negative rate of benign diagnosis was found only at RFNA, which was 2% (1/51). The area under the curve of CNB was significantly larger than that of RFNA for the diagnosis of malignancy with criteria 2 in the overall AUS/FLUS nodules by ROC analysis (p = 0.03).
Table 3.
Diagnostic values of RFNA and CNB for thyroid malignancy in nodules with final diagnoses (n = 91)
| Diagnostic values | All (n = 91) |
NA (n = 58) |
FOA (n = 33) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RFNA, % | CNB, % | p value | RFNA, % | CNB, % | p value | RFNA, % | CNB, % | p value | |
| Criteria 1 | |||||||||
| Sensitivity | 22.2 (4/18) | 44.4 (8/18) | 0.125 | 23.1 (3/13) | 53.8 (7/13) | 0.125 | 20 (1/5) | 20 (1/5) | 1 |
| Specificity | 100 (73/73) | 100 (73/73) | 100 (45/45) | 100 (45/45) | 100 (28/28) | 100 (28/28) | |||
| PPV | 100 (4/4) | 100 (4/4) | 100 (3/3) | 100 (7/7) | 100 (1/1) | 100 (1/1) | |||
| NPV | 83.9 (73/87) | 88 (73/83) | 81.8 (45/55) | 88.2 (45/51) | 87.5 (28/32) | 87.5 (28/32) | |||
| Accuracy | 84.6 (77/91) | 89 (81/91) | 82.8 (48/58) | 89.7 (52/58) | 87.9 (29/33) | 87.9 (29/33) | |||
| Criteria 2 | |||||||||
| Sensitivity | 44.4 (8/18) | 83.3 (15/18) | 0.021 | 53.8 (7/13) | 84.6 (11/13) | 0.125 | 20 (1/5) | 80 (4/5) | 0.25 |
| Specificity | 98.6 (73/73) | 97.4 (71/73) | 100 (45/45) | 97.8 (44/45) | 96.4 (27/28) | 96.4 (27/28) | |||
| PPV | 88.9 (8/9) | 88.2 (15/17) | 100 (7/7) | 91.7 (11/12) | 50 (1/2) | 80 (4/5) | |||
| NPV | 87.8 (72/82) | 95.9 (71/74) | 88.2 (45/51) | 95.7 (44/46) | 87.1 (27/31) | 96.4 (27/28) | |||
| Accuracy | 87.9 (80/91) | 95.4 (86/91) | 89.7 (52/58) | 94.8 (55/58) | 84.8 (28/33) | 93.9 (31/33) | |||
Data in parentheses are the raw data for the percentages. Criteria 1 indicates malignant diagnostic results as the criteria for the diagnosis of malignancy. Criteria 2 indicates diagnostic results of FN/SFN, suspicious for malignancy or malignant as the criteria for the diagnosis of malignancy. PPV = Positive predictive value; NPV = negative predictive value.
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of RFNA and CNB with criteria 2 (BSRTC category IV/V/VI) for follicular neoplasm among nonneoplastic nodules and follicular neoplasm was 28.6, 100, 100, 90.9 and 91.2% for RFNA, and 71.4, 100, 100, 96.2 and 96.5% for CNB, respectively. The sensitivity of CNB for follicular neoplasm was higher than that of RFNA, but was statistically insignificant (p = 0.375).
In 91 nodules with final diagnoses, the malignancy rate of repeated AUS/FLUS result by RFNA was similar or slightly higher than that by CNB overall in the nodules (29.2 vs. 20%, respectively) and at each subcategory (NA 26.7 vs. 25%, and FOA 33.3 vs. 14.3%, respectively).
Discussion
Our data demonstrates that the rate of overall management decision determined by CNB was significantly higher than that by RFNA in both the NA and FOA subcategories. The rate of surgery decision was significantly higher at CNB than that at RFNA in both the NA and FOA subcategories and the rate of observation decision was marginally higher only in the NA subcategory. Furthermore, CNB demonstrated a better diagnostic performance than RFNA overall in the AUS/FLUS nodules, and a higher sensitivity for malignancy with the criteria 2 (BSRTC category IV/V/VI) in both the NA and FOA subcategories, thus maintaining the high specificity compared with RFNA.
This study reproduces the result of a previous report [17] that CNB can reduce the inconclusive diagnostic results (nondiagnostic and AUS/FLUS) and has a higher sensitivity for thyroid malignancy than RFNA overall in AUS/FLUS nodules, and the present study additionally demonstrates a higher efficacy of CNB for management decisions at each subcategory of AUS/FLUS nodules.
