Abstract
The solute carrier 27A (SLC27A) gene family encodes fatty acid transport proteins (FATPs) and includes 6 members. During fetal and postnatal periods of development, the growing brain requires a reliable supply of fatty acids. Because autism spectrum disorders (ASD) are now recognized as disorders caused by impaired early brain development, it is possible that functional abnormalities of SLC27A genes may contribute to the pathogenesis of ASD. Here, we confirmed the expression of SLC27A3 and SLC27A4 in human neural stem cells derived from human induced pluripotent stem cells, which suggested their involvement in the developmental stage of the central nervous system. Additionally, we resequenced the SLC27A3 and SLC27A4 genes using 267 ASD patient and 1140 control samples and detected 47 (44 novel and 29 nonsynonymous) and 30 (17 novel and 14 nonsynonymous) variants for the SLC27A3 and SLC27A4, respectively, revealing that they are highly polymorphic with multiple rare variants. The SLC27A4 Ser209 allele was more frequently represented in ASD samples. Furthermore, we showed that a SLC27A4 Ser209 mutant resulted in significantly higher fluorescently-labeled fatty acid uptake into bEnd3 cells, a mouse brain capillary-derived endothelial cell line, compared with SLC27A4 Gly209, suggesting that the functional change may contribute to ASD pathophysiology.
Autism spectrum disorders (ASD) are complex neurodevelopmental disorders characterized by impairments in social orientation and communication and repetitive or restricted patterns of interests or behaviors1,2,3. Although ASD pathogenesis is not completely understood, recent evidence suggests that abnormal fatty acid metabolism may play a role in the pathophysiology of ASD4,5,6. In addition, a recent report suggested that fatty acid homeostasis may be altered in ASD as a result of insufficient dietary supplementation, genetic defects, and the dysfunction of enzymes involved in fatty acid metabolism7.
The brain is a lipid-rich organ, with nearly 50% its dry weight consisting of lipids8. Brain lipids are primarily phospholipids, sphingolipids, and glycolipids. These lipids, which are rich in highly polyunsaturated fatty acids (PUFAs), have essential structural and signaling roles to support normal neural function. In addition, PUFAs are critical structural components of the brain and are essential for normal brain development9,10,11. Because fetal synthesis of PUFAs is thought to be limited, transport of PUFAs from the maternal plasma to the growing fetus is particularly important for fetal brain growth and development.
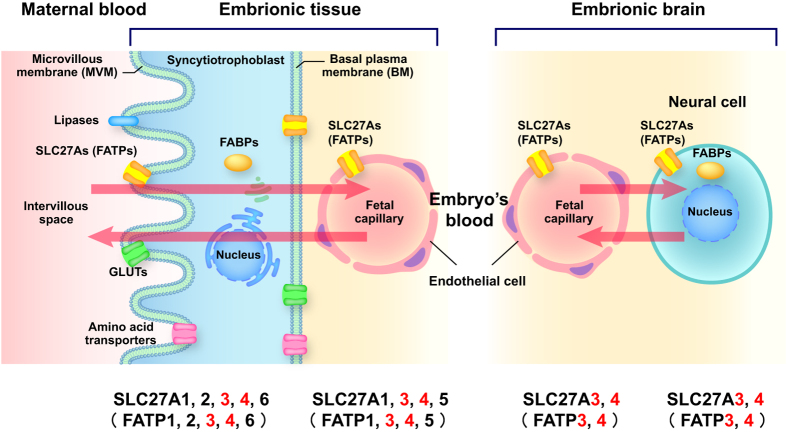
Fatty acid transport proteins (FATPs) solute carrier 27A (SLC27A) family includes six members in humans and mice (SLC27A1/Slc27a1 to SLC27A6/Slc27a6). Among FATP family members, SLC27A3/Slc27a3 (also know as FATP3/Fatp3) is highly expressed in the adrenal gland, testis, ovary, lung, and the neonatal and adult brain. SLC27A4/Slc27a4 (also know as FATP4/Fatp4) is expressed in the intestine, skin, brain, kidney, liver and heart, as well as trophoblasts of the placenta and endothelial cells. Additionally, only Slc27a3 and Slc27a4 were expressed in mouse brain capillary-derived endothelial cells (bEnd3)12 and while Slc27a3 is strongly expressed in the fetal mouse brain13, Slc27a4 is only weakly expressed14. SLC27A1, SLC27A3, SLC27A4, and SLC27A5 are expressed in human umbilical vein endothelial cells (HUVEC)12. Because nutrients derived from the mother’s plasma and delivered to the fetus have to be transported through the vascular endothelium of the placenta and brain, we hypothesized that SLC27A3 and SLC27A4, both of which are expressed in placenta and brain endothelial cells, are the most relevant fatty acid transporters for supplying fatty acids to the fetal brain (Fig. 1)12,13,14,15.
Figure 1. Schematic illustration of FATPs’ involvement in the placenta-fetal brain axis.
Fatty acid supply from the mother to the fetal brain is carried out through SLC27As (FATPs) at individual barriers shown in the figure. At all the barriers, SLC27As (FATPs) 3 and 4 are thought to be commonly expressed (see text). Arrows show direction of fatty acid transport. The part of this figure is made using the modification of Fig. 1 in ref. 15.
Although it is known that Slc27a3 mRNA levels are significantly higher in embryonic mouse brain than in newborn or adult mice13, an in vivo model of Slc27a3 loss of function has not yet been reported. The expression pattern of Slc27A3 protein suggests that it may play an important role in brain development13. Mishima et al.16 reported that Slc27a4 deficient embryos exhibited normal fetal growth. However, they did not perform any neuronal analyses including behavioral assessment, and it is worthy of examining a potential role of the SLC27A4 gene in functional brain disorders including ASD.
Thus, in this study, we investigated possible roles for SLC27A3 and SLC27A4 in ASD. First, we investigated the role of SLC27A3 and SLC27A4 in neural development by examining the expression of these genes during neuronal differentiation from human induced pluripotent stem cells (hiPSCs) and also in mouse fetal brain. Further, we resequenced SLC27A3 and SLC27A4 in 267 ASD patients and 1140 control samples. We focused on identifying nonsynonymous variants, and assessed the associations of these variants with ASD, along with the functional evaluation of the associated variant.
