Abstract
Background
It is well known that thyrotoxicosis causes rhythm disorders including sinus tachycardia, atrial fibrillation, and atrial flutter. Atrial fibrillation is the most common arrhythmia in thyrotoxicosis, occurring in 5-15% of patients over 60 years of age, whereas ventricular arrhythmia is an unusual manifestation.
Case Report
An 18-year-old Japanese woman was admitted to our emergency department because of loss of consciousness caused by ventricular fibrillation. She had been diagnosed with Graves' disease only 5 days earlier and had no other past medical history. Blood examination showed no obvious abnormality except thyrotoxicosis, and coronary angiography revealed patent coronary arteries. She was diagnosed with thyroid storm due to Graves' disease and is currently healthy during outpatient follow-up.
Conclusion
This case highlights that thyrotoxicosis can, albeit extremely rarely, cause ventricular fibrillation even in the absence of hypokalemia or underlying cardiovascular disease.
Key Words: Graves’ disease, Thyrotoxicosis, Ventricular fibrillation, Coronary artery vasospasm, Early repolarization syndrome
What Is Known about This Topic?
• Thyrotoxicosis is associated with an increased risk of arrhythmia, especially supraventricular arrhythmias.
What Does This Case Report Add?
• This case suggests that thyrotoxicosis can cause ventricular fibrillation even in the absence of hypokalemia, heart failure, or underlying cardiovascular disease.
Introduction
Thyroid hormone plays an important role in the cardiovascular system by increasing the heart rate and left ventricular systolic function and reducing systemic vascular resistance [1]. Thyrotoxicosis is associated with an increased risk of arrhythmia, especially supraventricular arrhythmias. Genomic and nongenomic actions of thyroid hormone may contribute to paroxysmal atrial tachycardia, atrial fibrillation (AF), and atrial flutter. Among these arrhythmias, AF is the most common, occurring in 9-22% of patients with hyperthyroidism [2]. Complications of AF in patients with hyperthyroidism include heart failure and thrombus formation, which can be life threatening [3]. Other atrial arrhythmias caused by thyrotoxicosis are often asymptomatic and are mostly detected on electrocardiographic monitoring.
In contrast to the high incidence of atrial arrhythmias, ventricular arrhythmias are uncommon, and the etiology of ventricular fibrillation (VF) in thyrotoxic subjects is unknown. Only a few isolated cases have been described to date, the majority of which showed severe hypokalemia as the cause of VF associated with thyrotoxicosis [4]. However, some reports have demonstrated other causes of VF [5,6]. We report here a case of Graves' disease with VF as the result of thyrotoxicosis and review the literature on VF associated with thyrotoxicosis. This case suggests that thyrotoxicosis can, albeit extremely rarely, cause VF even in the absence of hypokalemia, heart failure, or underlying cardiovascular disease.
Case Report
A previously healthy 18-year-old Japanese woman presented with a 2-day history of fever, dyspnea, and chest pain that was exacerbated by movement and deep inspiration. Physical examination revealed tenderness of the left lateral thoracic region and rales at the base of the left lung. The chest X-ray findings showed lobar consolidation in the inferior lobe of the left lung. Consequently, she was given a diagnosis of bacterial pneumonia with pleuritis and treated with antibiotics in an outpatient setting. Although she recovered from fever and respiratory symptoms after the course of antibiotics, she re-presented to the hospital with a 7-day history of palpitations and thyroid swelling. She was taking no other medications and her family and social history were noncontributory. Thyroid ultrasound showed markedly increased blood flow. Laboratory investigation revealed thyrotoxicosis (free T3: 42.2 pmol/l; free T4: 99.1 pmol/l) with suppressed thyroid-stimulating hormone, strongly suggestive of Graves' disease. She was started on thiamazole (30 mg/day) and propranolol hydrochloride (30 mg/day), and was managed in an outpatient setting.
