Abstract
Introduction
Kettlebell (KB) swing exercises have been proposed as a possible method to improve hip and spinal motor control as well as improve power, strength, and endurance.
Purpose
To describe electromyographic (EMG) and sagittal plane kinematics during two KB exercises: the two‐handed KB swing (THKS) and the single‐handed KB swing (SHKS). In addition, the authors sought to investigate whether or not hip flexor length related to the muscular activity or the kinematics of the exercise.
Methods
Twenty‐three healthy college age subjects participated in this study. Demographic information and passive hip flexor length were recorded for each subject. A maximum voluntary isometric contraction (MVIC) of bilateral gluteus maximus (GMAX), gluteus medius (GMED), and biceps femoris (BF) muscles was recorded. EMG activity and sagittal plane video was recorded during both the THKS and SHKS in a randomized order. Normalized muscular activation of the three studied muscles was calculated from EMG data.
Results
During both SHKS and THKS, the average percent of peak MVIC for GMAX was 75.02% ± 55.38, GMED 55.47% ± 26.33, and BF 78.95% ± 53.29. Comparisons of the mean time to peak activation (TTP) for each muscle showed that the biceps femoris was the first muscle to activate during the swings. Statistically significant (p < .05), moderately positive correlations (r = .483 and .417) were found between passive hip flexor length and % MVIC for the GMax during the SHKS and THKS, respectively.
Conclusions
The THKS and SHKS provide sufficient muscular recruitment for strengthening of all of the muscles explored. This is the first study to show significant correlations between passive hip flexor length and muscular activation of hip extensors, particularly the GMax. Finally, the BF consistently reached peak activity before the GMax and GMed during the SHKS.
Level of Evidence
Level 3
Keywords: Electromyography, hip flexor, hip extension, kettlebell
INTRODUCTION
Muscular imbalance and neuromuscular activation order have been implicated in numerous musculoskeletal disorders including low back pain (LBP),1–3 lower extremity injury,4 and upper extremity injury.5 Vladimir Janda is a well‐known pioneer who studied neuromuscular patterns. Among his contributions to rehabilitation is the concept of the lower crossed syndrome (also known as the crossed pelvis syndrome) described as an imbalance between tight and short hip flexors and lumbar erector spinae (ES) and weak or inhibited gluteal and abdominal muscles.1 Decreased hip flexor range of motion (ROM) has been described in individuals with a history of LBP,6 however evidence linking decreased hip flexor ROM with decreased gluteal function is limited. Janda described the “optimal” activation order of muscles contributing to the movement of hip extension, beginning with gluteus maximus activation followed by hamstring activation in coordination with the contralateral ES.3 However, no consensus exists regarding which side of the ES is activated first.7,8 In addition, the long held clinical belief that the gluteals activate prior to the hamstrings in the asymptomatic population has proven false, with consistent evidence showing that the hamstrings activate first during hip extension.8–10 Despite this trend, an increased delay between hamstring activation and gluteal activation appears to be present in patients with LBP compared to the healthy population.11
Preliminary evidence indicates that there may be a relationship between dysfunction of the hip musculature and the low back.4,12 However, the exact cause/effect relationship is not yet understood. The role of the strength, power, or endurance of the gluteus maximus (GMAX) in low back pain remains unknown. Cooper et al13 demonstrated weakness (measured by manual muscle testing) of the gluteus medius (GMED) in subjects with chronic, non‐specific LBP when compared to controls. Additionally, decreased hip abduction and external rotation strength has been implicated as predictors of LBP and lower extremity injury.4,12,13
Both Janda and Sahrmann have suggested that attempting to improve gluteal activation through therapeutic exercise may reduce stress on the hip and spine.13,14 Numerous methods to improve gluteal activation have been proposed, including the use of cueing10 and abdominal hollowing,16,17 as mechanisms to decrease the delay in gluteal activation. A less common neuromuscular reeducation tool that has become popular in recent years is the use of kettlebell (KB) exercise.18 Education for the correct performance of kettlebell swinging exercises involves verbal cueing geared toward gluteal activation during hip hinging. In addition, the swinging motion may provide kinesthetic and proprioceptive feedback regarding the desired performance of the technique. Finally, the kettlebell exercise provides training stresses for improving power, strength, and endurance of the hip and lumbopelvic musculature.18,19
Deficits of both strength and muscular endurance have been implicated in acute and chronic musculoskeletal dysfunction.20,21 Of these, muscular endurance has been suggested as more important than strength in patients with LBP.20,21 Jay et al demonstrated that using the kettlebell for targeted training for muscular strength and endurance resulted in decreased neck/shoulder pain and LBP when used in a work hardening program.19
Despite a lack of evidence in the literature, many clinicians utilize GMAX and GMED strengthening with perceived positive outcomes for patients with LBP. It is generally accepted that muscle activation of a minimum of 50% to 60% of maximum voluntary isometric contraction (MVIC) is necessary as a stimulus to facilitate muscle strengthening.22‐24 Therefore it would appear that if strengthening the GMAX and medius is the goal, an exercise should provide a stimulus that allows the patient to exceed 50% of MVIC of these muscles during performance of the exercise. A systematic review by Reiman, Bolga, and Loudon identified that the forward step‐up yielded the highest electromyographic (EMG) activity of the GMAX (74% ± 43%) MVIC and that the side‐bridge into a neutral spine position resulted in the highest EMG activity of the GMED (74% ± 30%) MVIC.25 McGill and Marshall demonstrated that the two handed kettlebell swing was able to produce an average peak GMAX activation of 76.1% ± 36.6% and an average peak GMED activation of 70.1% ± 23.6% in healthy subjects.18 This early evidence appears to indicate that the kettlebell swing may provide a viable alternative for both GMAX and GMED strengthening.
The purpose of this study was to describe EMG and sagittal plane kinematic data during two KB exercises: the two‐handed KB swing (THKS) and single‐handed KB swing (SHKS). In addition, the authors sought to investigate whether or not hip flexor length related to the muscular activity or the kinematics of the exercise.
METHODS
Participants
A convenience sample of 23 healthy individuals between the ages of 18‐30 was recruited for this study. Of this sample, the EMG data of four subjects was found to be unusable due to excessive noise artifact that could not be rectified, leaving a total of 19 subjects for final analysis. This selection was based both upon the availability of age eligible individuals at the institution and the age‐relative decreased risk of cardiovascular and musculoskeletal related injury in the group.40,41 Exclusion criteria included: preexisting cardiopulmonary conditions which prevented safe exercise participation, musculoskeletal injury or condition that had occurred within eight weeks of study participation, preexisting bleeding or clotting disorder which could increase risk of bleeding during skin preparation, and those who had ample experience using a KB. Ample experience with the KB was defined as any formal instruction on the use of the KB or using the KB on a weekly basis over the period of four weeks or more. Participants were required to have consistently maintained an active lifestyle over the previous month before participating in the physical demands of the current study. An active lifestyle was defined as at least 30 minutes of exercise (i.e. running, walking, resistance training, etc.) performed at least two times per week. The Human Research Review Committee of Grand Valley State University granted IRB approval for this project. All subjects read and signed the informed consent document.
Methods
The participants’ gender, age, height, and weight measurements were recorded. The flexibility of the hip flexor group was measured using a bubble inclinometer (Baseline, Fabrication Enterprises Inc, White Plains, New York 10602 USA) during performance of the modified Thomas test.