Results
SLC27A3 and SLC27A4 gene expression in hiPSC-derived cells and mouse fetal brain
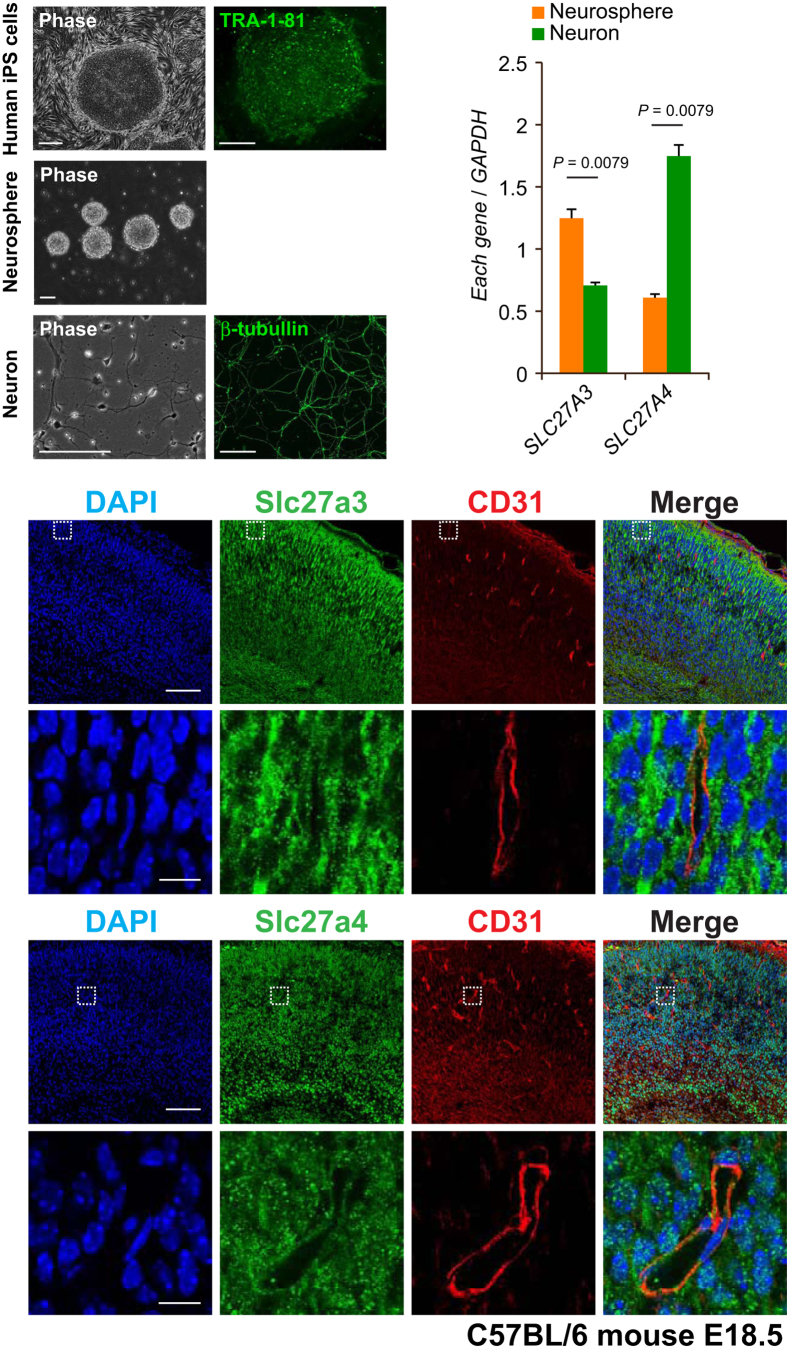
We focused on two FATP proteins, FATP3 (encoded by SLC27A3) and FATP4 (encoded by SLC27A4), because these proteins may be key molecules for fatty acid uptake into the fetal brain (Fig. 1). We first measured the relative SLC27A3 and SLC27A4 mRNA expression levels in human induced pluripotent stem cell (hiPSC) (201B7) derived-neurosphere cells and hiPSC derived-differentiated neurons (Fig. 2A) to examine the expression of these genes during the differentiation processes of the human brain. Both SLC27A3 and SLC27A4 were expressed in hiPSC-derived neurosphere cells and in hiPSC-derived differentiated neurons. SLC27A3 was expressed with higher level in hiPSC-derived neurospheres than in hiPSC-derived differentiated neurons, whereas SLC27A4 was expressed with higher level in hiPSC-derived differentiated neurons than in hiPSC-derived neurospheres (Fig. 2B). We also confirmed that the SLC27A3 and SLC27A4 proteins were expressed in the hiPSC-derived neurospheres and neurons (Supplementary Figure S1).
Figure 2. SLC27A3 and SLC27A4 expression.
(A) Establishing human iPS cells (201B7), iPS cells-derived neurospheres, and iPS cells-derived neurons. iPS cells stained positive for the pluripotency marker TRA-1-81. Neurons expressed the neuronal marker β-tubulin. Scale bars: 200 μm. (B) Relative SLC27A3 and SLC27A4 expression levels in human iPS cells, standardized using the internal control GAPDH (Y-axis) by the standard curve method. The relevant parameters obtained were: SLC27A3 (slope = -3.21, Y-intercept = 29.23), GAPDH (slope = -3.38, Y-intercept = 23.01); SLC27A4 (slope = -3.27, Y-intercept = 28.10), GAPDH (slope = −3.39, Y-intercept = 23.07). Error bars indicate mean ± SE’s. P values were determined by the Mann Whitney test. (C) Immunofluorescent labeling of Slc27a3 and Slc27a4 and CD31 (endothelial cell marker) in mouse fetal brain (E18.5). DAPI, 4',6-diamidino-2-phenylindole, was used to stain nuclei. Lower panels are magnified images of white dotted squares in upper panels. Scale bars (low magnification): 100 μm, Scale bars (high magnification): 10 μm.
In the sequencing analysis, we found that the iPSC clone does not have any mutations in the SLC27A3 gene and has only one SLC27A4 heterozygous missense mutation (Asn351Ser: rs111417655). This mutation is predicted to be benign by “PolyPhen2”17, PANTHER 9.0”18,19, “Mutation Assessor release 2”20,21 and “PMut”22 (see below for these algorithms). In addition, no relationship between this mutation and any diseases has been reported.
Furthermore, we assessed the Slc27a3 (Fatp3) and Slc27a4 (Fatp4) protein expression patterns in mouse fetal brain (E18.5). Slc27a3 and Slc27a4 proteins were expressed in both endothelial cells (CD31 positive cells) and cortical cells (CD31 negative cortical cells) in the mouse brain (Fig. 2C). The expressions of SLC27A3 (FATP3) and SLC27A4 (FATP4) in the brain are represented in the Human Protein Atlas database (http://www.proteinatlas.org/). These results suggest that fatty acids, including PUFA, may be taken up into endothelial cells and cortical cells through SLC27A3 (Slc27a3) and SLC27A4 (Slc27a4) during brain development.
Identifying SLC27A3 and SLC27A4 gene polymorphisms in ASD and control samples
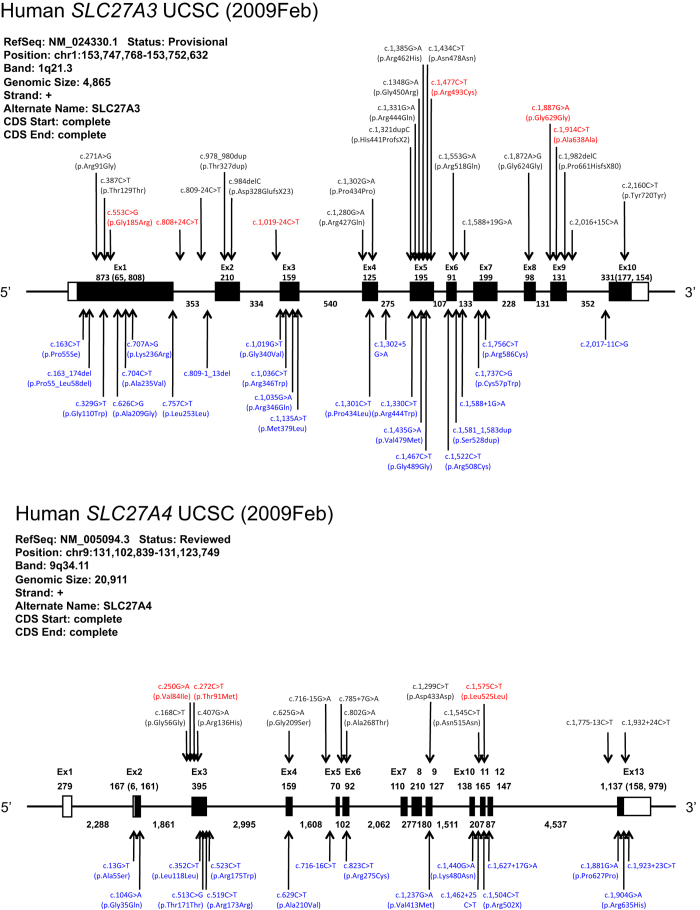
Because PUFA uptake into the fetal brain is necessary for brain development, genetic variations of the two SLC27A genes encoding fatty acid transporter proteins may modify the risk for neurodevelopmental disorders, including ASD. We first performed a resequencing analysis for the SLC27A3 and SLC27A4 genes in 267 ASD samples. Polymorphism screening detected a total of 24 and 13 different variants of SLC27A3 and SLC27A4, respectively (Table 1, Fig. 3). For the SLC27A3 gene, 21 variants were novel; additionally, 7 were synonymous and 12 were nonsynonymous. For the SLC27A4 gene, 3 were novel, 4 were synonymous and 5 were nonsynonymous (Table 1, Fig. 3).