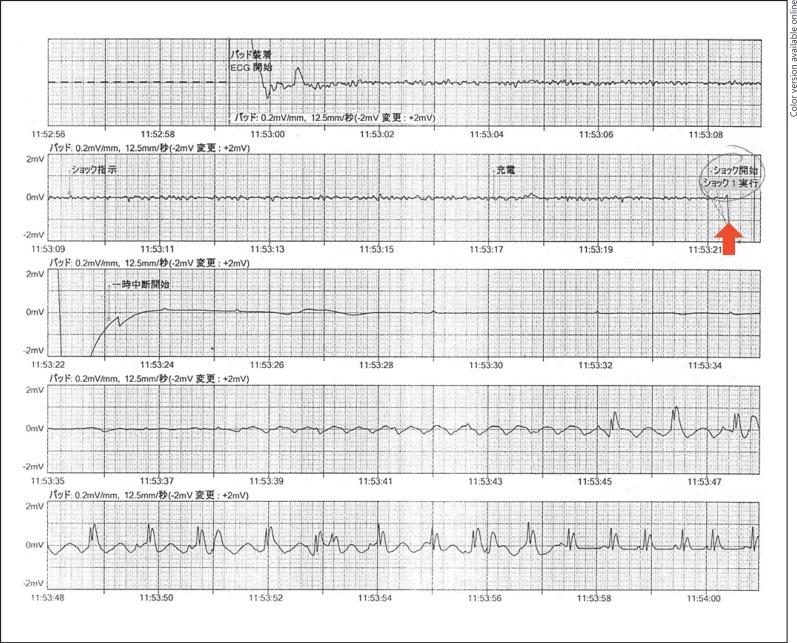
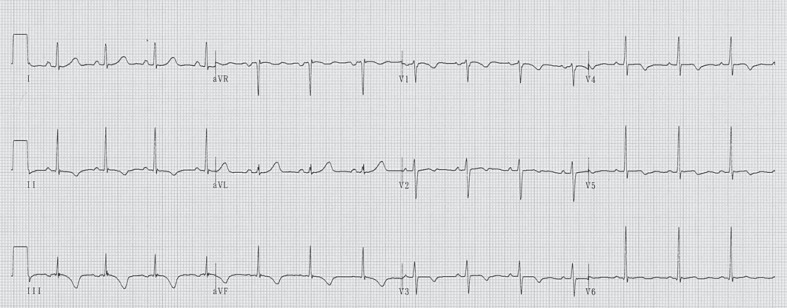
After 5 days of treatment for Graves' disease, she suddenly collapsed during a physical education class at her senior high school. She had a cardiopulmonary arrest and bystander cardiopulmonary resuscitation was immediately initiated while the emergency medical system was alerted. On ambulance arrival 17 min later, the patient was immediately placed on an automated external defibrillator monitor and the initial cardiac rhythm was VF (fig. 1). After basic life support for 4 min, including chest compressions and three defibrillation attempts, sinus rhythm and spontaneous circulation were restored. On arrival at our intensive care unit, her Glasgow Coma Scale score was 6 and physical examination revealed a blood pressure of 127/106 mm Hg, pulse of 124 beats/min, and a respiratory rate of 24 breaths/min. Laboratory findings were as follows: white blood cell count, 9,900/μl; hemoglobin, 10.6 g/dl; platelets, 150,000/μl; D-dimer, 4.4 µg/ml; aspartate aminotransferase, 71 IU/l; alanine aminotransferase, 103 IU/l; lactic dehydrogenase, 217 IU/l; creatine kinase, 61 IU/l; creatine kinase MB, 46 IU/l; troponin T, 0.05 ng/ml; blood urea nitrogen, 9.0 mg/dl; creatinine, 0.45 mg/dl; blood glucose, 168 mg/dl; sodium, 139 mmol/l; potassium, 4.1 mmol/l; chloride, 104 mmol/l; calcium, 9.0 mg/dl; free T3, 31.7 pmol/l (normal 3.1-5.9); free T4, 53.8 pmol/l (normal 10.3-19.3); thyroid-stimulating hormone, 0.01 μIU/l (normal 0.34-3.8); TRAb, 8.2 U/l (normal <2), and troponin I, 0.37 ng/ml (normal <0.1). She was intubated and therapeutic hypothermia was applied to reduce the likelihood of brain injury. An echocardiogram revealed a structurally intact heart with adequate biventricular function. ST segment depression in leads V3-5 and T-wave inversion in inferior leads were present on the electrocardiogram (fig. 2).