Modified Thomas Test
Subjects sat as close to the end of the table as possible to begin the modified Thomas test. They then grasped the front of both knees while the examiner stabilized the patient and assisted them back into a supine position on the table with both hips maximally flexed to the chest to ensure the lumbar spine was flexed and flattened to the table.42 The subject then slowly lowered the leg to be examined into an extended position with the end of the limb hanging off of the end of the table.42 The inclinometer was leveled on top of evaluation table in parallel with the floor. The examiner positioned the inclinometer on top of the thigh approximately halfway between the superior patellar border and the anterior superior iliac spine (ASIS) of the pelvis. The angle of hip flexion was recorded as follows: the subject's thigh lined up with the horizontal level of the table = 0 degrees, test thigh was above the horizontal = negative value (measured in degrees), and test thigh was below the horizontal = positive value (measured in degrees). Horizontal was defined as parallel with the plane of the table. For the purpose of this study, a value of 10 degrees or greater from the horizontal was indicative of 10 degrees of hip flexion, which was considered a “tight” hip flexor (iliopsoas). If the hip flexor was noted to be tight, the examiner passively flexed the knee to examine the contribution of rectus femoris in decreasing hip extension. If the lumbar spine increased in lordosis to the point in which a 1.27 cm piece of PVC tubing could be inserted under the low back, rectus femoris involvement was considered positive. This procedure was repeated on the opposite limb.42
Although some questions exist regarding the reliability of the modified Thomas test in determining rectus femoris muscle ROM at the knee joint,43 it has been shown to have both high intra‐rater and inter‐rater reliability for hip extension ROM.44 In addition, a study comparing both goniometric and inclinometer measures of hip extension ROM demonstrated high inter‐rater parallel‐forms reliability between the two devices, which demonstrates evidence for their interchangeable use.44 Validity of the modified Thomas test has not been studied.
Two Dimensional (2‐D) Kinematic Analysis
Video was captured by two Canon HV20A (Melville, New York, USA) cameras filming at 720p at a rate of 30 frames per second. Dartfish ProSuite version 6.0 (Alpharetta, Georgia, USA) was utilized for frame‐by‐frame analysis (30 frames per second) and measurement of relative hip and knee angles of movement during the KB swing. The purpose of the kinematic analysis was to determine kinematic variations, which can be identified in a clinical setting from a sagittal view. The use of sagittal plane video analysis has been shown to be a valid and reliable measure of hip and knee joint motion when compared to goniometric measures.39 In the analysis, the authors provided a novel attempt to produce a standardized nomenclature for key points of movement during the KB swing, and chose not to utilize frontal plane video data. Sagittal plane kinematic analysis of the ankle, knee, and hip were emphasized.
The camera was placed 4.3 meters away from the position of the subject. Markers were placed at anatomical landmarks in order to provide reference points for consistency in measurements of trunk and lower extremity joint angles. To accomplish this, a 1” strip of colored Kinesio© Tex Tape™ (Kinesio USA, LLC. Albuquerque, NM) was placed along the mid‐axillary line on both sides of the subject at the level of rib 10, and smaller pieces (2.5 cm square pieces) placed over the greater trochanters of both femurs, lateral femoral epicondyles, and the lateral malleoli of the tibia at the ankle. Upon completion of the study, videos were removed from the recording cameras and laptop and archived to DVD and/or external storage device for analysis. A single investigator interpreted the results of hip flexion angles obtained during sagittal plane kinematic analysis in attempt to maximize intra‐rater reliability.
Electromyography
The Biopac Tel‐100 EMG System (Biopac Systems, Goleta, CA) was used to measure electromyographic activity during the KB swings. Acqknowledge software version 3.9.1 was used to analyze electromyography (EMG) data. Bipolar adhesive surface electrodes (Noraxon Dual Electrodes, Ag‐AgCl, spacing 2.0 cm, Noraxon USA, Inc, Scottsdale, AZ) were placed over relevant muscle bellies.
Data were collected by placing electrodes as described by Dondelinger36 over the following muscles: right and left GMAX muscle bellies between the 1st‐3rd sacral the level of the greater trochanter; the GMED electrode was placed parallel to the muscle fibers over the proximal third of the distance between the iliac crest and greater trochanter; the biceps femoris placement had an electrode over the muscle belly midway between the knee and hip.
Each subject (when appropriate) had the area shaved of hair with a disposable razor and skin was cleansed, abraded, and dried36 prior to electrode placement by an investigator of the same gender to maintain modesty. To elicit the maximum isometric contraction for each muscle, the participants were placed into the positions described by Kendall37 and Dondelinger,36 and their maximum contraction during tester‐induced resistance was recorded. The MVIC for each subject was established by measuring the average peak EMG activity over two trials of a resisted isometric contraction, as a measure of each muscle's maximal strength. The percent of MVIC for each muscle was calculated by averaging the peak EMG activity of each muscle, collected during three repetitions of the right and left SHKS and the THKS. The highest percent of MVIC of each muscle group was used to establish average values for statistical analysis. Time to peak (TTP) was measured from the bottom swing, or ending of the loading phase, to peak contraction during the same three reps used to establish percent of MVIC during each set of swings. The lowest average value for TTP, which is the fastest value, between right and left muscle groups was used during statistical analyses. The fastest value was utilized because the authors believed that it represented the best performance during the testing session. Raw EMG data were filtered using a band‐pass determination with a low frequency of 20 Hz and a high pass frequency of 500 Hz. This was done in order to eliminate as much noise artifact as possible without compromising the relevant electrical signals given off by the muscles.
KB Swing Standardization Device
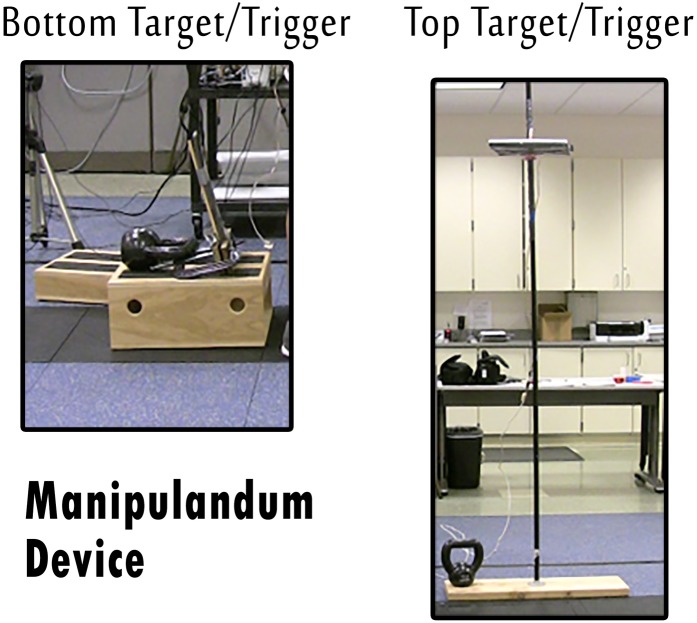
This device, henceforth referred to as the “manipulandum,” was used as a method to standardize performance of the KB swing and to electronically mark the bottom and top phases of the KB swinging motion. Pressure sensitive electrical switches were attached to foam boards, which served as contact points for the KB. (Figure 1)
Figure 1.
The manipulandum devices, created by the authors to denote the bottom and top of the kettlebell swings.