Table 1. Polymorphisms identified in the SLC27A3 and SLC27A4 genes using 267 ASD samples.
| Gene | Nucleotidechange | Amino acid change | *dbSNP ID | Minor allelehomo/hetero/majorallele homo | **MAF | ***HWE | ****De novo |
|---|---|---|---|---|---|---|---|
| SLC27A3 | c.271 A>G | p.Arg91Gly | New (rs138225868) | 0/1/261 | 0.2% | 0.98 | No |
| c.387 C>T | Synonymous | rs36064263 | 0/2/265 | 0.4% | 0.95 | No | |
| c.553 G>C | p.Gly185Arg | New (rs147251588) | 0/1/260 | 0.2% | 0.98 | No | |
| c.808+24 C>T | — | New (ss1399952609) | 0/1/265 | 0.2% | 0.98 | No | |
| c.809−24 C>T | — | New (ss1399952610) | 0/1/266 | 0.2% | 0.98 | No | |
| c.980_981 dup | p.Thr327dup | New (rs149047357) | 0/2/260 | 0.4% | 0.95 | No | |
| c.984 delC | p.Asp328GlufsX23 | New (rs143078987) | 0/4/258 | 0.8% | 0.90 | No | |
| c.1,019−24 C>T | — | New (ss1399952611) | 0/1/266 | 0.2% | 0.98 | No | |
| c.1,280 G>A | p.Arg427Gln | rs77673307 | 0/13/249 | 2.7% | 0.05 | No | |
| c.1,302 G>A | Synonymous | New (rs139037399) | 0/1/266 | 0.2% | 0.98 | No | |
| c.1,321 dupC | p.His441ProfsX2, p.Lys442Stop | New (rs144727289) | 0/1/261 | 0.2% | 0.98 | No | |
| c.1,331 G>A | p.Arg444Gln | New (rs141932545) | 0/1/261 | 0.2% | 0.98 | No | |
| c.1,348 G>A | p.Gly450Arg | New (rs146128753) | 0/11/251 | 2.1% | 0.73 | No | |
| c.1,385 G>A | p.Arg462His | New (rs143908472) | 0/5/257 | 1.0% | 0.88 | No | |
| c.1,434 C>T | Synonymous | New (rs146407808) | 0/2/265 | 0.4% | 0.95 | No | |
| c.1,477 C>T | p.Arg493Cys | New (rs140637267) | 0/1/261 | 0.2% | 0.98 | No? | |
| c.1,553 G>A | p.Arg518Gln | New (rs142414300) | 0/3/259 | 0.6% | 0.93 | No | |
| c.1,588+19 G>A | — | New (ss1399952612) | 1/0/265 | 0.4% | 0.00 | No? | |
| c.1,872 A>G | Synonymous | rs80014940 | 0/1/264 | 0.2% | 0.98 | No? | |
| c.1,887 G>A | Synonymous | New (rs138193292) | 0/1/264 | 0.2% | 0.98 | No | |
| c.1,914 C>T | Synonymous | New (rs146109147) | 0/1/264 | 0.2% | 0.98 | No | |
| c.1,982 delC | p.Pro661HisfsX80 | New (rs142189417) | 0/4/256 | 0.8% | 0.90 | No | |
| c.2,016+15 C>A | — | New (ss1399952613) | 0/2/263 | 0.4% | 0.95 | No | |
| c.2,160 C>T | Synonymous | New (rs147511607) | 0/4/261 | 0.8% | 0.90 | No | |
| SLC27A4 | c.168 C>T | Synonymous | rs181020996 | 0/3/264 | 0.6% | 0.93 | — |
| c.250 G>A | p.Val84Ile | New (ss1399952594) | 0/1/266 | 0.2% | 0.98 | No | |
| c.272 C>T | p.Thr91Met | rs138443340 | 0/1/266 | 0.2% | 0.98 | No | |
| c.407 G>A | p.Arg136His | rs148684713 | 0/3/264 | 0.6% | 0.93 | No | |
| c.625 G>A | p.Gly209Ser | rs2240953 | 15/87/165 | 21.9% | 0.43 | No | |
| c.716−15 G>A | — | rs17848327 | 2/68/197 | 13.5% | 0.13 | — | |
| c.785+7 G>A | — | rs17848328 | 0/13/254 | 2.4% | 0.68 | — | |
| c.802 G>A | p.Ala268Thr | rs17848330 | 0/2/265 | 0.4% | 0.95 | No | |
| c.1,299 C>T | Synonymous | rs78415617 | 0/2/265 | 0.4% | 0.95 | — | |
| c.1,545 C>T | Synonymous | rs2240952 | 0/8/259 | 1.5% | 0.80 | — | |
| c.1,575 C>T | Synonymous | New (ss1399952595) | 0/1/266 | 0.2% | 0.98 | — | |
| c.1,775−13 C>T | — | New (ss1399952596) | 0/4/263 | 0.7% | 0.90 | — | |
| c.1,932+24 G>A | — | rs138008274 | 0/2/265 | 0.4% | 0.95 | — |
*The NCBI database (http://www.ncbi.nlm.nih.gov/SNP/) was searched for known SNPs.
**MAF: minor allele frequency.
***HWE: Hardy-Weinberg equilibrium.
****267 pedigrees (201 complete trios and 66 incomplete trios) were examined. “No?” means that one of parents was not available.
Figure 3. Genomic structures and polymorphic sites in the SLC27A3 and SLC27A4 genes.
Exons are denoted as boxes, with coding regions in black and 5′-/3′-untranslated regions in white. The sizes (base pairs) of each exon and intron are also shown. Red: SNPs that were found only in ASD samples. Black: SNPs that were found in both ASD and control samples. Blue: SNPs that were found only in control samples.
We then resequenced 1140 control samples, from which 40 variants were detected exclusively in control samples; 23 for SLC27A3 and 17 for SLC27A4 (Fig. 3, Supplementary Table S1). All of these variants of SLC27A3 were novel. Two and 17 variants were synonymous and nonsynonymous, respectively. Regarding SLC27A4, 14 were novel, and 4 and 9 variants were synonymous and nonsynonymous, respectively (Fig. 3, Supplementary Table S1).
All together, we found 47 variants of SLC27A3 and 30 variants of SLC27A4. The data showed that these two genes are highly polymorphic in nature with most of the variants being rare (minor allele frequency <1%). Since we identified a large number of variants, especially in the SLC27A3 gene, we suspected the presence of duplications in this gene locus. We performed genomic quantitative PCR for the SLC27A3 genomic interval to determine whether the SLC27A3 gene had any copy number variations. We found no evidence of duplications (or deletions) in the genomic region that spanned the SLC27A3 gene (Supplementary Table S2). In addition, there was no evidence that the variants detected for the SLC27A3 and SLC27A4 genes were de novo mutations, after the examination of sequences of available parental genomes (Table 1).