Fig. 1.
Automated external defibrillator record showing VF before defibrillation. Arrow indicates defibrillation.
Fig. 2.
Twelve-lead electrocardiogram on hospital day 33.
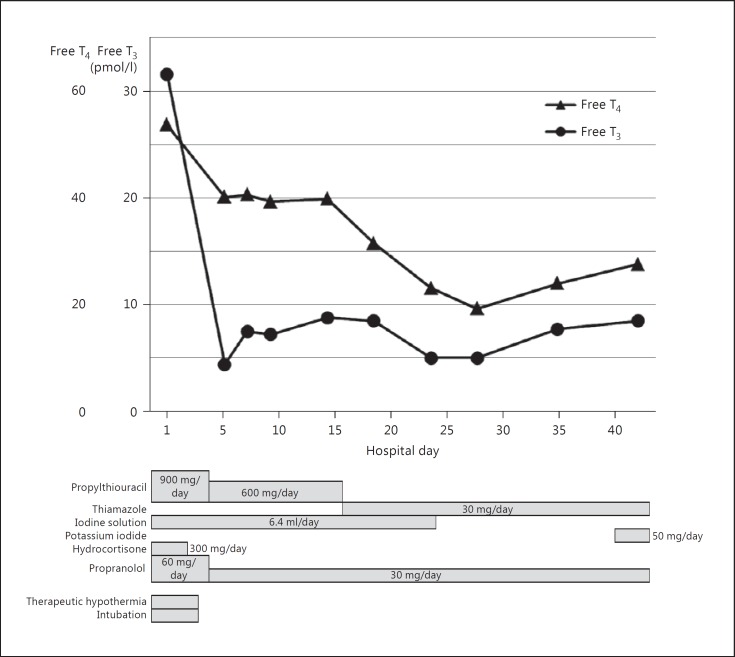
On the day of admission, we started propylthiouracil (900 mg/day), iodine solution (6.4 ml/day), hydrocortisone (300 mg/day), and propranolol (60 mg/day) to control thyroid storm. Her general condition gradually improved, accompanied by a dramatic decrease in the level of thyroid hormone, and therapeutic hypothermia was discontinued on hospital day 5 (fig. 3).
Fig. 3.
Clinical course of the patient.
Since myocardial ischemia was suspected from her acute presentation, coronary angiography was performed which showed neither vasospasm nor coronary occlusion. Cardiac magnetic resonance imaging (MRI) showed late gadolinium enhancement in the posteroseptal wall, suggesting the presence of acute myocardial damage. She underwent implantable cardioverter defibrillator placement 1 month after admission for secondary prevention. Her condition stabilized with no emergent symptoms and she was discharged on day 43.
No signs of recurrent VF or other cardiac arrest rhythms have been observed during 3 years of outpatient follow-up.
Discussion
This case suggests that thyrotoxicosis can cause VF even in the absence of hypokalemia and underlying cardiovascular disease. Thyroid hormone plays an important role in cardiac muscle, systemic circulation, and the sympathetic nervous system that affect cardiovascular hemodynamics. The action of thyroid hormone is mediated by the binding of T3 to nuclear receptors [2]. The subsequent binding of the T3-receptor complex to DNA regulates the expression of genes coding for cardiac proteins, such as sarcoplasmic reticulum calcium adenosine triphosphatase, myosin heavy chain-α, β1-adrenergic receptors, guanine nucleotide-regulatory proteins, sodium/potassium adenosine triphosphatase, and voltage-gated potassium channels [7]. In addition, some effects of thyroid hormone appear to be mediated by nongenomic mechanisms. The mechanism of action is not well understood, but may involve changes in the functional properties of membrane ion channels and pumps. For this reason, thyroid hormone has been shown to have cardiovascular effects.