One foam board, sized 16” × 5.5” or 12” × 5.5”, was placed vertically on the ground 1‐2” behind the participants heels and stabilized between 2 clamped wooden boards. For further height adjustment, the stabilized boards were on an adjustable 2” × 8” plank with holes spaced 1.5” apart that could be elevated or lowered based on participant height. The other foam board, 12” × 12”, was mounted on height‐adjustable copper rivets attached to a 7.5’ tall ¾” thick pole. The height of this foam board was placed at a height of 2‐3” above eye level of the participant.
KB Swing Procedures
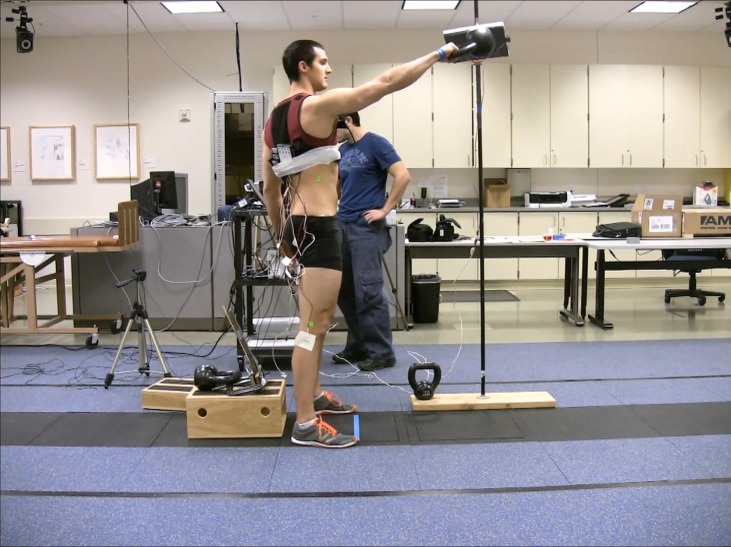
An instructional session based on a standardized script for performing the THKS and SHKS was individually delivered for each of the subjects. Each instruction session lasted no more than 10 minutes and subjects were allowed a maximum of 10 repetitions of each type of swing during this time. This session served three purposes: First, to teach the appropriate KB swing technique utilizing a standardized script conducted by one investigator. Second, to determine the appropriate KB weight (8 kg, 12 kg, or 16 kg). This was based on the participants’ body weight, performance ability, and personal preference. Third, this session served to provide an active warm‐up. Prior to data collection, subjects performed a brief re‐warm‐up after EMG electrode placement consisting of 10 KB deadlifts and 5 THKS followed by two minutes of rest. Subjects then performed 10 repetitions of THKS, left SHKS, and right SHKS, in randomly assigned order determined by a coin flip. Both the THKS and each of the SHKS were performed with the same subject self‐selected weight. These swings were performed at a rate of 45 beats per minute (BPM) by following a computerized metronome. Each subject performed all three KB swing variations, with 60‐second rest intervals between conditions. EMG data were recorded and subjects were video recorded in the sagittal plane while performing the swings. (Figure 2)
Figure 2.
Subject performing the R Single handed kettlebell swing, with full EMG set up shown.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Version 20. The independent variables were the sagittal plane hip extension findings (active hip extension) from video analysis, Thomas test measurements (passive hip extension or ROM), and gender. The dependent variables were TTP and percent of MVIC. Independent sample t‐tests were used to establish gender differences in percent of MVIC during SHKS and THKS as well as TTP, and therefore activation order. Paired t‐tests were utilized to establish differences in TTP between LSHKS, RSHKS, and THKS. Finally, multi‐linear regression analyses were performed to determine existence of relationships between hip flexor tightness (Thomas Test), hip extension ROM values (kinematic analysis), and gender and percent of MVIC and TTP. Significant differences were recorded at a value of p < .05. Strength of correlations was described using the following scale outlined by Portney and Watkins: 0.0‐.29 = weak correlation, .30‐.59 = a moderate correlation, and .60 and above was considered a strong correlation.45
RESULTS
Descriptive Data
Subject Demographics
The mean subject age was 24 years (SD+/−1.97), average weight in kilograms was 70.7 kg, (SD+/−13.3), and average height was 178 cm, (SD+/−8.25). Average Thomas test measurements were 15.5 (left) to 16.8 (right) degrees of extension across the sample (SD of 6.27‐7.58 left to right, respectively). Therefore, the subjects were able to, on average, extend 15.5° on the left and 16.8° on the right below the horizontal during the Thomas test and demonstrated no limitations in hip flexor flexibility.
Descriptive Data for Percent of MVIC
For both males and females, SHKS yielded 74.2‐81.4% of MVIC for the GMAX, SD of 49.0‐61.3% and 56.0‐57.4% of MVIC for GMED, with an SD of 25.9‐27.7% for the entire sample. Two‐handed kettlebell swings yielded an average of 69.4% of MVIC for the GMAX and 53.04% of MVIC for the GMED. For these means, the highest percent of MVIC between the left and right sides for each of the 19 subjects was utilized. For further demographic information and gender specific means across the sample, please refer to Table 1.
Table 1.
Subject demographics, including means and standard deviations of all subjects and separated by gender.
| Age (Mean Years/Std. Deviation) | Weight (kg) | Height (cm) | SHKS % MVIC GMAX | THKS % MVIC GMAX | SHKS % MVIC GMED | THKS % MVIC GMED | Thomas Test Left (degrees) | Thomas Test Right (degrees) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | ||||||||
| Sample (N=19) | 24/1.97 | 70.7/13.3 | 178/8.25 | 74.2/49.0 | 81.4/61.3 | 69.4/55.9 | 57.4/25.9 | 56.0/27.7 | 53.0/25.4 | 15.5/6.27 | 16.8/7.58 |
| Men (N=9) | 24.1/2.57 | 80.5/10.0 | 181/6.78 | 64.5/30.7 | 61.4/28.2 | 54.6/26.5 | 60.8/28.6 | 56.5/26.8 | 43.0/12.9 | 14.1/5.62 | 12.6/6.91 |
| Women (N=10) | 23.9/1.37 | 61.8/8.79 | 171/6.47 | 83.0/61.5 | 99.4/77.7 | 82.8/77.2 | 54.3/24.4 | 55.6/29.9 | 62.0/30.8 | 16.7/6.86 | 20.8/6.05 |
SHKS = single‐handed kettlebell swing; THKS = two‐handed kettlebell swing; percent of MVIC = Percent of maximal voluntary isometric contraction; Gluteus Maximus = GMAX; and Gluteus Medius = GMED.
Descriptive Analysis of Hip Flexion and Extension ROM
Kinematic analysis of average terminal hip extension during SHKS (left and right averages combined) revealed an average of −8.5° ( ± 5.3°) for both genders (males = −8.8° ± 5.58° / females = −8.1° ± 5.32°) and an average of −9.7° ( ± 7.8°) for both genders (males = −8.4° ± 7.72° / females = −10.9° ± 8.08°) for THKS. To clarify, the negative value during the kinematic analysis represents a position of hip extension and a positive value is indicative of a position of hip flexion. Kinematic analysis of average terminal hip flexion during SHKS (left and right averages combined) revealed an average of 84.8° ( ± 10.7°) for both genders (males = 84.7 ± 12.5 / females = 84.8 ± 9.3) and an average of 85.4° ( ± 9.0°) for both genders (males = 84.8 ± 9.42 / females = 85.9 ± 9.03 ) for THKS.