SLC27A3 and SLC27A4 genetic association studies
We focused on the nonsynonymous variants of SLC27A3 and SLC27A4 detected in ASD samples, for their possible associations with ASD because of their potential functional importance. Because the incidence of ASD is sex-dependent (more common in males than in females), we performed association analyses separately for males and females (Table 2). SLC27A3 c.1,982delC (p.Pro661HisfsX80) was significantly less frequent among male ASD patients as compared with male control samples. However, this significant result was not maintained after correcting for multiple testing (n = 12 × 2). SLC27A4 c.625G>A (p.Gly209Ser) was significantly less frequent among male ASD patients and was significantly more frequent among female ASD patients as compared with control samples. The significant result found for males was not maintained after correcting for multiple testing (n = 5 × 2). In contrast, the significant result for females was maintained after correcting for multiple testing (n = 5 × 2) (Table 2). We confirmed the results using a larger number of control samples (n = 2270) (Supplementary Table S3). To investigate whether there is the effect of background mutations, we performed copy number variation (CNV) analysis for 16p11.2 and 15q11.2, which are reported as the two most prevalent CNVs in ASD23. Two ASD patients had the 15q11.2 duplication and one ASD patient had the16p11.2 duplication. But none of SLC27A4 p.Ser209 carriers had those CNVs. We compared the Autism Diagnostic Interview-Revised (ADI-R) scores among genotypes (G209/G209, G209/S209, S209/S209) of the SLC27A4 variant in female ASD patients. There was no significant genotype-dependency on the phenotypes (Supplementary Table S4). When both male and female samples were combined, none of the variants fulfilled the criteria for statistical significance (data not shown).
Table 2. Association analysis results.
| Gene | Missense Variants | Sample | Male |
Female |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Allele frequency | *P-value | N | Allele frequency | *P-value | |||||
| SLC27A3 | p.Arg91Gly (A/G) | A | G | A | G | |||||
| Control | 426 | 851 | 1 | 1.0000 | 682 | 1364 | 0 | 1.0000 | ||
| ASD | 221 | 441 | 1 | 41 | 82 | 0 | ||||
| p.Gly185Arg (C/G) | C | G | C | G | ||||||
| Control | 430 | 860 | 0 | 1.0000 | 689 | 1378 | 0 | 0.0562 | ||
| ASD | 220 | 440 | 0 | 41 | 81 | 1 | ||||
| p.Thr327-Thr-Thr (InsAAC) | — | AAC | — | AAC | ||||||
| Control | 435 | 869 | 1 | 1.0000 | 688 | 1375 | 1 | 0.1094 | ||
| ASD | 221 | 441 | 1 | 41 | 81 | 1 | ||||
| p.Asp328- frameshift (C/−) | C | — | C | — | ||||||
| Control | 435 | 869 | 1 | 0.1140 | 688 | 1368 | 8 | 0.4069 | ||
| ASD | 221 | 439 | 3 | 41 | 81 | 1 | ||||
| p.Arg427Gln (G/A) | G | A | G | A | ||||||
| Control | 413 | 808 | 18 | 0.9712 | 642 | 1241 | 43 | 0.2046 | ||
| ASD | 221 | 433 | 9 | 41 | 77 | 5 | ||||
| p.His441Pro, Lys442Stop (−/C) | — | C | — | C | ||||||
| Control | 413 | 825 | 1 | 1.0000 | 642 | 1281 | 3 | 1.0000 | ||
| ASD | 221 | 441 | 1 | 41 | 82 | 0 | ||||
| p.Arg444Gln (G/A) | G | A | G | A | ||||||
| Control | 413 | 819 | 7 | 0.2741 | 642 | 1281 | 3 | 1.0000 | ||
| ASD | 221 | 441 | 1 | 41 | 82 | 0 | ||||
| p.Gly450Arg (G/A) | G | A | G | A | ||||||
| Control | 413 | 812 | 14 | 0.6238 | 642 | 1270 | 14 | 0.6068 | ||
| ASD | 221 | 432 | 10 | 41 | 81 | 1 | ||||
| p.Arg462His (G/A) | G | A | G | A | ||||||
| Control | 413 | 824 | 2 | 0.0542 | 642 | 1279 | 5 | 1.0000 | ||
| ASD | 221 | 437 | 5 | 41 | 82 | 0 | ||||
| p.Arg493Cys (C/T) | C | T | C | T | ||||||
| Control | 413 | 826 | 0 | 0.3486 | 642 | 1284 | 0 | 1.0000 | ||
| ASD | 221 | 441 | 1 | 41 | 82 | 0 | ||||
| p.Arg518Gln (G/A) | G | A | G | A | ||||||
| Control | 432 | 858 | 6 | 1.0000 | 689 | 1372 | 6 | 1.0000 | ||
| ASD | 221 | 439 | 3 | 41 | 82 | 0 | ||||
| p.Pro661-frameshift (C/−) | C | — | C | — | ||||||
| Control | 371 | 724 | 18 | 0.0378 | 612 | 1201 | 23 | 1.0000 | ||
| ASD | 219 | 435 | 3 | 41 | 81 | 1 | ||||
| SLC27A4 | Val84Ile (G/A) | G | A | G | A | |||||
| Control | 440 | 880 | 0 | 0.3373 | 700 | 1400 | 0 | 1.0000 | ||
| ASD | 224 | 447 | 1 | 43 | 86 | 0 | ||||
| p.Thr91Met (C/T) | C | T | C | T | ||||||
| Control | 440 | 880 | 0 | 0.3373 | 700 | 1400 | 0 | 1.0000 | ||
| ASD | 224 | 447 | 1 | 43 | 86 | 0 | ||||
| p.Arg136His (G/A) | G | A | G | A | ||||||
| Control | 440 | 876 | 4 | 1.0000 | 700 | 1392 | 8 | 0.4161 | ||
| ASD | 224 | 446 | 2 | 43 | 85 | 1 | ||||
| p.Gly209Ser (G/A) | G | A | G | A | ||||||
| Control | 440 | 649 | 231 | 0.0054 | 700 | 1097 | 303 | 0.0030 | ||
| ASD | 224 | 362 | 86 | 43 | 55 | 31 | ||||
| p.Ala268Thr (G/A) | G | A | G | A | ||||||
| Control | 440 | 875 | 5 | 1.0000 | 700 | 1393 | 7 | 1.0000 | ||
| ASD | 224 | 446 | 2 | 43 | 86 | 0 | ||||
*Fisher’s exact test.
Four rare nonsynonymous variants, SLC27A3 c.553G>A (p.Gly185Arg), c.1,477C>T (p.Arg493Cys), SLC27A4 c.250G>A (p.Val84Ile), and c.272C>T (p.Thr91Met) were exclusively found in ASD patients (Table 1, Table 2), which suggested a possible role for these genes in the pathogenesis of ASD although statistical assessment was not feasible in the current study due to small sample size.
Maternally transmitted effects of SLC27A3 and SLC27A4 alleles
SLC27A3 and SLC27A4 are expressed in the mouse fetal brain13,14. In addition, we confirmed that they are expressed in human placenta tissues using RT-PCR (Supplementary Figure S2). Since the placenta consists of tissues derived from both the mother and the fetus, and plays a key role in fetal nutrition, we speculated that the risk of developing ASD among children with functional mutations in the SLC27A genes may increase, when their mothers also harbored the same nonsynonymous mutations. To test this, we investigated the effect of maternal transmission by using 201 patient-parent complete trio and 66 incomplete trio samples, and performing TDT against 12 variants of SLC27A3 and 5 variants of SLC27A4. A significant transmission disequilibrium for the variant Arg462His in SLC27A3 was observed (P = 0.0455); however, this significance did not withstand correction for multiple testing (n = 12) (Supplementary Table S5).