Excess thyroid hormone is known to be an important factor in the etiology of atrial arrhythmia. The mechanism of AF in patients with hyperthyroidism is a shortening of the action potential duration in the atrial myocardium and a decrease in the refractoriness of cardiomyocytes. This mechanism enhances the formation of multiple reentry circuits [8]. Some reports have shown that the pulmonary veins of several species have pacemaker cells, and thyrotoxicosis enhances the physiological activity of pulmonary vein myocytes [9]. Increased automaticity and enhanced triggered activity may increase arrhythmias arising from the pulmonary vein in hyperthyroidism. In contrast to atrial arrhythmia, VF is an unusual manifestation of thyrotoxicosis. The majority of cases of VF induced by thyrotoxicosis occur in the context of thyrotoxic periodic paralysis with severe hypokalemia [4]. In our literature review, we identified only 9 cases (in 7 case reports) of VF caused by thyrotoxicosis without hypokalemia (table 1) [5,6,10,11,12,13,14]. Among these, all of the patients except Brooks' case had neither previous history of cardiac disease nor family history of sudden death. All were diagnosed with Graves' disease or amiodarone-induced thyrotoxicosis at almost exactly the same time as VF. Half of the patients were active smokers.
Table 1.
Literature on VF caused by thyrotoxicosis without hypokalemia
| Study (first author) | Year reported | Sex | Age, years | Diagnosis | Free T3, pmol/l | Free T4, pmol/l | TRAb | Pathophysiology | Smoking history |
|---|---|---|---|---|---|---|---|---|---|
| This case | 2015 | female | 18 | Graves' disease | 31.7 | 53.8 | 8.2 U/l | unknown | nonsmoker |
| Nakashima [10] | 2014 | male | 23 | Graves' disease | >46.2 | 64.7 | 30.2% | unknown | unknown |
| Brooks [6] | 2013 | male | 55 | Amiodarone induced | 14.9 | 74.9 | – | vasospasm | smoker |
| Ando [11] | 2011 | male | 38 | Graves' disease | 19.6 | 106.8 | 21.4% | unknown | smoker |
| Ando [11] | 2011 | male | 45 | Graves' disease | 7.1 | 38.6 | 25 U/l | unknown | smoker |
| Ando [11] | 2011 | male | 43 | Graves' disease | 13.9 | 33.5 | 3.1 U/l | unknown | smoker |
| Ueno [5] | 2010 | male | 69 | Graves' disease | 37.0 | 77.2 | – | Early repolarization | nonsmoker |
| Chatterjee [12] | 2009 | male | 74 | Graves' disease | 6.5 | 222.7 | – | unknown | nonsmoker |
| Jao [13] | 2004 | female | 34 | Graves' disease | 1,925 (T3) | 54.3 | – | unknown | nonsmoker |
| Wei [14] | 1979 | female | – | – | – | – | – | unknown | unknown |
Two possible mechanisms for VF associated with thyrotoxicosis were considered in these cases. The first mechanism is coronary vasospasm. Previous reports have demonstrated that the mechanisms of vasospasm include endothelial dysfunction and primary hyperreactivity of vascular smooth muscle cells [15]. Vascular smooth muscle cell hyperreactivity is related to several intracellular pathways that regulate vascular resistance. Thyroid hormones may affect those intracellular pathways and cause hyperreactivity, leading to spasms, followed by myocardial ischemia and possibly VF. The characteristics of vasospasm due to thyrotoxicosis are as follows: age <55 years [16], higher prevalence in woman [16], mild symptoms of thyrotoxicosis [17], and smoking history [11]. In the present case, although we could not demonstrate vasospasm induced by acetylcholine in the coronary arteriography, coronary artery spasm is one of the possible causes of VF as suggested by the patient's clinical history and late gadolinium enhancement on cardiac MRI.
The second possible mechanism of VF due to thyrotoxicosis is associated with early repolarization. Although the mechanism responsible for J-point elevation observed in early repolarization syndrome is not fully understood, involvement of the transmural voltage gradient between the endocardium and epicardium has been implicated in ventricular activation of the action potential. β-Blockers and parasympathetic stimulation enhance the elevation of J-point and ST segment through attenuation of ionized calcium and increase in the voltage gradient between the endocardium and epicardium, thereby causing arrhythmia [18]. Therefore, treatment of thyroid storm in patients with early repolarization can exaggerate the J-point elevation and induce VF [5]. In our case, J-point elevation was not observed and the relation between VF and early repolarization was thought to be irrelevant in this case.