The overall lower rate of repeated AUS/FLUS result at CNB may be explained mainly by two factors – sufficient biopsy tissue sample and better information of nodule architecture at CNB (fig. 2, 3, 4). First, the cytology diagnosis of AUS/FLUS is strongly related to a paucicellular FNA specimen that manifests as scenarios of NA, architectural atypia and oncocytic pattern in paucicellular aspirates [3], and, therefore, this causative factor for AUS/FLUS diagnosis at FNA may be overcome by a large tissue sample of CNB. Second, CNB can provide more information of nodule architecture by tissue sampling, including the nodule, nodule margin (information of capsule) and adjacent normal glandular tissue [19]. This factor may also be a major cause for a higher rate of FN/SFN diagnosis at CNB in both the NA and FOA subcategories compared with RFNA.
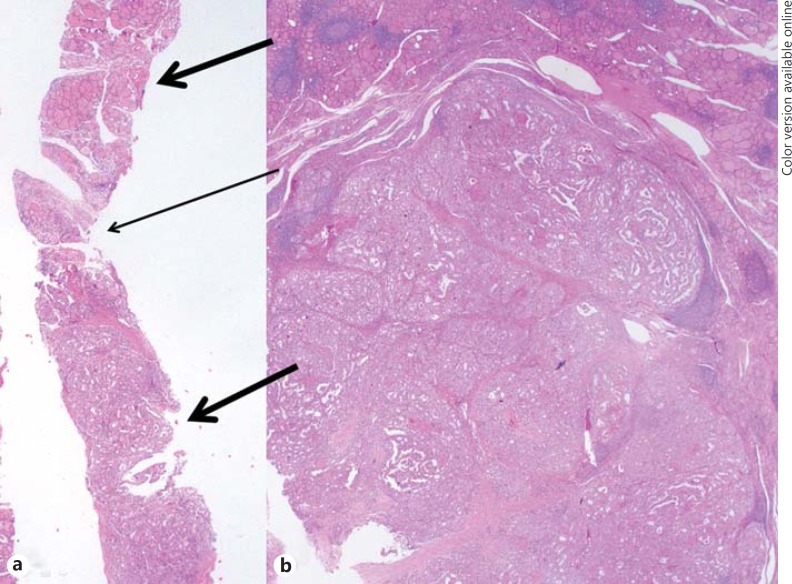
Fig. 2.
CNB histology and surgical pathology of a papillary thyroid carcinoma. a This CNB histology specimen shows tumor cells with an atypical papillary growth pattern (lower thick arrow), diagnosed as papillary thyroid carcinoma. The specimen also shows a normal gland (upper thick arrow) and intervening fibrous capsule-like structure between the tumor and normal gland (thin arrow). b Histology of the surgical specimen demonstrates tumor cells and an adjacent normal gland corresponding to the histology of CNB, diagnosed as papillary thyroid carcinoma. The diagnosis of the initial and repeat FNA was NA (not shown). HE stain. ×10.
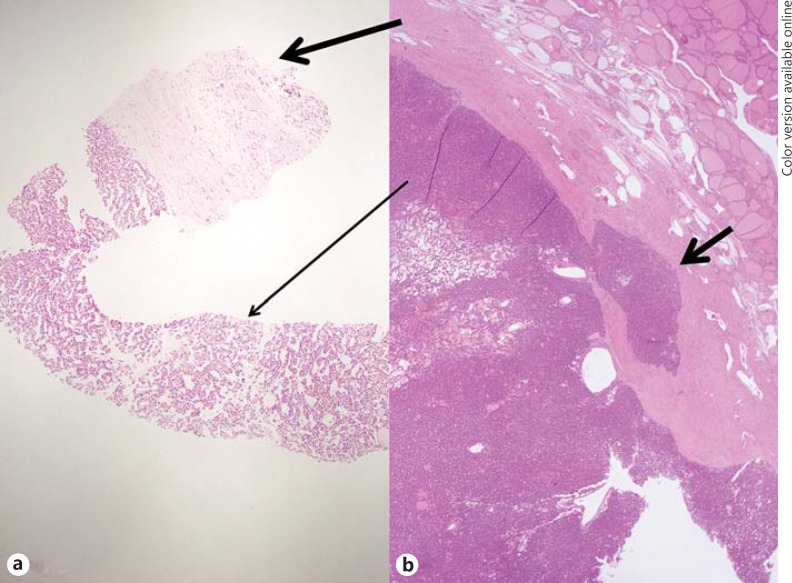
Fig. 3.