Fatty acid uptake analysis for SLC27A4 Gly209 and Ser209
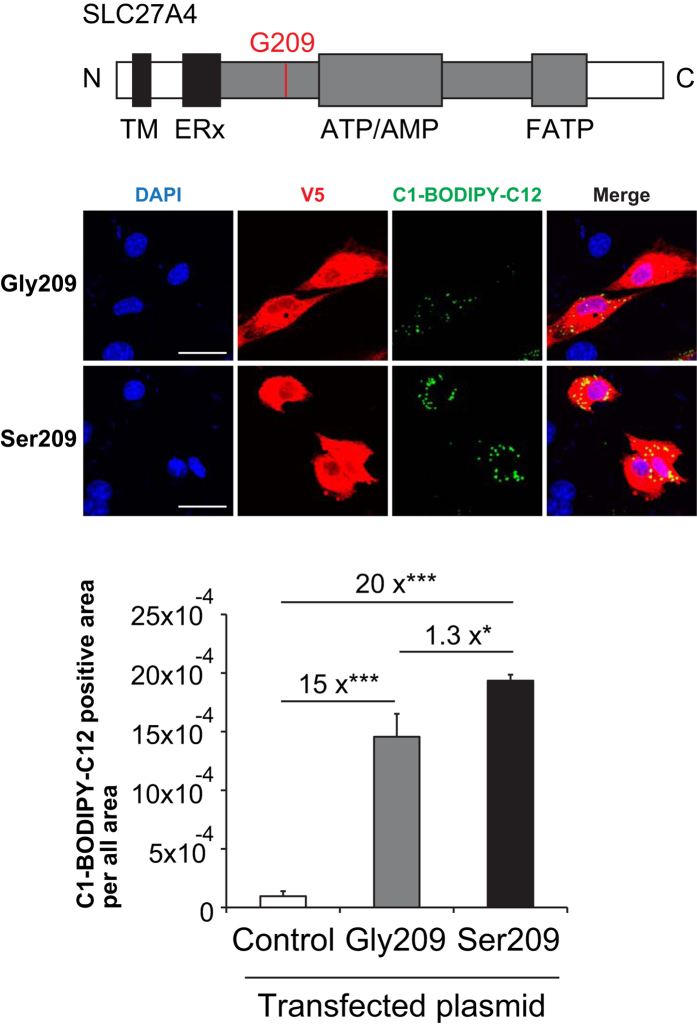
The SLC27A4 p.Ser209 was the only variant that showed an empirical association with ASD in the current genetic study (Table 2), and it is known to be genetically associated with insulin resistance syndrome24. Insulin abnormality has been well discussed in ASD25. Therefore, we focused on the SLC27A4 Gly209Ser substitution in terms of functional consequence and examined whether it affected the accumulation of long chain fatty acids (LCFAs) in cells (Fig. 4A). We transfected the endothelial cell line bEnd3 with expression plasmids encoding either SLC27A4 Gly209, Ser209, or a control construct, and measured the cellular uptake of a 4,4-difluoro-5-methyl-4-bora-3a, which is a 4a-diaza-s-indacene-3-dodecanoic acid-labeled saturated LCFA analogue (C1-BODIPY-C12). We used bEnd3 cells, mouse brain capillary-derived endothelial cells, to investigate the mechanism of Slc27a4, because these cells express only Slc27a3 and Slc27a4 and not other members of the Slc27a family. Additionally, the contribution of Slc27a3 to the fatty acid uptake is small12. SLC27A4 Gly209 transfection facilitated C1-BODIPY-C12 uptake into these cells, which formed bright, dot-like structures (Fig. 4B). Strikingly, SLC27A4 Ser209 more potently induced C1-BODIPY-C12 uptake than SLC27A4 Gly209 (Fig. 4B,C). These results suggested that the SLC27A4 substitution may induce functional alterations with respect to fatty acid uptake in the fetal brain and may have a role in ASD pathophysiology.
Figure 4. Fatty acid uptake analysis for SLC27A4 (p.Gly209Ser).
(A) Schematic of the mutant SLC27A4 protein. Red lines indicate the position of a missense mutation. Gray indicated the AMP binding domain. Abbreviation: TM = N-terminal transmembrane region; ERx = ER localization signal; ATP/AMP = ATP/AMP motif involved in ATP binding and adenylate formation; FATP = conserved FATP motif of importance for fatty acid binding. (B) Subcellular localization of C1-BODIPY-C12 and V5-tagged SLC27A4 in bEnd3 cells after transfection with different plasmids. Control cells were transfected with the vector containing inverted SLC27A4 cDNA. Nuclei were stained blue using DAPI. Scale bars: 30 μm (C) C1-BODIPY-C12 uptake into bEnd3 cells transfected with different plasmids. Values are mean + SE. Statistical comparisons were made by one-way ANOVA, followed by Bonferroni’s post hoc multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Abnormal fatty acid metabolism has been suggested to play a crucial role in ASD pathophysiology7. In our previous studies we found that the cytosolic fatty acid binding genes FABP7, FABP5, and FABP3 were associated with ASD24,25. It is also interesting to note that Fabp7 is abundantly expressed in neural progenitor cells during early brain development in mice26. We have demonstrated multiple psychiatric illnesses-related phenotypes in Fabp7 knockout mice (e.g., reduced prepulse inhibition)27. In addition to FABPs (Fabps), cell-surface fatty acid transport proteins probably play pivotal roles in brain development and neurodevelopmental dysfunction in humans. Thus, in the present study, we examined genetic polymorphisms and possible genetic associations of the two SLC27A genes (SLC27A3 and SLC27A4) that are expressed in the fetal brain with ASD.
One of the limitations of the current genetic study is that the sample size is rather small. Bearing this in mind, our results showed that SLC27A4 p.Gly209Ser was significantly more frequent in ASD patients and that this substitution caused increased fatty acid uptake into bEnd3 cells in vitro. SLC27A4 Ser209 is a known variant that is associated with insulin resistance syndrome20. It was recently suggested that metabolic syndrome, including obesity, diabetes, and hypertension, in pregnant women may increase the risk of autism among their children28. Also insulin abnormality has been well discussed in ASD20. Thus, our findings of an association between the SLC27A4 Ser209 allele and ASD may link metabolic syndrome to ASD at the molecular level.
It remains unknown how the SLC27A4 p.Gly209Ser substitution increases the intrinsic transporter activity of a protein. One possibility is that this substitution affects CoA synthase activity that is coupled to transporter activity. Indeed, the Gly209 residue is located in the AMP binding domain (Fig. 4A), which is important for transport and the activation of fatty acids29,30. According to the structural modeling analysis (Supplementary Methods, Supplementary Figures S3 and S4), the substituted site is predicted to be located at the surface of the protein (Supplementary Figure S5), suggesting that it may affect the interactions with other molecules, e.g., transporters forming homo- and/or hetero-dimers31, although this remains to be experimentally validated. Moreover, C1-BODIPY-C12 was more efficiently transported by SLC27A4 Ser209 into bEnd3 cells than by SLC27A4 Gly209 (Fig. 4B,C). This amino acid substitution could disturb the balance of fatty acid supply in the brain during the developmental stage, which could disrupt fine-tuning during brain development.