Descriptive Analysis of 1D Sagittal Kinematics
Although not objectively measured, a number of subjectively descriptive phases of movement during all KB swings were noted using videographic recordings of sagittal plane kinematics. These phases are clearly visible both with the naked eye and with the use of simple video recording software. Furthermore, although not objectively measured, a consistent curvilinear path of movement of the KB was subjectively observed on video analysis of the subjects performing the swinging tasks. A notable limitation to the analysis of phases is the authors’ inability to objectively synchronize kinematic and EMG data, and this will be further discussed in the limitations section. Based on the kinematic data, the authors propose a standardized nomenclature for defining KB swings. Consisting of five phases, the authors define terminal hip flexion of the KB swing as the “pre‐swing phase”, the transition from terminal hip flexion toward hip extension as “acceleration”, the phase between maximal hip flexion and hip extension as the “swing phase”, terminal hip extension as the “terminal swing phase”, and the return from terminal hip extension phase to hip flexion as the “return phase”. (Figure 3)
Figure 3.
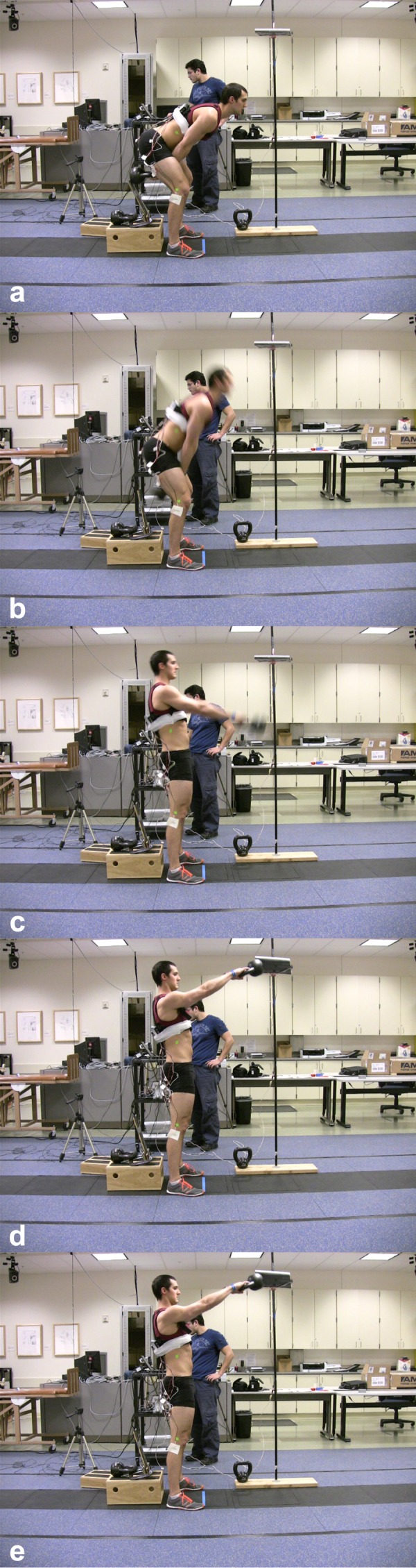
Phases of the kettlebell swing, from top to bottom: a) terminal hip flexion phase, b) acceleration phase, c) swing phase, d) terminal swing phase, and e) return phase.
Statistical Results
Mean Differences in Time to Peak between Muscle Groups During All Swings
Significant differences were observed between the mean TTP of the GMAX and BF with the left SHKS (p=.006), the GMAX and BF with the right SHKS (p=.010), between the TTP of the BF of the THKS and both left and right SHKS (p=.016 and .028, respectively), and TTP of the GMED during left SHKS in comparison to the THKS (p=.043). These statistically significant findings demonstrate that the first muscle to be activated of those investigated was the BF, across single‐handed swings and that the BF had a faster time to peak in SHKS than THKS. The mean differences between the GMAX and BF during the THKS were not statistically significant, but did show that the GMAX had a longer TTP than the BF did, as evidenced by the positive mean value in column 2 (.051 seconds). The full results can be seen in Table 3.
Table 3.
Paired Samples Tests – Mean differences in time to peak EMG output (TTP) in seconds between muscle pairs during the SHKS and THKS. A positive value in Column 2 indicates that the first listed variable had a longer TTP than the second variable (was activated second). A negative value indicates the first listed variable had a shorter TTP than the second variable (was activated first). Statistically significant differences are denoted with an asterisk.
| Muscle Pair |
Mean Difference (seconds) |
Std. Deviation |
2 Tailed Sig. (p < .05) |
|---|---|---|---|
| GMAX ‐L ‐ BF ‐L | .0992105 | .1381029 | .006* |
| GMAX ‐L ‐ GMED ‐L | .0238947 | .0853990 | .238 |
| GMAX‐R ‐ BF‐R | .0884211 | .1346759 | .010* |
| GMAX‐R ‐ GMED‐R | −.0210000 | .0852767 | .297 |
| GMAX‐T ‐ BF‐T | .0514737 | .1427384 | .133 |
| GMAX‐T ‐ GMED‐T | −.0268947 | .0830608 | .175 |
| BF ‐L ‐ BF‐T | −.0716316 | .1169208 | .016* |
| BF‐R ‐ BF‐T | −.0554737 | .1010354 | .028* |
| GMAX ‐L ‐ GMAX‐T | −.0238947 | .1469221 | .487 |
| GMAX‐R ‐ GMAX‐T | −.0185263 | .0918588 | .391 |
| GMED ‐L ‐ GMED‐T | −.0746842 | .1491647 | .043* |
| GMED‐R ‐ GMED‐T | −.0244211 | .0682889 | .136 |
EMG=electromyographic; SHKS = single handed kettlebell swing; THKS = two handed kettlebell swing; GMAX‐L = left gluteus maximus during SHKS; BF‐L = left biceps femoris during SHKS; GMED‐L = left gluteus medius during SHKS; GMAX‐R = right gluteus maximus during SHKS; BF‐R = right biceps femoris during SHKS; GMED‐R = right gluteus medius during SHKS; BF‐T = mean activation of both biceps femoris muscles during the THKS; GMED‐T = mean activation of both gluteus medius muscles during the THKS; GMAX‐T = mean activation of both gluteus maximus muscles during the THKS.
Table 2.
Descriptive Phases of the Kettlebell swing
| Phase Title | Phase Description |
|---|---|
| Pre‐Swing Phase | Terminal hip flexion with the KB between and behind the legs with its momentum halted. The lowest amount of muscle activity occurs during this phase. |
| Acceleration Phase | Transition from terminal hip flexion toward hip extension with the KB forcibly accelerated in a pendular anterior/superior trajectory. The highest GMAX , GMED, and BF muscle activity occurs during this phase. |
| Swing Phase | Transition from acceleration through hip extension with the KB travelling in an anterior/superior curvilinear path. Variable pulsatile muscle activity occurs during this phase. |
| Terminal Swing Phase | Terminal hip extension with the KB suspended at the top of its peak trajectory in line with the eyes of performer. Low level GMAX, GMED, and BF activity occurs during this phase. |
| Return Phase | Return from terminal hip extension towards hip flexion with the KB reversing its curvilinear trajectory either by the effect of gravity or through forcible concentric contraction (an advanced technique not performed by our subjects). Low level GMAX, GMED, and BF activity occurs during the non‐advanced form of this exercise performed in this study during this phase. |
Sagittal Hip Extension, and Percent of MVIC
A statistically significant strong and negative correlation existed between hip flexor length (as measured by the Thomas test) and GMAX muscular activation (r = −.651) for female subjects but not for males. The types of swings are indicated in the descriptions below each table. During the THKS, moderately positive correlations were found between GMAX percent of MVIC and Thomas test measurements, with a correlation coefficient of .417. Weak and positive correlations were found between active sagittal plane hip extension (r = .190), while weak, negative correlations were found between percent of MVIC and gender (r = −.259). Correlations are presented in Table 4.