CNB histology and surgical pathology of a minimally invasive follicular carcinoma. a This CNB histology specimen shows a microfollicular proliferative lesion (thin arrow) with a fibrous capsule (thick arrow), diagnosed as FN/SFN. b Histology of the surgical specimen demonstrates tumor, encapsulation and focal minimal tumor invasion to the fibrous capsule (small arrows), diagnosed as minimally invasive follicular carcinoma. The diagnoses of the initial and repeat FNA were FOA and NA, respectively (not shown). HE stain. ×10.
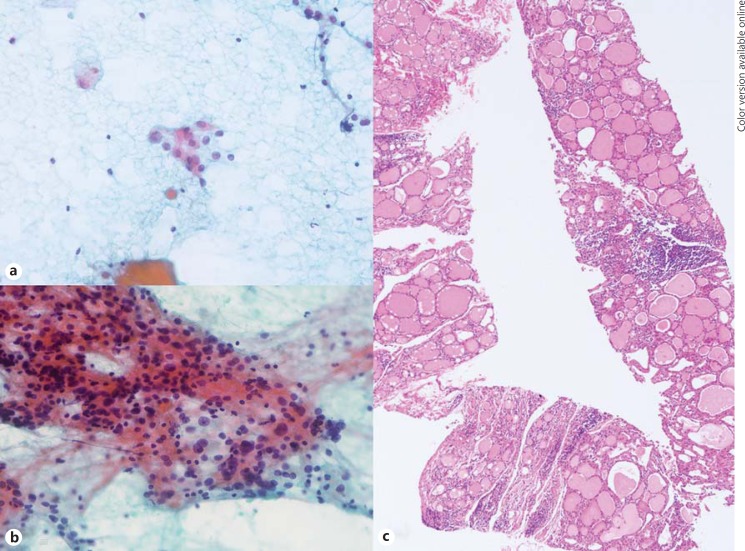
Fig. 4.
FNA cytology and CNB histology of focal lymphocytic thyroiditis manifested as a thyroid nodule. a The initial FNA cytology shows a few scattered atypical follicular epithelial cells with nuclear enlargement, size variation and pale chromatin, with the diagnosis of NA. b The repeat FNA cytology shows some atypical follicular epithelial cells with enlarged overlapping nuclei, occasional nuclear grooves, pale chromatin and some lymphocytes, repeatedly diagnosed as NA. c The CNB histology specimen shows variable-sized follicles with mild oncocytic change, and lymphocytic infiltration (arrow) without capsulation, diagnosed as lymphocytic thyroiditis. HE stain. ×10.
However, the data of subcategory analysis at CNB show different results between the NA and FOA subcategories. First, in contrast to the NA subcategory, there was no significant difference in the rate of malignant and benign diagnoses between RFNA and CNB in the FOA subcategory. Second, CNB showed a higher rate of FN/SFN diagnosis in the FOA subcategory than that in the NA subcategory. These differences between the NA and FOA subcategories seem closely related to the difference of histopathology because the FOA subcategory included mostly follicular lesions showing predominant microfollicle formation and rarely showing NA for papillary thyroid carcinoma. Our results also suggest that CNB is superior to RFNA in the diagnosis of follicular neoplasm in both the NA and FOA subcategories, which is supported by recent reports [25,26] that CNB is more sensitive and predictive of follicular neoplasm, and which could be explained by an advantage of CNB in providing better information of nodule architecture.
Our data demonstrated that CNB was more useful for management decisions relating to AUS/FLUS nodules than RFNA due to fewer inconclusive results at each NA and FO subcategory as well as overall AUS/FLUS nodules. Although management decisions of surgery or observation could be made more effectively at CNB compared with RFNA in the NA subcategory, only the management decision of surgery could be made more effectively at CNB in the FOA subcategory. This might be partly caused by a difficulty in categorizing a cellular hyperplastic nodule without a capsule in CNB specimens as benign, which was usually categorized as AUS/FLUS in the present study due to a concern of follicular variant papillary thyroid carcinoma or follicular carcinoma. Further investigations are required to establish the standardized criteria for a histology diagnosis in CNB. In our study, the sensitivity of CNB for malignancy with criteria 2 was 30.8% higher than RFNA in the NA subcategory and 60% higher in the FOA subcategory (table 3). The results of a higher rate of management decision and higher diagnostic accuracy for malignancy at CNB suggest that CNB may reduce the rate of unnecessary diagnostic surgery due to repeated inconclusive results, and has the potential to be a first-line alternative diagnostic method in AUS/FLUS nodules.
However, the optimal strategy for management decisions in the nodules showing repeated AUS/FLUS results at CNB remains to be investigated. In our study, the malignancy risk of repeated AUS/FLUS results at CNB was not substantially higher than the malignancy risk of the initial AUS/FLUS results, which suggests that a decision for surgery needs to be made conservatively after estimating the overall malignancy risk with clinical features, US findings [20,27] and other ancillary tests such as immunohistochemical studies [19,28,29].