Interestingly, an association between the SLC27A4 p.Gly209Ser substitution and ASD was detected only in samples from females. In the fetal brain, the estrogen supply from the peripheral circulation is blocked by α-fetoprotein in the blood. However, as compared to females, males produce significantly more testosterone32,33, which triggers a reaction cascade that culminates in the masculinization of genital tissues and of the developing nervous system. Testosterone may be converted into other sex hormones, including estrogen. Estrogen synthesis is catalyzed by the converting enzyme aromatase34. Once converted and generated by aromatase, estrogen binds to its receptors. We found one consensus sequence for an estrogen receptor-binding site in the promoter region of SLC27A4 by using gene regulation analysis software, TRANSFAC Professional (https://portal.biobase-international.com/cgi-bin/portal/login.cgi). Estrogen produced by the actions of aromatase may promote SLC27A4 expression in males. Therefore, the differences in balance of sex hormone levels between males and females during a critical period of brain development may underlie the different effects of the SLC27A4 polymorphism for the risk of ASD. In addition to the p.Gly209Ser mutation, a excess of potentially functional variants (missense and ins/del) of SLC27A4 was observed in the female ASD group (Supplementary Table S6). Additionally, an excess of potentially functional variants of both SLC27A3 and SLC27A4 was observed in the female ASD patients (Supplementary Table S7). The mechanistic evaluation of other variants besides the p.Gly209Ser of SLC27A4 would be necessary.
In invertebrates, it is known that polymorphisms are frequently found for proteins involved in cell membrane structure and composition35. In the present study, we observed this highly polymorphic nature for the SLC27A3 and SLC27A4 genes, which encode fatty acid transporters in the plasma membrane. There are other examples of genes that are highly polymorphic and are reported to be associated with diseases like the SLC274A36,37,38.
To conclude, in the present study we found that: (1) the SLC27A3 and SLC27A4 genes encoding fatty acid transporters, which potentially have important roles in the developing brain, were highly polymorphic; (2) SLC27A4 p.Gly209Ser was associated with ASD although a caveat the small sample size of this study; and (3) this variant had functional consequences with regard to controlling endothelial long-chain fatty acid transport. Further studies with larger sample sizes and more extensive functional assessments including a comparison of fatty acid uptake activity between SLC27A4 G209 and Ser209-harboring hiPSCs and acyl-CoA ligase activity for long-chain fatty acids will be needed to determine the precise roles of SLC27A3 and SLC27A4 in ASD pathogenesis.
Methods
Human iPS cell culture
Human-induced pluripotent stem cells (hiPSCs) (201B7: provided by Shinya Yamanaka, M.D., Ph.D., Kyoto University)39 were cultured in standard hiPSC culture medium of DMEM/F12 (Wako, Osaka, Japan) supplemented with 20% Knockout serum replacement (Life Technologies, Carlsbad, CA), 2 mM L-glutamine (Sigma-Aldrich, St Louis, Missouri), 1:100 diluted nonessential amino acids (Sigma-Aldrich), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), and 5 ng/ml of basic fibroblast growth factor (bFGF) (PeproTech, Rocky Hill, New Jersey). For induction of neurospheres from hiPSCs, hiPSCs were incubated with TrypLETM Select (Life Technologies) and dissociated into single cells by pipetting. Cells were plated into a T75 flask and cultured in medium hormone mix (MHM) supplemented with B27 (Life Technologies), 20 ng/mL of bFGF, 10 μM Y-27632 (Wako), and 10 ng/mL of human leukemia inhibitory factor (hLIF) (Millipore, Darmstadt, Germany) in 4% oxygen for 14 days40. For neural differentiation, dissociated neurospheres were allowed to adhere to poly-L-ornithine (Sigma-Aldrich) and fibronectin (Sigma-Aldrich) coated dishes and cultured in MHM that contained B27 but without bFGF and LIF for 10 days (Fig. 2A,B) and 14 days (Supplementary Figure S1)41.
Real-time quantitative RT-PCR
Total RNA was extracted from hiPSC derived neurosphere cells and hiPSC derived-differentiated neurons using a miRNeasy Mini Kit (Qiagen, Venlo, Netherlands). Single stranded cDNA was synthesized using SuperScript VILO Master Mix (Invitrogen, Carlsbad, CA). mRNA expression levels were determined by real-time quantitative PCR by using TaqMan Gene Expression Master Mix, transcript-specific minor groove binding (MGB) probes (Applied Biosystems, Foster City, CA) (GAPDH: Hs02758991_g1, SLC27A3: Hs00950760_g1, SLC27A4: Hs00192700_m1), and an ABI 7900 sequence detection system, according to the manufacturer’s instructions. The GAPDH gene was used as an internal control (Applied Biosystems). A PCR assay was performed with test and standard samples simultaneously and with no template controls on the same plate. A standard curve was generated by plotting the cycle of threshold values against input quantity (log scale) for both the GAPDH gene and the target genes for each PCR assay. All real-time quantitative PCR data were acquired using ABI PRISM 7900 Sequence Detection System (SDS) v2.4 (Applied Biosystems). The expression level of a target gene relative to that of the GAPDH gene (target gene/GAPDH gene) was determined.
Immunohistochemistry and Immunocytochemistry
C57BL/6NCrlCrlj (B6) inbred mouse strains were obtained from Japan’s Charles River Laboratories. Mice were housed in groups of four in standard cages in a temperature and humidity-controlled room with a 12-h light/dark cycle (lights on at 08:00) and provided free access to standard lab chow and tap water. All experiments were performed between 10:00 and 14:00. Our experimental procedures were approved by the RIKEN Animal Ethics Committee. The animal experiments were carried out in "accordance" with the approved guidelines. For immunohistochemical studies, at least five mice at E18.5 were examined. Mice were deeply anesthetized with sodium pentobarbital and then transcardially perfused with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS). Brains were removed and further immersion-fixed in the same fixative at 4 °C for 16 h. Coronal sections (14 μm thick) were prepared using a cryostat (CM3050, Leica, Germany). These sections were washed with Tris-buffered saline that contained Tween 20 (TBST; pH 7.4). For immunostaining, cryostat sections were incubated with primary antibodies at 4 °C for 18 h. To detect antigen localization, sections were incubated with appropriate secondary antibodies at 4 °C for 2 h. For immunocytochemical studies, hiPS cells, hiPS-derived neurospheres and hiPS-derived neurons were used. They were fixed with 4% paraformaldehyde in 0.01 M PBS at room temperature (RT) for 20 min, and washed with TBST followed by incubated with primary antibodies at RT for 1 h. After that, they were incubated with appropriate secondary antibodies at RT for 1 h. Information on the primary and secondary antibodies and other reagents are given in Supplementary Table S8. Fluorescent signals were detected using a confocal laser-scanning microscope (FV1000, OLYMPUS, Japan).
Human subjects
For resequencing and association analyses of all the protein-coding exons of the SLC27A3 and SLC27A4 genes, we evaluated 267 Japanese ASD patients (225 men, 42 women; mean age: 11.91 ± 5.20 years) and 1140 Japanese control subjects (440 men, 700 women; mean age; 44.10 ± 13.63 years). In the analysis of SLC27A4 p. Gly209Ser, we examined an expanded control sample set (total 2270: 889 men, 1281 women; mean age: 42.40 ± 14.22 years). For the transmission disequilibrium test and de novo mutation analysis, 201 patient-parents trio samples (603 samples) and 267 pedigree samples that included 66 incomplete trios (only single parents were available) were examined, respectively. ASD patients from pedigrees were the same as those used for resequencing and case-control association analyses. All of our study subjects resided in central Japan. A diagnosis of ASD was made using DSM-IV and Interview-Revised (ADI-R) criteria42 based on a consensus by at least two experienced psychiatrists. Control subjects were recruited from among hospital staff and volunteers who had no present or previous evidence of psychoses during brief interviews conducted by psychiatrists. Written informed consent was obtained from all participants after explaining our study protocols and purposes. This study was approved by the Ethics Committees of RIKEN and Hamamatsu University School of Medicine and was conducted according to the principles of the Declaration of Helsinki (http://www.wma.net).