Table 4.
Gluteus Maximus Correlations –Correlation between GMAX % of MVIC and Thomas test findings, gender, and sagittal plane hip extension. P‐values for the correlations between each of the two variables being compared are also presented.
| Correlations | |||||
|---|---|---|---|---|---|
| GMAX % MVIC | Thomas Test | Gender | THKS ‐ Hip Extension | ||
| Pearson Correlation | GMAX % MVIC | 1.000 | .417 | −.259 | .190 |
| Thomas Test | .417 | 1.000 | −.651 | −.331 | |
| Gender | −.259 | −.651 | 1.000 | .294 | |
| THKS ‐ Hip Extension | .190 | −.331 | .294 | 1.000 | |
| Sig. (1‐tailed) | GMAX % MVIC | . | .038 | .142 | .218 |
| Thomas Test | .038 | . | .001* | .083 | |
| Gender | .142 | .001 | . | .111 | |
| THKS ‐ Hip Extension | .218 | .083 | .111 | . | |
GMAX % MVIC = gluteus maximus percent maximum voluntary isometric contraction; THKS‐ Hip Extension = two handed kettlebell swing measurement of hip extension in the sagittal plane.
denotes statistically significant correlation at p<0.05.
Table 5.
Gluteus Medius Correlations –Correlation between GMED % MVIC and Thomas test findings, gender, and sagittal plane hip extension. P‐values for the correlations between each of the two variables being compared are also presented.
| Correlations | |||||
|---|---|---|---|---|---|
| Glute med % MVIC | Thomas Test | Gender | THKS ‐ Hip Extension | ||
| Pearson Correlation | Glute med % MVIC | 1.000 | .396 | −.384 | −.348 |
| Thomas Test | .396 | 1.000 | −.651 | −.331 | |
| Gender | −.384 | −.651 | 1.000 | .294 | |
| THKS ‐ Hip Extension | −.348 | −.331 | .294 | 1.000 | |
| Sig. (1‐tailed) | Glute med % MVIC | . | .047 | .052 | .072 |
| Thomas Test | .047 | . | .001* | .083 | |
| Gender | .052 | .001 | . | .111 | |
| THKS ‐ Hip Extension | .072 | .083 | .111 | . | |
GMED % MVIC = gluteus medius percent maximum voluntary isometric contraction; THKS‐ Hip Extension = two handed kettlebell swing measurement of hip extension in the sagittal plane.
denotes statistically significant correlation at p<0.05.
DISCUSSION AND CONCLUSION
The purpose of this study was to describe EMG and sagittal plane kinematic data during two KB exercises: the THKS and the SHKS and to investigate whether or not hip flexor length related to the muscular activity or the kinematics of the exercise. KB swings have been suggested as a beneficial technique for neuromuscular education.17 It is widely believed that KB swings emphasize gluteal activation. Education for performance of KB exercises involves verbal cueing directed specifically toward gluteal activation, and, subsequently, the swinging motion provides kinesthetic and proprioceptive feedback regarding appropriate performance of the technique. Recent evidence from McGill and Marshall demonstrated that the two handed KB swing was able to produce an average peak GMAX activation of 76.1% ± 36.6% MVIC and an average peak GMED activation of 70.1% ± 23.6% MVIC in healthy subjects.18 This is well within the 50% to 60% of MVIC suggested to be a sufficient stimulus to facilitate muscle strengthening.21–23 This early evidence appears to indicate that the KB swing may provide a viable alternative for both GMAX and GMED strengthening. Beyond McGill and Marshall's recent examination of the EMG, ground reaction forces (GRFs), and 3D kinematic data of the THKS on male subjects,18 limited descriptive studies have examined EMG and kinematics of the THKS and SHKS across both genders.
Vladimir Janda previously discussed an association between hip flexors ROM and gluteal activation.1 Evidence for decreased hip flexor ROM exists in individuals with a history of LBP,5 however, to the best of the authors’ knowledge, no literature has previously demonstrated associations between hip flexor tightness and gluteal activation. Although flexibility and ROM of the hip flexor was not evaluated, previous research has demonstrated that greater MVIC activation of the GMAX occurs at 0° of hip extension compared to 30°, 60°, or 90° of hip flexion.46 Theoretical implications of the existence of this imbalance are that gluteal activation may be modified by alterations in hip flexor flexibility.
Kinematics
Measurement of passive ROM via the modified Thomas test demonstrated an average of 14° ( ± 9.8°) of hip extension for both genders, indicating extension of the hip beyond neutral in the test position. Despite the notable availability of passive hip extension ROM, kinematic analysis of video taken during the data collection revealed that none of the subjects obtained neutral hip position while performing any of the KB swings. Average terminal hip extension during the SHKS (left and right averages combined) lacked a mean of 8.5° ( ± 5.3°) from neutral hip extension for both genders (males = −8.8° ± 5.58° / females = −8.1° ± 5.32°) and lacked a mean of 9.7° ( ± 7.8°) from neutral hip extension for both genders (males = −8.4° ± 7.72° / females = −10.9° ± 8.08°) during the THKS. This indicates that for both the SHKS and the THKS neither gender performed throughout their full available hip extension, despite cueing during the instructional sessions. Kinematic analysis of average terminal hip flexion during the SHKS (left and right averages combined) revealed a mean of 84.8° ( ± 10.7°) for both genders (males = 84.7° ± 12.5° / females = 84.8° ± 9.3°) and a mean of 85.4° ( ± 9.0°) for both genders (males = 84.8° ± 9.42° / females = 85.9° ± 9.03°) during the THKS. This indicates that during performance of both the SHKS and THKS, hip flexion near 90° was obtained at active terminal flexion. These hip flexion ROM values are greater than those observed by McGill and Marshall whose subjects performed an average of 75° of hip flexion at the bottom of the swing, however, they only used male subjects.18 Furthermore, McGill and Marshall found their subjects performed greater hip extension than the subjects within the current study, with an average of 1° of hip extension while performing a KB swing.18 The 8.5‐9.8° difference in both hip flexion and hip extension between subjects may in part be due to differences in verbal cues given in the instructional sessions, McGill and Marshall emphasized a “squat” based KB swing and gave a target of chest height, while the instructions emphasized in this study emphasized a “pendular motion” through hip hinging with a target of eye height and controlled hip ROM for both the bottom and the top of the swing through the targets of the manipulandum. Furthermore, the authors of the current study attempted to control the velocity of the technique through the use of a metronome, which may have influenced the motor program utilized to reach terminal ranges based on higher or lower velocities, which were not discussed in the McGill and Marshall study. In addition, some of the variance may have been related to differences in accuracy of measurements obtained during 3‐D versus one‐dimensional kinematics.
