Abstract
We report on a 79-year-old woman with staghorn calculi who presented with severe hypercalcemia. She was later found to have humoral hypercalcemia of malignancy caused by a rare tumor, squamous cell carcinoma of the renal pelvis. Chronic irritation, infection and inflammation from staghorn stones cause squamous metaplasia, leading to squamous cell carcinoma of the renal collecting system. The prognosis is very poor, with a 5-year survival rate of <10%. This case highlights the importance of awareness of a very rare and aggressive carcinoma in a patient with long-standing nephrolithiasis.
Key Words: Hypercalcemia, Malignancy, Squamous cell carcinoma, Renal pelvis, Staghorn calculi
Introduction
Staghorn calculi are branched calculi that fill the entire or part of the renal pelvis and extend into the renal calices. If untreated or inadequately treated, they may lead to various complications such as deterioration of renal function and risk of developing urosepsis [1]. In addition, chronic irritation, inflammation and infection from these stones can cause squamous metaplasia of the renal pelvis epithelium that may progress to squamous cell carcinoma [2]. Squamous cell carcinoma of the renal pelvis is a rare tumor, with a prevalence of <1% of urinary tract neoplasms. However, there is a high incidence of renal stones worldwide [3]. Here we demonstrate an older woman with staghorn calculi presenting with severe hypercalcemia caused by squamous cell carcinoma of the renal pelvis.
Case Report
A 79-year-old female presented with gradual deterioration of mental status for 1 month. For 2 months prior to admission, she had had intermittent right upper abdominal pain, vomiting, constipation, anorexia and significant weight loss. Her medical conditions were hypertension and dyslipidemia, and she was receiving treatment with amlodipine and simvastatin. She was a passive smoker and did not drink alcohol. There was no history of malignancy in her family.
The patient was admitted to another hospital for investigation. Kidney, ureter and bladder (KUB) X-ray visualized a large right staghorn stone (fig. 1a), and a retrograde pyelogram showed right staghorn renal calculi with partial obstruction and left middle ureter stricture (fig. 1b). A diuretic renal scan was interpreted as a nonfunctioning right kidney and fair renal function of the left kidney. Serum blood urea nitrogen and creatinine were 52 mg/dl (reference range 7–18) and 6.8 mg/dl (reference range 0.67–1.17), respectively. She was rehydrated with normal saline and then referred to our hospital for relief of left ureteric obstruction.
Fig. 1.
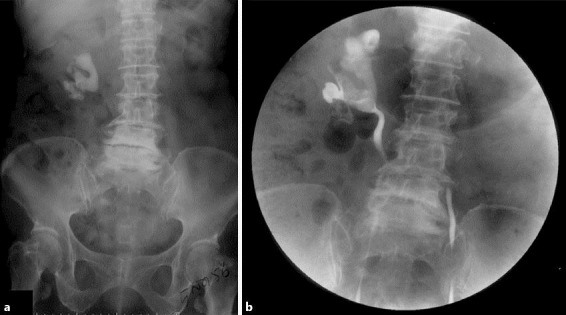
a KUB X-ray visualized a large right staghorn stone. b Retrograde pyelogram showed right staghorn renal calculi with partial obstruction and left middle ureter stricture.
On examination, the patient appeared lethargic and dehydrated. Her blood pressure was 160/87 mm Hg and her pulse rate was 93 beats/min. She had no fever and mild pallor. Two lymph nodes, 0.7 and 1 cm in diameter, were palpated in the left supraclavicular area. She had nonpitting edema on both legs that was more pronounced on the right leg with the presence of Homan's sign. Deep vein thrombosis was suspected.
Urinary examination showed a pH of 5, white blood cells of 1–2/high-power field and red blood cells of 0–1 cells/high-power field. Furthermore, blood chemistries revealed serum total calcium of 14.8 mg/dl (reference range 8.5–10.1), serum phosphorus of 3 mg/dl (reference range 2.5–4.9) and serum creatinine of 2 mg/dl (reference range 0.67–1.17). Her corrected calcium adjusted with albumin was 15.93 mg/dl. Serum intact parathyroid hormone (PTH) was investigated and found to be 11.5 pg/ml (reference range 15–65). In the settings of old age, weight loss and deep vein thrombosis, humoral hypercalcemia of malignancy was mostly suspected. Unfortunately, we could not confirm the diagnosis with PTH-related protein (PTH-rP) due to test unavailability in our country.
Renal ultrasound visualized large right staghorn calculi and moderate left hydronephrosis with proximal hydroureter (fig. 2). Doppler ultrasound showed acute extensive deep vein thrombosis of both legs along the external iliac vein, the common femoral vein, the proximal deep femoral vein to the popliteal vein. Thrombosis completely occluded the right leg and partially the left leg. There was minimal right pleural effusion on chest X-ray, with no abnormal pulmonary nodules. In searching for the primary site of malignancy, a left supraclavicular lymph node biopsy was performed. Histopathology was compatible with metastatic squamous cell carcinoma. Computed tomography of the chest and abdomen showed an infiltrative tumor with extensive involvement of the right kidney, the right pelvocalyceal system, the right adrenal gland, the right lobe of the liver and the adjacent right hemidiaphragm and psoas muscle (fig. 3). There were inferior vena cava invasion and multiple metastases in both hepatic lobes and intra-abdominal lymph nodes. The final diagnosis was advanced-stage squamous cell carcinoma of the right renal pelvis.