We tried to obtain qualified cytology or histology specimens at each FNA or CNB procedure. The nondiagnostic rate of FNA was less than 10%, which supports the adequacy of FNA results in our study. Although the procedural safety of CNB is still a concern for some, previous studies [24,30,31] suggest that CNB is safe and tolerable, and the procedural technique of CNB is similarly feasible compared with FNA for a radiologist or thyroidologist experienced in thyroid intervention. This study has several limitations. First, there was an inevitable patient selection bias due to the retrospective nature of the study. Second, the results of CNB may have been influenced by the technique and experience of the operator and pathologist.
In conclusion, CNB may be more useful for guiding management decisions than RFNA in both the NA and FOA subcategories, and has a potential to be a first-line alternative diagnostic tool in initially diagnosed AUS/FLUS nodules.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 2.Faquin WC, Baloch ZW. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol. 2010;38:731–739. doi: 10.1002/dc.21292. [DOI] [PubMed] [Google Scholar]
- 3.Bongiovanni M, Krane JF, Cibas ES, Faquin WC. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol. 2012;120:73–86. doi: 10.1002/cncy.20178. [DOI] [PubMed] [Google Scholar]
- 4.Ho AS, Sarti EE, Jain KS, Wang H, Nixon IJ, Shaha AR, Shah JP, Kraus DH, Ghossein R, Fish SA, Wong RJ, Lin O, Morris LG. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS) Thyroid. 2014;24:832–839. doi: 10.1089/thy.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyeon J, Ahn S, Shin JH, Oh YL. The prediction of malignant risk in the category ‘atypia of undetermined significance/follicular lesion of undetermined significance' of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014;122:368–376. doi: 10.1002/cncy.21396. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan PS, Hirschowitz SL, Fung PC, Apple SK. The impact of atypia/follicular lesion of undetermined significance and repeat fine-needle aspiration: 5 years before and after implementation of the Bethesda System. Cancer Cytopathol. 2014;122:866–872. doi: 10.1002/cncy.21468. [DOI] [PubMed] [Google Scholar]
- 7.Renshaw AA. Does a repeated benign aspirate change the risk of malignancy after an initial atypical thyroid fine-needle aspiration? Am J Clin Pathol. 2010;134:788–792. doi: 10.1309/AJCPRA9Y2XQVFOFV. [DOI] [PubMed] [Google Scholar]
- 8.VanderLaan PA, Marqusee E, Krane JF. Clinical outcome for atypia of undetermined significance in thyroid fine-needle aspirations: should repeated FNA be the preferred initial approach? Am J Clin Pathol. 2011;135:770–775. doi: 10.1309/AJCP4P2GCCDNHFMY. [DOI] [PubMed] [Google Scholar]
- 9.Renshaw AA. Should ‘atypical follicular cells' in thyroid fine-needle aspirates be subclassified? Cancer Cytopathol. 2010;118:186–189. doi: 10.1002/cncy.20091. [DOI] [PubMed] [Google Scholar]
- 10.VanderLaan PA, Marqusee E, Krane JF. Usefulness of diagnostic qualifiers for thyroid fine-needle aspirations with atypia of undetermined significance. Am J Clin Pathol. 2011;136:572–577. doi: 10.1309/AJCPO0BQ2YSKPXXP. [DOI] [PubMed] [Google Scholar]
- 11.Olson MT, Clark DP, Erozan YS, Ali SZ. Spectrum of risk of malignancy in subcategories of ‘atypia of undetermined significance. Acta Cytol. 2011;55:518–525. doi: 10.1159/000333232. [DOI] [PubMed] [Google Scholar]
- 12.Luu MH, Fischer AH, Stockl TJ, Pisharodi L, Owens CL. Atypical follicular cells with equivocal features of papillary thyroid carcinoma is not a low-risk cytologic diagnosis. Acta Cytol. 2011;55:526–530. doi: 10.1159/000333227. [DOI] [PubMed] [Google Scholar]
- 13.Horne MJ, Chhieng DC, Theoharis C, Schofield K, Kowalski D, Prasad ML, Hammers L, Udelsman R, Adeniran AJ. Thyroid follicular lesion of undetermined significance: evaluation of the risk of malignancy using the two-tier sub-classification. Diagn Cytopathol. 2012;40:410–415. doi: 10.1002/dc.21790. [DOI] [PubMed] [Google Scholar]