Peak Muscle Activation
On an average, peak muscle activation for GMAX was 75.02% ± 55.38% (THKS = 69.44% ± 55.90 / LSHKS = 74.24% ± 48.98% / RSHKS = 81.39% ± 61.27%) in all KB swing techniques. It has been previously suggested that between 50% to 60% of MVIC is necessary to facilitate muscle strengthening.22–24 This indicates that both forms of KB swings may induce sufficient muscular activation to potentially promote muscle strengthening of the GMAX. Although the GMAX activation results of this present study were slightly lower on average, McGill and Marshall similarly demonstrated peak GMAX activation over 60% of MVIC (76.1% ± 36.6%).18 The current results for GMAX MVIC are higher than a single leg squatting activity (57% ± 44%)23 and similar to the highest level of GMAX activation previously identified in a systematic review by Reiman et al during performance of the step‐up activity (74% ± 43% MVIC).25 Curiously, although the back squat is popularly thought of as an exercise to strengthen the GMAX, it fails to exceed peak muscle activation of 35%, regardless of depth, stance, or weight utilized.47–49
Average peak muscle activation for GMED also met the minimum of 50% of MVIC, which meets the current suggested standard for muscle strengthening. Average peak percent of MVIC for GMED was 55.47% ± 26.33% (THKS = 53.0% ± 25.4% / LSHKS = 57.4% ± 25.9%, / RSHKS = 56.0% ± 27.7%, This average is less than the results of McGill and Marshall who demonstrated average peak GMED activation of 70.1% ± 23.6% in males.18 It is possible that an additional training effect was present in McGill and Marshall's work, as most of their subjects had experience with the KB, whereas none of subjects in this study were formally trained in the KB, nor did they frequently utilize a KB in their training.18 Despite having lower values than those described in the work of McGill and Marshall, values were greater than many clinically utilized GMED activities, including the clam shell exercise (38% ± 29% for a 60° clamshell to 40% ± 38% in a 30° clamshell), the side lunge (39% ± 19%), and the forward lunge (42% ± 21%) exercises as evaluated by Distefano et al.22 The GMED activation in both the SHKS and THKS were close to both the lateral band walk (61% ± 34%) and single limb squat (64% ± 24%), but not as high as the sidelying hip abduction exercise (81% ± 42%).21 Regardless, it is clear that the THKS and SHKS can be utilized for a sufficient stimulus for GMED strengthening.
Perhaps the most interesting comparison that can be made between the current work and the work of McGill and Marshall18 was the difference in average percent of MVIC of the BF, which may be related to the training effect. The subjects included in the McGill and Marshall study had familiarity with the KB compared to the novice subjects in the current study. Subjects familiar with the KB swing may have been more efficient with the technique and used musculature differently than novices, or verbal cues used over time in an individual trained in KB swinging may have affected muscular recruitment. Average percent of MVIC for BF in the current study was 78.95% ± 53.29%, significantly greater than the subjects in the McGill and Marshall study 32.6% ± 24.1%18 but less than Zebis et al, who demonstrated an average peak MVIC for BF of 93% ± 31% with female subjects during a THKS.34 No information was provided by Zebis et al regarding the familiarity of their subjects with the use of a KB.34 Regardless, the current values are well above the 50‐60% of MVIC needed for strengthening and are comparable to that of the seated leg curl exercise (81.0% ± 28.0%) and greater than the stiff leg deadlift (49.0% ± 27.0%), the single leg stiff leg deadlift (48.0% ± 39.0%), the Good Morning exercise (43.0% ± 16.0%), and the squat exercise (27.0% ± 20.0%) for average peak hamstring activation.50
Correlations
During the THKS, a moderate positive correlation was found between average peak GMAX percent of MVIC and Thomas test measurements, with a correlation coefficient of 0.417. Weak and positive correlations were found between active hip extension (sagittal kinematic hip extension) and GMAX activation (r = 0.246), while weak and negative correlations (r = −0.318) were found between percent of MVIC and gender (females had greater GMAX activation). Based on these results, it appears that the greater the available passive or active hip extension ROM (the less “tight” or “short” the hip flexor), the greater the GMAX percent of MVIC. Although not strong, evidence of moderate positive correlation between GMAX activation and passive hip extension (the Thomas test) as well as weak positive correlation to dynamic/active hip extension (kinematic sagittal hip extension) is an interesting finding, as the original hypothesis was that no correlation would be seen due to a lack of existing evidence associated with static hip flexor length/static hip extension ROM. However, the association between hip extension ROM may indirectly have been demonstrated in the work of Worrell et al, who demonstrated that peak MVIC was weakest at 90° of hip flexion but progressively increased until the highest peak activation was obtained at zero degrees of hip extension.46 To the best of the authors’ knowledge, this is the first study to demonstrate an association between hip ROM/flexibility and gluteal activation with a closed chain activity. Mauntel et al examined numerous lower extremity PROM measures and their influence on lower extremity muscle activation during a single leg squat but found no relationship between passive hip extension and muscle activation.51 Although speculative and requiring further research, the current results may provide some clinical value regarding the relationship between hip extension ROM and muscle performance, and support the premise which Janda laid in his description of an association between tight hip flexors and a under recruited GMAX.
Moderate and negative correlations were found between average peak GMED percent of MVIC and hip extension (r = −0.348) and gender (r = −0.384), indicating a higher percent of GMED MVIC activation among women during the THKS. Although not an objective of this study, it could be postulated that this increased activation may be the result of increased effort relative to the proportion of lean body mass to the mass of the kettlebell, which may be gender specific. Thomas test measurements had a moderately positive relationship (r = 0.396) with percent of GMED MVIC during the THKS. This finding is truly novel, as no proposed associations between passive hip ROM/flexibility and GMED activity has previously been made. Similar to the GMAX, the less hip extension ROM available, the weaker the GMED activation.
Although no statistically significant differences were noted, females consistently had higher average peak percent of MVIC of GMAX in all swings, and a higher means of percent of MVIC of GMED during THKS. Curiously, the only significant statistical finding (p = 0.001) in correlational analysis was the strong negative correlation (r = −0.651 ) between gender and Thomas test results (females had greater ROM in the Thomas test), and based on other moderate and weak GMAX correlations and previous evidence regarding maximal GMAX MVIC occurring with greater hip flexor length, there may be some subtle relationships not statistically detected in the current study due to sample size. Conversely, males had slightly higher percent of MVIC values for GMED recruitment during both SHKS trials.
Overall, a theme of positive correlations related to a greater percentage of MVIC activation of the GMAX and GMED occurred with greater passive hip flexor range (Thomas test) and active hip extension range (kinematic hip extension). Previous research has demonstrated that greater MVIC activation of the GMAX occurs at 0° of hip extension than at 30°, 60°, or 90° of hip flexion.46
Muscle Activation Order
In agreement with previous research regarding the activation order of hamstrings compared to gluteals throughout the movement of hip extension,9–11 the hamstrings activated first in all forms of KB swings across gender, hip flexor ROM, and kinematic hip flexion or extension. Significant differences (p < 0.05) were calculated between the mean TTP of the GMAX and BF between left (p = 0.006) and right (p = 0.010) sided SHKS (BF activated first in both swings), between the TTP of the BF of the THKS and both SHKS (BF activated earlier in both the left SHKS p = 0.016 and right SHKS p = 0.028 and THKS). This is despite emphasis during the education of the swings on conscious activation of the gluteals. These results are in agreement with McGill and Marshall, where the BF was also activated prior to the GMAX (at 52% of the swing cycle versus 57% respectively).18
Regarding comparing GMED activation between THKS and SHKS, the TTP of the GMED was earlier in the left SHKS (p = 0.043) than GMED of THKS. Although not officially recorded, it was noted during the instructional sessions that the majority of the subjects were right handed, which may partially explain for the differences in earlier activation with the left SHKS than the right due to the time of pelvic control necessary to control the KB in the non‐dominant hand.