Fig. 2.
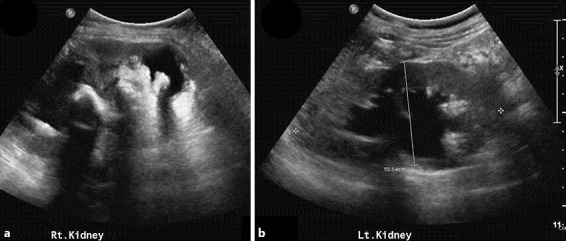
Renal ultrasound of the right kidney (a) and the left kidney (b) revealed large right staghorn calculi and moderate left hydronephrosis and proximal hydroureter.
Fig. 3.
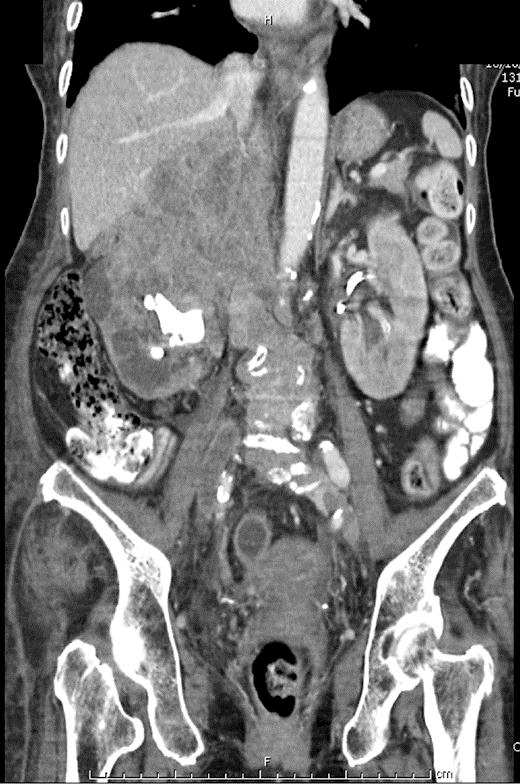
A coronal section of computed tomography of the abdomen demonstrated an infiltrative tumor with extensive involvement of the right kidney, the right pelvocalyceal system, the right adrenal gland, the right lobe of the liver and the adjacent right hemidiaphragm and psoas muscle.
To rescue left kidney function, a left retrograde pyelogram with double J stent was performed. The treatment of hypercalcemia was first intravenous saline rehydration. After restoration of volume status, serum creatinine levels decreased from 2.0 to 1.7 mg/dl. Then the patient was prescribed 60 mg of pamidronate, an intravenous bisphosphonate. Four days later, serum calcium level subsided to 10.16 mg/dl. It was decided that long-term therapy would be the best supportive care. The patient was discharged to a primary care hospital and passed away 1 month later.
Discussion
Hypercalcemia of malignancy is not an uncommon etiology of hypercalcemia in an inpatient setting. The tumor generally presents with abrupt onset, severe symptoms and high serum calcium concentration of >14 mg/dl. The prognosis is very poor and associated with an advanced stage of malignancy [4]. Humoral hypercalcemia of malignancy mediated by PTH-rP is a predominant cause. Even though various cells in the body have the potential to produce PTH-rP, tumors that commonly produce PTH-rP include squamous cell carcinomas of the head and neck, esophagus, cervix and lung; adenocarcinomas of the breast and ovary and renal cell carcinoma. Endogenous PTH secretion is suppressed by PTH-rP-mediated hypercalcemia. Thus, serum intact PTH levels are suppressed, as demonstrated in our case. The definite diagnosis can be confirmed by detection of high serum PTH-rP, but this is not necessary for diagnosis in most situations.
Most renal pelvic tumors are transitional cell carcinomas. Squamous cell carcinoma of the renal pelvis is a very rare tumor, accounting for only 0.5–8.0% of malignant renal tumors [5, 6]. It usually occurs at the age of 50–70 years. Clinical manifestations include abdominal or flank pain, hematuria and abdominal mass. It is frequently involved with hydronephrosis. Humoral hypercalcemia of malignancy is rarely found [7]. The coexistence of renal calculi has been reported in 87–100% of cases [6, 8, 9]. It has also been associated with tuberculosis, chronic pyelonephritis, radiation therapy, chronic rejection in a transplanted kidney, analgesic abuse with phenacetin, immunosuppression with azathioprine and previous percutaneous nephrolithotomy [10].