A limitation in this case is the lack of an electrophysiologic study as the patient did not wish to undergo the test.
In summary, although VF is a very rare complication of hyperthyroidism and rarely reported in the literature, it is clinically important as well as potentially life threatening. We therefore suggest that clinicians should exclude hyperthyroidism in patients presenting with VF fibrillation.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 2.Marrakchi S, Kanoun F, Idriss S, Kammoun I, Kachboura S. Arrhythmia and thyroid dysfunction. Herz. 2014;40(suppl 2):101–109. doi: 10.1007/s00059-014-4123-0. [DOI] [PubMed] [Google Scholar]
- 3.Petersen P. Thromboembolic complications in atrial fibrillation. Stroke. 1990;21:4. doi: 10.1161/01.str.21.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Fisher J. Thyrotoxic periodic paralysis with ventricular fibrillation. Arch Intern Med. 1982;142:1362–1364. [PubMed] [Google Scholar]
- 5.Ueno A, Yamamoto T, Sato N, Tanaka K. Ventricular fibrillation associated with early repolarization in a patient with thyroid storm. J Interv Card Electrophysiol. 2010;29:93–96. doi: 10.1007/s10840-010-9507-3. [DOI] [PubMed] [Google Scholar]
- 6.Brooks MJ, Pattison DA, Teo EP, Price S, Gurvitch R. Amiodarone-induced destructive thyroiditis associated with coronary artery vasospasm and recurrent ventricular fibrillation. Eur Thyroid J. 2013;2:65–67. doi: 10.1159/000345528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 8.Freedberg AS, Papp JG, Williams EM. The effect of altered thyroid state on atrial intracellular potentials. J Physiol. 1970;207:357–369. doi: 10.1113/jphysiol.1970.sp009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, Lin CI. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima Y, Kenzaka T, Okayama M, Kajii E. A case of thyroid storm with cardiac arrest. Int Med Case Rep J. 2014;7:89–92. doi: 10.2147/IMCRJ.S63475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ando T, Henmi T, Haruta D, Haraguchi A, Ueki I, Horie I, Imaizumi M, Usa T, Maemura K, Kawakami A. Graves' disease complicated by ventricular fibrillation in three men who were smokers. Thyroid. 2011;21:1021–1025. doi: 10.1089/thy.2010.0368. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Nautiyal A, Mukherjee JT, Sweeney AT, Chaudhry MG. Life threatening ventricular fibrillation – an initial manifestation of Graves' disease. Resuscitation. 2009;80:1085–1086. doi: 10.1016/j.resuscitation.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Jao YT, Chen Y, Lee WH, Tai FT. Thyroid storm and ventricular tachycardia. South Med J. 2004;97:604–607. doi: 10.1097/00007611-200406000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Wei JY, Genecin A, Greene HL, Achuff SC. Coronary spasm with ventricular fibrillation during thyrotoxicosis: response to attaining euthyroid state. Am J Cardiol. 1979;43:335–339. doi: 10.1016/s0002-9149(79)80023-5. [DOI] [PubMed] [Google Scholar]
- 15.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 16.Patel R, Peterson G, Rohatgi A, Ghayee HK, Keeley EC, Auchus RJ, Chang AY. Hyperthyroidism-associated coronary vasospasm with myocardial infarction and subsequent euthyroid angina. Thyroid. 2008;18:273–276. doi: 10.1089/thy.2007.0131. [DOI] [PubMed] [Google Scholar]
- 17.Featherstone HJ, Stewart DK. Angina in thyrotoxicosis. Thyroid-related coronary artery spasm. Arch Intern Med. 1983;143:554–555. doi: 10.1001/archinte.143.3.554. [DOI] [PubMed] [Google Scholar]
- 18.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]