Limitations
The sample population included only young healthy subjects; therefore, the generalizability of the results is limited to like populations. Next, the fashion in which hip extension (as a measure of hip flexor length) was measured could lend itself to slightly increased variance as two separate investigators took these measurements based on the subject's gender in order to maintain modesty. However, to decrease possible variance in inter‐rater measurements, a PVC pipe was used under the subject's lumbar spine to determine when movement occurred. Due to all of the subjects falling within the definition of “normal” hip flexor length, the authors developed a numerical scale to categorically rank levels of increasing hip flexor length. If the scale was too narrow, it may have masked any underlying correlations between hip flexor length and gluteal activation that may have been present otherwise.
As previously noted, and in contrast to the study by McGill et al,18 the subjects in this study were inexperienced KB users and therefore were allowed to self‐select the KB weight for safety and comfort purposes. Varying the KB weight based on bodyweight and subject preference may have increased variability in percent MVIC values. Furthermore, although not examined, some of the outliers may have utilized greater ES activation or upper back and arm musculature than hip musculature in order to perform the KB swings, and this could account for some of the lower activation of recorded muscles for some subjects.
Finally, the inability to synchronize the outputs from the Dartfish and EMG software into one cohesive data set limited the authors’ ability to directly associate kinematics with EMG activity. During each swing cycle (defined as beginning with pre‐swing and ending with the return phase) a significant delay between the frequency of video collection (29.97 frames per second) and the frequency of EMG recording (1,000 data points per second) limits the authors’ ability to associate the exact timing of kinematic and muscle activity.
Conclusions
The THKS and SHKS are versatile exercises that demonstrate the ability to provide a stimulus sufficient to strengthen the GMAX, GMED and BF during a closed kinetic chain task. The terminal flexion and extension movements of the hip are fairly consistent and are easily observed or recorded from a sagittal plane view, using a video camera in a clinical or gym environment, and can be divided into five phases for education and training purposes. It appears that both passive and active hip extension ROM are associated with muscle activation of the GMAX, GMED, and BF. In addition, females appear to have greater GMAX activation in all KB swinging activities than their male counterparts. The descriptive data provided in this study supports the use of and clinical value of the KB swinging exercises in order to address a number of clinical strength and endurance impairments specific to the GMAX, GMED, and BF.
References
- 1.Janda V. Muscles and motor control in low back pain: Assessment and management. In: Twomey LT, ed. Physical therapy of the low back. New York: Churchill Livingstone; 1987:238‐278. [Google Scholar]
- 2.Janda V. Muscle weakness and inhibition (pseudoparesis) in back pain syndromes. Modern Manual Therapy of the Vertebral Column. 1986:197‐201. [Google Scholar]
- 3.Janda V. Motor learning impairment and back pain. J Man Med. 1984;22:74‐78. [Google Scholar]
- 4.Leetun DT Ireland ML Willson JD Ballantyne BT Davis IM. Core stability measures as risk factors for lower extremity injury in athletes. Med Sci Sports Exerc. 2004;36(6):926‐934. [DOI] [PubMed] [Google Scholar]
- 5.Page P. Shoulder muscle imbalance and subacromial impingement syndrome in overhead athletes. Int J Sports Phys Ther. 2011;6(1):51‐58. [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dillen LR McDonnell MK Fleming DA Sahrmann SA. Effect of knee and hip position on hip extension range of motion in individuals with and without low back pain. J Orthop Sports Phys Ther. 2000;30(6):307‐316. [DOI] [PubMed] [Google Scholar]
- 7.Lehman GJ Lennon D Tresidder B Rayfield B Poschar M. Muscle recruitment patterns during the prone leg extension. BMC Musculoskeletal Disorders. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nygren Pierce M Lee WA. Muscle firing order during active prone hip extension. J Orthop Sports Phys Ther. 1990;12(1):2‐9. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto ACL Teixeira‐Salmela LF de Paula‐Goulart FR de Morais Faria Christina Danielli Coelho Guimarães CQ. Muscular activation patterns during active prone hip extension exercises. J Electromyography and Kinesiology. 2009;19(1):105‐112. [DOI] [PubMed] [Google Scholar]
- 10.Lewis CL Sahrmann SA. Muscle activation and movement patterns during prone hip extension exercise in women. J Athl Train. 2009;44(3):238‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruno PA Bagust J. An investigation into motor pattern differences used during prone hip extension between subjects with and without low back pain. Clinical Chiropractic. 2007;10(2):68‐80. [Google Scholar]
- 12.Nadler SF Malanga GA Bartoli LA Feinberg JH Prybicien M Deprince M. Hip muscle imbalance and low back pain in athletes: Influence of core strengthening. Med Sci Sports Exerc. 2002;34(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 13.Cooper NA Scavo KM Strickland KJ Tipayamongkol N Nicholson JD Bewyer DC Sluka KA. Prevalence of gluteus medius weakness in people with chronic low back pain compared to healthy controls. Eur Spine J. 2015. (Epub ahead of print 26006705). [DOI] [PubMed] [Google Scholar]
- 14.Sahrmann SA. Diagnosis and treatment of movement impairment syndromes. St Louis, MO: Mosby, Inc.; 2002. [Google Scholar]
- 15.Janda V. Muscle strength in relation to muscle length, pain, and muscle imbalance. International Perspectives in Physical Therapy. 1993:83‐97. [Google Scholar]
- 16.Chance‐Larsen K Littlewood C Garth A. Prone hip extension with lower abdominal hollowing improves the relative timing of gluteus maximus activation in relation to biceps femoris. Man Ther. 2010;15(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 17.Oh JS Cynn HS Won JH Kwon OY Yi CH. Effects of performing an abdominal drawing‐in maneuver during prone hip extension exercises on hip and back extensor muscle activity and amount of anterior pelvic tilt. J Orthop Sports Phys Ther. 2007;37(6):320‐324. [DOI] [PubMed] [Google Scholar]
- 18.McGill SM Marshall LW. Kettlebell swing, snatch, and bottoms‐up carry: Back and hip muscle activation, motion, and low back loads. J Strength Cond Res. 2012;26(1):16‐27. [DOI] [PubMed] [Google Scholar]
- 19.Jay K Frisch D Hansen K, et al. Kettlebell training for musculoskeletal and cardiovascular health: A randomized controlled trial. Scand J Work Environ Health. 2011;37(3):196‐203. [DOI] [PubMed] [Google Scholar]