Resequencing analyses of SLC27A3 and SLC27A4
Genomic DNA was isolated from blood samples obtained from our human subjects using standard methods. All the coding exons and exon/intron boundaries of the SLC27A3 or SLC27A4 genes were screened for polymorphisms by direct sequencing of polymerase chain reaction (PCR) products. The primers used for amplification are listed in Supplementary Table S9. PCR was performed with an initial denaturation at 95 °C for 10 min, followed by 35 cycles at 95 °C for 15 sec, 61–61.5 °C (optimized for each primer pair) for 15 sec, 72 °C for 30 sec, and a final extension at 72 °C for 10 min, with AmpliTaqGold (Applied Biosystems). Direct sequencing of PCR products was performed with a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) and an ABI PRISM 3730xl Genetic Analyzer (Applied Biosystems). Polymorphisms were detected using the SEQUENCHER program (Gene Codes Corporation, Ann Arbor, MI). The genomic structures of SLC27A3 (RefSeq: NM_024330.1) and SLC27A4 (RefSeq: NM_005094.3) were based on the UCSC February 2009 draft assembly of the human genome database (http://www.genome.ucsc.edu), and the NCBI database (http://www.ncbi.nlm.nih.gov/) was searched for known single nucleotide polymorphisms (SNPs). Custom TaqMan SNP Genotyping Assays (Applied Biosystems) were used to score the identified missense SNPs using the TaqMan assay method43, along with an ABI PRISM 7900 and SDS v2.4 software (Applied Biosystems).
Genomic quantitative PCR
Insertions/deletions within the genomic length of SLC27A3 were analyzed by real-time genomic quantitative PCR for exons 1, 4, and 10 using the TaqMan method (Applied Biosystems). MLC1 at chromosome 22q13.33 was used as a normal copy number control gene. For quality control, PFKFB1 on chromosome Xp11.21 was used to determine whether our genomic quantitative PCR accurately detected differential dosages of the X chromosome between male and female control samples. For the analysis of CNVs in the 16p11.2 and 15q11.2 regions, the RNaseP gene was used as a normal copy number control gene. No copy number polymorphisms have been documented in the MLC1, PFKFB1 and RNaseP genes within the Japanese population. For genomic quantitative PCR, DNA solutions were first quantified using an ultraviolet spectrophotometer and further quantified using a TaqMan RNase P Detection Reagent kit (Applied Biosystems). Primers sequences used for these analyses are listed in Supplementary Table S10.
Semi-quantitative RT-PCR
We performed semi-quantitative analysis of SLC27A3 and SLC27A4 mRNA expression using Human Placenta Marathon-Ready cDNA (Clontech, CA, USA). The primers used for amplification are listed in Supplementary Table S11. PCR was performed with an initial denaturation at 95 °C for 10 min, followed by 35 cycles at 95 °C for 30 sec, 61 °C for 30 sec, 72 °C for 1 min, and a final extension at 72 °C for 10 min, with AmpliTaqGold (Applied Biosystems). GAPDH was used for quality control. The PCR products were separated on an agarose gel.
Fatty acid uptake analysis with SLC27A4 p.Gly209Ser
The mouse endothelial cell line bEnd3 (ATCC, Manassas, VA) was cultured in high glucose DMEM (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 100 U/ml of penicillin, and 100 U/ml of streptomycin. Human SLC27A4 cDNA that covered the open reading frame was generated by PCR using cDNA derived from established lymphoid cell lines of ASD children who were heterozygous for wild-type (Gly209) and mutant (Ser209) SLC27A4 alleles and the following primer set; SLC27A4 forward: 5′-ATGCTGCTTGGAGCCTCTCTGG-3′, SLC27A4 reverse: 5′-CAGCTTCTCCTCGCCTGCCT-3′. Each amplified cDNA was cloned into the mammalian expression vector pcDNA3.1/V5-His-TOPO (Life Technologies), which is driven by the CMV promoter and enables TA-cloning of the PCR products and adding V5 and His tags (epitopes) to the C-termini of expressed proteins. Because the vector is supplied as a linearized TA cloning vector, it is very difficult to obtain an empty vector. Therefore, we used the inverted SLC27A4 cDNA-containing construct as a control, which was obtained in a single transformation along with the construct containing the SLC27A4 cDNA in the normal direction. The inverted cDNA is predicted to encode a very short peptide due to the early termination codon, suggesting that no functional or biologically active protein can be produced. Plasmid structures were verified by sequencing. Cells were seeded in 8-well chamber slides (Nalge Nunc, Penfield, NY) and cultured as described above using growth medium that contained 1% fatty acid-free bovine serum albumin (FF-BSA) and no fetal calf serum. bEnd3 cells were transfected with human SLC27A4-Gly209-pcDNA3.1/V5-His-TOPO and human SLC27A4-Ser209-pcDNA3.1/V5-His-TOPO simultaneously or with the control construct using a Lipofectamine LTX transfection system together with Plus-reagent (both from Life Technologies), according to the manufacturer’s instructions. At 24 h after transfection, cells were washed with PBS-1% FF-BSA and incubated at 37 °C for 3 min with PBS that contained 1% FF-BSA and 20 μM C1-BODIPY-C12 (Sigma-Aldrich). Cells were washed vigorously, and fixed with 4% paraformaldehyde at room temperature (RT) for 10 min. After fixation, cells were first incubated with 0.2% Triton X-100 in PBS (PBST) at RT for 5 min and then incubated with 1% blocking reagent (Roche, Basel, Switzerland) in PBST at RT for 30 min12.
For immunocytochemistry, cells were incubated with primary antibodies (Supplementary Table S9) at RT for 1 h. Slides were then washed three times (5 min each) with PBS at RT. To detect antigen localization, cells were incubated with appropriate secondary antibodies (Supplementary Table S9) at RT for 1 hour. Cells were analyzed using an all-in-one fluorescence microscope, BZ-8000 (KEYENCE, Osaka, Japan). For each experiment, two or three wells were photographed diagonally using a 20× objective and equal exposure times. All frames (n > 30) were used for computer-assisted quantification of green fluorescent pixels using ImageJ software (National Institute of Health, Bethesda, Maryland: http://imagej.nih.gov/ij/). These experiments were repeated at least three times12.
Statistical Analyses
P values for gene expression levels in hiPSC-derived neurospheres and neurons were determined using a Mann Whitney test. P values for our genetic association analysis were determined by Fisher’s exact test. A transmission disequilibrium test (TDT) was performed using PLINK v1.07 software (http://pngu.mgh.harvard.edu/~purcell/plink/). P values for fatty acid uptake analysis were determined using Bonferroni’s multiple comparisons test. P < 0.05 was considered significant.
Additional Information
How to cite this article: Maekawa, M. et al. Investigation of the fatty acid transporter-encoding genes SLC27A3 and SLC27A4 in autism. Sci. Rep. 5, 16239; doi: 10.1038/srep16239 (2015).
Supplementary Material
Acknowledgments
We thank Dr. Naruya Saito (Division of Population Genetics, National Institute of Genetics, Mishima, Japan) for helpful discussions, Dr. Kazuo Yamada (RIKEN Brain Science Institute, Saitama, Japan) for his help with statistical analysis, and Dr. Kenji J Tsuchiya (Department of Psychiatry and Neurology, Hamamatsu University School of Medicine, Shizuoka, Japan) for his help with clinical evaluations. We also thank the Research Resource Center of the RIKEN Brain Science Institute (BSI) for sequencing support. This study was supported, in part, by grants-in-aid for Scientific Research and by a grant-in-aid for Scientific Research on Innovative Areas (TY) from the Japan Society for the Promotion of Science (JSPS), Japan. This study was also supported by RIKEN Brain Science Institute Funds (TY), and a part of this study was the result of the “Development of Biomarker Candidates for Social Behavior” (TY) and “Integrated Research on Neuropsychiatric Disorders” (NM) projects, carried out under the Strategic Research Program for Brain Sciences, and a grant “Platform for Drug Discovery, Informatics,and Structural Life Science” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Japan Agency for Medical Research and development (AMED) (MO and SF). This study was also supported by grants from the Mitsubishi Pharma Research Foundation (MM) and by the Program for Intractable Disease Research Utilizing Disease-specific iPS Cells funded by the Japan Science and Technology Agency (JST) and MEXT to HO.