- 14.Wu HH, Inman A, Cramer HM. Subclassification of ‘atypia of undetermined significance' in thyroid fine-needle aspirates. Diagn Cytopathol. 2014;42:23–29. doi: 10.1002/dc.23052. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Moon JH, Yom CK, Kim KH, Choi JY, Choi SI, Ahn SH, Jeong WJ, Lee WW, Park SY. Thyroid ‘atypia of undetermined significance' with nuclear atypia has high rates of malignancy and BRAF mutation. Cancer Cytopathol. 2014;122:512–520. doi: 10.1002/cncy.21411. [DOI] [PubMed] [Google Scholar]
- 16.Park KT, Ahn SH, Mo JH, Park YJ, Park do J, Choi SI, Park SY. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011;33:160–165. doi: 10.1002/hed.21414. [DOI] [PubMed] [Google Scholar]
- 17.Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, Yoo H. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012;22:468–475. doi: 10.1089/thy.2011.0185. [DOI] [PubMed] [Google Scholar]
- 18.Hahn SY, Shin JH, Han BK, Ko EY, Ko ES. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol. 2013;86:20130007. doi: 10.1259/bjr.20130007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasrollah N, Trimboli P, Guidobaldi L, Cicciarella Modica DD, Ventura C, Ramacciato G, Taccogna S, Romanelli F, Valabrega S, Crescenzi A. Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate: a new sampling technique. Endocrine. 2013;43:659–665. doi: 10.1007/s12020-012-9811-z. [DOI] [PubMed] [Google Scholar]
- 20.Lee KH, Shin JH, Oh YL, Hahn SY. Atypia of undetermined significance in thyroid fine-needle aspiration cytology: prediction of malignancy by US and comparison of methods for further management. Ann Surg Oncol. 2014;21:2326–2331. doi: 10.1245/s10434-014-3568-y. [DOI] [PubMed] [Google Scholar]
- 21.Hakala T, Kholová I, Sand J, Saaristo R, Kellokumpu-Lehtinen P. A core needle biopsy provides more malignancy-specific results than fine-needle aspiration biopsy in thyroid nodules suspicious for malignancy. J Clin Pathol. 2013;66:1046–1050. doi: 10.1136/jclinpath-2013-201559. [DOI] [PubMed] [Google Scholar]
- 22.Choi YJ, Baek JH, Ha EJ, Lim HK, Lee JH, Kim JK, Song DE, Shong YK, Hong SJ. Differences in risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance or follicular lesion of undetermined significance: the role of ultrasound-guided core-needle biopsy. Thyroid. 2014;24:494–501. doi: 10.1089/thy.2012.0635. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH, Korean Society of Thyroid Radiology (KSThR), Korean Society of Radiology Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015;16:391–401. doi: 10.3348/kjr.2015.16.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, Baek JH. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012;22:1564–1572. doi: 10.1007/s00330-012-2405-6. [DOI] [PubMed] [Google Scholar]
- 25.Min HS, Kim JH, Ryoo I, Jung SL, Jung CK. The role of core needle biopsy in the preoperative diagnosis of follicular neoplasm of the thyroid. APMIS. 2014;122:993–1000. doi: 10.1111/apm.12244. [DOI] [PubMed] [Google Scholar]
- 26.Yoon RG, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, Kim JK, Yoon JH, Kim WB. Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid. 2014;24:1612–1617. doi: 10.1089/thy.2014.0140. [DOI] [PubMed] [Google Scholar]
- 27.Rosario PW. Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda category III): importance of ultrasonography and cytological subcategory. Thyroid. 2014;24:1115–1120. doi: 10.1089/thy.2013.0650. [DOI] [PubMed] [Google Scholar]
- 28.de Matos LL, Del Giglio AB, Matsubayashi CO, de Lima Farah M, Del Giglio A, da Silva Pinhal MA. Expression of CK-19, galectin-3 and HBME-1 in the differentiation of thyroid lesions: systematic review and diagnostic meta-analysis. Diagn Pathol. 2012;7:97. doi: 10.1186/1746-1596-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip L. Molecular diagnostic testing and the indeterminate thyroid nodule. Curr Opin Oncol. 2014;26:8–13. doi: 10.1097/CCO.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 30.Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, Abati A. The National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference: a summation. Cytojournal. 2008;5:6. doi: 10.1186/1742-6413-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasrollah N, Trimboli P, Rossi F, Amendola S, Guidobaldi L, Ventura C, Maglio R, Nigri G, Romanelli F, Valabrega S, Crescenzi A. Patient's comfort with and tolerability of thyroid core needle biopsy. Endocrine. 2014;45:79–83. doi: 10.1007/s12020-013-9979-x. [DOI] [PubMed] [Google Scholar]