- 20.Alaranta H Luoto S Heliovaara M Hurri H. Static back endurance and the risk of low‐back pain. Clin Biomech (Bristol, Avon). 1995;10(6):323‐324. [DOI] [PubMed] [Google Scholar]
- 21.Hamberg‐van Reenen HH Ariens GA Blatter BM Twisk JW van Mechelen W Bongers PM. Physical capacity in relation to low back, neck, or shoulder pain in a working population. Occup Environ Med. 2006;63(6):371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Distefano LJ Blackburn JT Marshall SW Padua DA. Gluteal muscle activation during common therapeutic exercises. J Orthop Sports Phys Ther. 2009;39(7):532‐540. [DOI] [PubMed] [Google Scholar]
- 23.Ayotte NW Stetts DM Keenan G Greenway EH. Electromyographical analysis of selected lower extremity muscles during 5 unilateral weight‐bearing exercises. J Orthop Sports Phys Ther. 2007;37(2):48‐55. [DOI] [PubMed] [Google Scholar]
- 24.Myers JB Pasquale MR Laudner KG Sell TC Bradley JP Lephart SM. On‐the‐field resistance‐tubing exercises for throwers: An electromyographic analysis. J Athl Train. 2005;40(1):15‐22. [PMC free article] [PubMed] [Google Scholar]
- 25.Reiman MP Bolgla LA Loudon JK. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiother Theory Pract. 2012;28(4):257‐268. [DOI] [PubMed] [Google Scholar]
- 26.Guimaraes CQ Sakamoto AC Laurentino GE Teixeira‐Salmela LF. Electromyographic activity during active prone hip extension did not discriminate individuals with and without low back pain. Rev Bras Fisioter. 2010;14(4):351‐357. [DOI] [PubMed] [Google Scholar]
- 27.Tsatsouline P. Enter the kettlebell. First ed. Dragon Door Publications; 2006. [Google Scholar]
- 28.Farrar RE Mayhew JL Koch AJ. Oxygen cost of kettlebell swings. J Strength Cond Res. 2010;24(4):1034‐1036. [DOI] [PubMed] [Google Scholar]
- 29.Husley CR Soto DT Koch AJ Mayhew JL. Comparison of kettlebell swings and treadmill running at equivalent RPE values. J Strength Cond Res. 2012;26(5):1203‐7. [DOI] [PubMed] [Google Scholar]
- 30.Jay K Jakobsen MD Sundstrup E, et al. Effects of kettlebell training on postural coordination and jump performance: A randomized controlled trial. J Strength Cond Res. 2012; 27(5):1202‐9. [DOI] [PubMed] [Google Scholar]
- 31.Manocchia P Spierer DK Lufkin AK Minichiello J Castro J. Transference of kettlebell training to strength, power and endurance. J Strength Cond Res. 2012; 27(2):477‐84. [DOI] [PubMed] [Google Scholar]
- 32.Lake JP Lauder MA. Mechanical demands of kettlebell swing exercise. J Strength Cond Res. 2011; 26(12):3209‐16. [DOI] [PubMed] [Google Scholar]
- 33.Otto WH Coburn JW Brown LE Spiering BA. Effects of weightlifting vs. kettlebell training on vertical jump, strength, and body composition. J Strength Cond Res. 2012. 26(5):1199‐202. [DOI] [PubMed] [Google Scholar]
- 34.Zebis MK Skotte J Andersen CH, et al. Kettlebell swing targets semitendinosus and supine leg curl targets biceps femoris: An EMG study with rehabilitation implications. British Journal of Sports Medicine. 2012; 47(18):1192‐8. [DOI] [PubMed] [Google Scholar]
- 35.Waters TR Putz‐Anderson V Garg A Fine LJ. Revised NIOSH equation for the design and evaluation of manual lifting tasks. Ergonomics. 1993;36(7):749‐776. [DOI] [PubMed] [Google Scholar]
- 36.Dondelinger RM. Electromyography‐‐an overview. Biomedical instrumentation & technology/Association for the Advancement of Medical Instrumentation. 2010;44(2):128‐132. [DOI] [PubMed] [Google Scholar]
- 37.Neuman DA. Knee: Synergy among monoarticular and biarticular muscles of the hip and knee. In: Kinesiology of the musculoskeletal system: Foundations for rehabilitation. Second ed. St. Louis, Missouri: Mosby Elsevier; 2010:563‐565. [Google Scholar]
- 38.Kendall FP McCreary EK Provance PG Rodgers MM Romani WA Muscles: Testing and function with posture and pain. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 39.Norris BS Olson SL. Concurrent validity and reliability of two‐dimensional video analysis of hip and knee joint motion during mechanical lifting. Physiother Theory Pract. 2011;27(7):521‐530. [DOI] [PubMed] [Google Scholar]
- 40.Havenetidis K Paxinos T. Risk factors for musculoskeletal injuries among greek army officer cadets undergoing basic combat training. Mil Med. 2011;176(10):1111‐1116. [DOI] [PubMed] [Google Scholar]
- 41.Vishram JK Borglykke A Andreasen AH, et al. Impact of age on the importance of systolic and diastolic blood pressures for stroke risk: The MOnica, risk genetics, archiving, and monograph (MORGAM) project. Hypertension. 2012. ;60(5):1117‐23. [DOI] [PubMed] [Google Scholar]
- 42.Harvey D. Assessment of the flexibility of elite athletes using the modified thomas test. Br J Sports Med. 1998;32(1):68‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peeler JD Anderson JE. Reliability limits of the modified thomas test for assessing rectus femoris muscle flexibility about the knee joint. J Athl Train. 2008;43(5):470‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clapis PA Davis SM Davis RO. Reliability of inclinometer and goniometric measurements of hip extension flexibility using the modified thomas test. Physiother Theory Pract. 2008;24(2):135‐141. [DOI] [PubMed] [Google Scholar]
- 45.Portney L Watkins M. Foundations of clinical research: Applications to practice. 3rd ed. Upper Saddle River, New Jersey: Prentice Hall; 2008. [Google Scholar]
- 46.Worrell TW Karst G Adamczyk D, et al. Influence of joint position on electromyographic and torque generation during maximal voluntary isometric contractions of the hamstrings and gluteus maximus muscles. J Orthop Sports Phys Ther. 2001;31(12):730‐740. [DOI] [PubMed] [Google Scholar]
- 47.Caterisano A Moss RF Pellinger TK, et al. The effect of back squat depth on the EMG activity of 4 superficial hip and thigh muscles. J Strength Cond Res. 2002;16(3):428‐432. [PubMed] [Google Scholar]
- 48.McCaw ST Melrose DR. Stance width and bar load effects on leg muscle activity during the parallel squat. Med Sci Sports Exerc. 1999;31(3):428‐436. [DOI] [PubMed] [Google Scholar]
- 49.Lubahn AJ Kernozek TW Tyson TL Merkitch KW Reutemann P Chestnut JM. Hip muscle activation and knee frontal plane motion during weight bearing therapeutic exercises. Int J Sports Phys Ther. 2011;6(2):92‐103. [PMC free article] [PubMed] [Google Scholar]
- 50.Ebben WP. Hamstring activation during lower body resistance training exercises. Int J Sports Physiol Perform. 2009;4(1):84‐96. [DOI] [PubMed] [Google Scholar]
- 51.Mauntel TC Begalle RL Cram TR, et al. The effects of lower extremity muscle activation and passive range of motion on single leg squat performance. J Strength Cond Res. 2013;27(7):1813‐1823. [DOI] [PubMed] [Google Scholar]
- 52.Delitto RS Rose SJ. An electromyographic analysis of two techniques for squat lifting and lowering. Phys Ther. 1992;72(6):438‐448. [DOI] [PubMed] [Google Scholar]