Footnotes
H.O. is a paid scientific advisory board member and a founding scientist for SanBio Co., Ltd. Other authors have no competing interests to disclose.
Author Contributions M.M., Y.O. and T.Y.: conceived and designed the experiments; M.M., Y.I., M.T., Y.H. and N.K.: performed the experiments; M.M., C.S., M.O. and S.F.: analyzed data; M.M., T.O., T.T., S.B., H.M., Y.I., S.T., K.Y., Y.O., W.A., M.T., H.O. and N.M.: contributed reagents/materials/analysis tools; M.M.: wrote the first manuscript draft; M.M., T.O., S.B., M.O., S.F. and T.Y.: contributed to manuscript writing.
References
- Ozonoff S. et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 49, 256–66 e1-2 (2010). [PMC free article] [PubMed] [Google Scholar]
- Goldberg W. A. et al. Language and other regression: assessment and timing. J Autism Dev Disord 33, 607–16 (2003). [DOI] [PubMed] [Google Scholar]
- Kurita H. Infantile autism with speech loss before the age of thirty months. J Am Acad Child Psychiatry 24, 191–6 (1985). [DOI] [PubMed] [Google Scholar]
- Ming X. et al. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot Essent Fatty Acids 73, 379–84 (2005). [DOI] [PubMed] [Google Scholar]
- Chauhan A., Chauhan V., Brown W. T. & Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin--the antioxidant proteins. Life Sci 75, 2539–49 (2004). [DOI] [PubMed] [Google Scholar]
- James S. J. et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80, 1611–7 (2004). [DOI] [PubMed] [Google Scholar]
- Tamiji J. & Crawford D. A. The neurobiology of lipid metabolism in autism spectrum disorders. Neurosignals 18, 98–112 (2010). [DOI] [PubMed] [Google Scholar]
- Makrides M., Neumann M. A., Byard R. W., Simmer K. & Gibson R. A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60, 189–94 (1994). [DOI] [PubMed] [Google Scholar]
- Uauy R. & Dangour A. D. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev 64, S24–33; discussion S72-91 (2006). [DOI] [PubMed] [Google Scholar]
- Innis S. M. Essential fatty acids in growth and development. Prog Lipid Res 30, 39–103 (1991). [DOI] [PubMed] [Google Scholar]
- Georgieff M. K. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 85, 614S–620S (2007). [DOI] [PubMed] [Google Scholar]
- Hagberg C. E. et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917–21 (2010). [DOI] [PubMed] [Google Scholar]
- Pei Z. et al. Mouse very long-chain Acyl-CoA synthetase 3/fatty acid transport protein 3 catalyzes fatty acid activation but not fatty acid transport in MA-10 cells. J Biol Chem 279, 54454–62 (2004). [DOI] [PubMed] [Google Scholar]
- Fitscher B. A., Riedel H. D., Young K. C. & Stremmel W. Tissue distribution and cDNA cloning of a human fatty acid transport protein (hsFATP4). Biochim Biophys Acta 1443, 381–5 (1998). [DOI] [PubMed] [Google Scholar]
- Lager S. & Powell T. L. Regulation of nutrient transport across the placenta. J Pregnancy 2012, 179827 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima T., Miner J. H., Morizane M., Stahl A. & Sadovsky Y. The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PLoS One 6, e25865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I. A. et al. A method and server for predicting damaging missense mutations. Nat Methods 7, 248–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. D. et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13, 2129–41 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. D. & Kejariwal A. Coding single-nucleotide polymorphisms associated with complex vs. Mendelian disease: evolutionary evidence for differences in molecular effects. Proc Natl Acad Sci USA 101, 15398–403 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva B., Antipin Y. & Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res 39, e118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva B., Antipin Y. & Sander C. Determinants of protein function revealed by combinatorial entropy optimization. Genome Biol 8, R232 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Costa C. et al. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics 21, 3176–8 (2005). [DOI] [PubMed] [Google Scholar]
- Malhotra D. & Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell 148, 1223–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertow K. et al. Genetic and structural evaluation of fatty acid transport protein-4 in relation to markers of the insulin resistance syndrome. J Clin Endocrinol Metab 89, 392–9 (2004). [DOI] [PubMed] [Google Scholar]
- Stern M. Insulin signaling and autism. Front Endocrinol ( Lausanne) 2, 54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto C. et al. Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum Mol Genet 23, 6495–511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M. et al. Polymorphism screening of brain-expressed FABP7, 5 and 3 genes and association studies in autism and schizophrenia in Japanese subjects. J Hum Genet 55, 127–30 (2010). [DOI] [PubMed] [Google Scholar]
- Arai Y. et al. Role of Fabp7, a downstream gene of Pax6, in the maintenance of neuroepithelial cells during early embryonic development of the rat cortex. Journal of Neuroscience 25, 9752–61 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A. et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol 5, e297 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaczynski W. Translational research and behavioral sciences in developmental medicine: metabolic conditions of pregnancy versus autism spectrum disorders. Med Wieku Rozwoj 16, 171–4 (2012). [PubMed] [Google Scholar]
- DiRusso C. C., Darwis D., Obermeyer T. & Black P. N. Functional domains of the fatty acid transport proteins: studies using protein chimeras. Biochim Biophys Acta 1781, 135–43 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z., DiRusso C. C., Ctrnacta V. & Black P. N. Fatty acid transport in Saccharomyces cerevisiae. Directed mutagenesis of FAT1 distinguishes the biochemical activities associated with Fat1p. J Biol Chem 277, 31062–71 (2002). [DOI] [PubMed] [Google Scholar]
- Richards M. R. et al. Oligomerization of the murine fatty acid transport protein 1. J Biol Chem 278, 10477–83 (2003). [DOI] [PubMed] [Google Scholar]
- Finegan J. A., Bartleman B. & Wong P. Y. A window for the study of prenatal sex hormone influences on postnatal development. J Genet Psychol 150, 101–12 (1989). [DOI] [PubMed] [Google Scholar]
- de Zegher F., Devlieger H. & Veldhuis J. D. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res 32, 605–7 (1992). [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. S. et al. Inhibition of estrogen biosynthesis and its consequences on gonadotrophin secretion in the male. J Steroid Biochem Mol Biol 41, 437–43 (1992). [DOI] [PubMed] [Google Scholar]
- Volkman S. K. et al. Excess polymorphisms in genes for membrane proteins in Plasmodium falciparum. Science 298, 216–8 (2002). [DOI] [PubMed] [Google Scholar]
- Cohen J. C. et al. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA 103, 1810–5 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. et al. Excess of rare variants in genes that are key epigenetic regulators of spermatogenesis in the patients with non-obstructive azoospermia. Sci Rep 5, 8785 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S. et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol Psychiatry 14, 6–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–72 (2007). [DOI] [PubMed] [Google Scholar]
- Maekawa M. et al. Utility of Scalp Hair Follicles as a Novel Source of Biomarker Genes for Psychiatric Illnesses. Biol Psychiatry 78, 116–25 (2015). [DOI] [PubMed] [Google Scholar]
- Chaddah R., Arntfield M., Runciman S., Clarke L. & van der Kooy D. Clonal neural stem cells from human embryonic stem cell colonies. J Neurosci 32, 7771–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M. & Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24, 659–85 (1994). [DOI] [PubMed] [Google Scholar]
- Ranade K. et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res 11, 1262–8 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.