Staghorn calculi are usually composed of magnesium ammonium phosphate (struvite) and/or calcium carbonate apatite. Cystine or uric acid mixed with other components can also grow in a staghorn configuration. Struvite/calcium carbonate apatite stones are frequently referred to as ‘infection stones’ because of the strong relation with urinary tract infection caused by urease-producing Gram-negative bacteria, e.g. Proteus, Morganella and Providencia spp. However, recent data found that 55% of cases with staghorn calculi were from metabolic stones, in particular of calcium phosphate composition [11]. Moreover, the most common component of staghorn calculi in patients in Southern Thailand was uric acid [12]. Left untreated, staghorn calculi may cause life-threatening sepsis and renal function impairment. Over time, staghorn stones cause squamous metaplasia and dysplasia of the uroepithelium. Therefore, most patients should require treatment of staghorn stones. The treatment options are medical therapy, surgery, percutaneous nephrolithotomy and shock wave lithotripsy. Analysis of the composition of the stone and metabolic evaluation are essential for preventing recurrence [13, 14]. Increased fluid intake to achieve a urine output of >2.5 l/day will decrease the risk of urinary supersaturation.
Early detection of the tumor by ultrasound would be difficult because the tumor appears as a nonspecific finding, such as a solid mass, calcification or hydronephrosis [2]. As a result, a computed tomography scan may be an option for cancer detection, with better yield for diagnosis [9]. Additionally, intravenous urography is beneficial for monitoring chronic calculi patients. Findings of filling defect in the collecting system, delayed appearance of pyelogram or renal parenchymal thickening should be definitely considered as renal tumor [8].
In conclusion, staghorn stone is a leading cause of squamous cell carcinoma of the renal pelvis. Squamous cell carcinoma of the renal pelvis has a grave prognosis. Humoral hypercalcemia of malignancy mediated by PTH-rP is an uncommon feature of the tumor. Removal of the staghorn stone is necessary to eliminate infection and prevent squamous metaplasia of the renal epithelium.
Statement of Ethics
Written informed consent was obtained from the next of kin for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editor of this journal.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Healy KA, Ogan K. Pathophysiology and management of infectious staghorn calculi. Urol Clin North Am. 2007;34:363–374. doi: 10.1016/j.ucl.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Holmäng S, Lele SM, Johansson SL. Squamous cell carcinoma of the renal pelvis and ureter: incidence, symptoms, treatment and outcome. J Urol. 2007;178:51–56. doi: 10.1016/j.juro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–e96. [PMC free article] [PubMed] [Google Scholar]
- 4.Ralston SH, Gallacher SJ, Patel U, Campbell J, Boyle IT. Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499–504. doi: 10.7326/0003-4819-112-7-499. [DOI] [PubMed] [Google Scholar]
- 5.Blacher EJ, Johnson DE, Abdul-Karim FW, Ayala AG. Squamous cell carcinoma of renal pelvis. Urology. 1985;25:124–126. doi: 10.1016/0090-4295(85)90526-6. [DOI] [PubMed] [Google Scholar]
- 6.Li MK, Cheung WL. Squamous cell carcinoma of the renal pelvis. J Urol. 1987;138:269–271. doi: 10.1016/s0022-5347(17)43116-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Sharifi R, Kurtzman NA. Humoral hypercalcemia due to squamous cell carcinoma of renal pelvis. Urology. 1988;32:250–253. doi: 10.1016/0090-4295(88)90395-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee TY, Ko SF, Wan YL, Cheng YF, Yang BY, Huang DL, Hsieh HH, Yu TJ, Chen WJ. Renal squamous cell carcinoma: CT findings and clinical significance. Abdom Imaging. 1998;23:203–208. doi: 10.1007/s002619900324. [DOI] [PubMed] [Google Scholar]
- 9.Raghavendran M, Rastogi A, Dubey D, Chaudhary H, Kumar A, Srivastava A, Mandhani A, Krishnani N, Kapoor R. Stones associated renal pelvic malignancies. Indian J Cancer. 2003;40:108–112. [PubMed] [Google Scholar]
- 10.Sivaramakrishna B, Aron M, Ansari MS, Seth A, Goel R, Mundada OP, Balchander H. Squamous cell carcinoma of the renal pelvis manifesting after percutaneous nephrolithotomy for long standing calculus. Int Urol Nephrol. 2004;36:149–151. doi: 10.1023/b:urol.0000034672.68658.fa. [DOI] [PubMed] [Google Scholar]
- 11.Viprakasit DP, Sawyer MD, Herrell SD, Miller NL. Changing composition of staghorn calculi. J Urol. 2011;186:2285–2290. doi: 10.1016/j.juro.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 12.Tanthanuch M. Staghorn calculi in southern Thailand. J Med Assoc Thai. 2006;89:2086–2090. [PubMed] [Google Scholar]
- 13.Schubert G. Stone analysis. Urol Res. 2006;34:146–150. doi: 10.1007/s00240-005-0028-y. [DOI] [PubMed] [Google Scholar]
- 14.Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97:1847–1860. doi: 10.1210/jc.2011-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]


