Abstract
Background
Motor control therapeutic exercise (MCTE) for the neck is a motor relearning program that emphasizes the coordination and contraction of specific neck flexor, extensor, and shoulder girdle muscles. Because motor imagery (MI) improves sensorimotor function and it improves several motor aspects, such as motor learning, neuromotor control, and acquisition of motor skills, the authors hypothesized that a combination of MCTE and MI would improve the sensorimotor function of the cervical spine more effectively than a MCTE program alone.
Purpose
The purpose of this study was to investigate the influence of MI combined with a MCTE program on sensorimotor function of the craniocervical region in asymptomatic subjects.
Study Design
This study was a single‐blinded randomized controlled trial.
Methods
Forty asymptomatic subjects were assigned to a MCTE group or a MCTE+MI group. Both groups received the same MCTE program for the cervical region (60 minutes), but the MCTE+MI group received an additional intervention based on MI (15 minutes). The primary outcomes assessed were craniocervical neuromotor control (activation pressure value and highest pressure value), cervical kinesthetic sense (joint position error [JPE]), and the subjective perception of fatigue after effort.
Results
Intra‐group significant differences were obtained between pre‐ and post interventions for all evaluated variables (p<0.01) in the MCTE+MI and MCTE groups, except for craniocervical neuromotor control and the subjective perception of fatigue after effort in the MCTE group. In the MCTE+MI group a large effect size was found for craniocervical neuromotor control (d between ‐0.94 and ‐1.41), cervical kinesthetic sense (d between 0.97 and 2.14), neck flexor muscle endurance test (d = ‐1.50), and subjective perception of fatigue after effort (d = 0.79). There were significant inter‐group differences for the highest pressure value, joint position error (JPE) extension, JPE left rotation, and subjective perception of fatigue after effort.
Conclusion
The combined MI and MCTE intervention produced statistically significant changes in sensorimotor function variables of the craniocervical region (highest pressure value, JPE extension and JPE left rotation) and the perception of subjective fatigue compared to MCTE alone. Both groups showed statistically significant changes in all variables measured, except for craniocervical neuromotor control and the subjective perception of fatigue after effort in the MCTE group
Level of Evidence
1b
Keywords: Cervical disorders, motor imagery, motor control, therapeutic exercise
INTRODUCTION
Neck pain is one of the most frequent musculoskeletal disorders, with a one‐year prevalence of around 37%, and a significant problem in healthcare.1 Neck pain has a high prevalence in triathletes and cyclists, especially recreational athletes.2‐6 Fortunately, athletic neck pain is usually the result of minor injury and most athletes can return to full activity.7
Therapeutic exercise is an effective intervention in neck pain management in both the short (<1 month) and intermediate (1‐6 months) terms.8 One of the most interesting approaches used to manage neck pain is therapeutic exercise with a focus on motor control; some authors describe altered movement patterns (e.g., less flexible movement patterns, reduced range motion, and/or poor accuracy in maintaining maximal voluntary isometric contraction) in the cervical spines of patients with neck pain.9‐11 Motor control can be defined as the capacity of how the central nervous system produces of useful movements that are coordinated and integrated with the rest of the body and the environment.12 Thus, motor control therapeutic exercises (MCTE) are relevant to improve the status of patients with neck pain. In fact, MCTE have been demonstrated to increase motor control and reduce pain and disability in patients with neck pain.13‐15 Changes in motor control that could cause pain or dysfunction require practitioners to work on the components of motor learning for a successful intervention capable of producing satisfactory motor learning and retention. Such an intervention requires repetitive training.16,17
Alternatively, motor imagery (MI), defined as the mental representation of movement without any body movement, can be employed to improve motor performance and learn motor tasks.18 This technique has usually been used in sports, but recent researchers show that it is also effective in treating patients with neurological diseases or chronic pain.18,19 By inducing the activation of different cortical areas,18,20,21 MI is useful for influencing the central nervous system and causing plastic changes in the brain. Thus, this method should be considered for use in rehabilitation, because its goals include the improvement of motor performance and learning.18,22,23
Considering that MI improves sensorimotor function and it improves several motor aspects, such as motor learning, neuromotor control, and acquisition of motor skills,24,25 the authors hypothesized that a combination of MCTE and MI would improve the sensorimotor function of the cervical spine more effectively than a MCTE program alone. Thus, the purpose of this study was to investigate the influence of MI combined with a MCTE program on sensorimotor function of the craniocervical region in asymptomatic subjects.
METHODS
Study Design
This study was a single‐blind, randomized, and controlled trial; the assessor responsible for obtaining the study outcomes was blinded to intervention group allocation. This study was planned and conducted in accordance with the CONSORT requirements (Consolidated Standards of Reporting Trials).26
Recruitment of Participants
A convenience sample of asymptomatic volunteers was obtained from a university campus and the local community through flyers, posters, and social media. Subjects were recruited between February and May 2014. The inclusion criteria were healthy subjects between 18 and 65 years old. The exclusion criteria included the following: a) subjects who experienced neck pain in the previous 6 months; b) subjects who had been treated for neck pain in the previous 6 months; c) subjects with other chronic pain conditions; and d) subjects with difficulty in communication or understanding.
Informed consent was obtained from all subjects before inclusion. All participants received an explanation about the procedures of the study, and each one completed a questionnaire with demographic data. All of the procedures were planned under the ethical norms of the Declaration of Helsinki and were approved by the ethics committee of the Center for Advanced Studies University La Salle.
Randomization
Randomization was performed using a computer‐generated random‐sequence table with a two‐balanced block design (GraphPad Software, Inc., CA, USA). A statistician generated the randomization list, and a member of the research team who was not involved in the assessment or treatment of the participants was in charge of the randomization and maintained the list.
Once the initial assessment and inclusion of the participants were complete, the included were randomly assigned to either of the two groups (MCTE alone or MCTE‐MI) using the random‐sequence list, ensuring concealed allocation.
Blinding
The assessor was blinded to the condition of the healthy subjects being assessed. The subjects were told to freely comment to the researcher in charge of performing the allocation about how they were feeling or regarding the intervention itself. Additionally, the subjects were asked not to make any comments to the assessor.
Interventions
MCTE in isolation (MCTE)
The subjects in the MCTE group received a prescription for MCTE for the cervical region. A physiotherapist instructed the participants regarding the MCTE program in one one‐to‐one session that lasted approximately 60 minutes. In this session, participants were taught each exercise and all the details of the training program were explained (sets, repetitions, rest periods, frequency, and common mistakes in the exercises). To ensure proper motor control, the session ended with the participant performing the entire training program supervised by a physiotherapist.
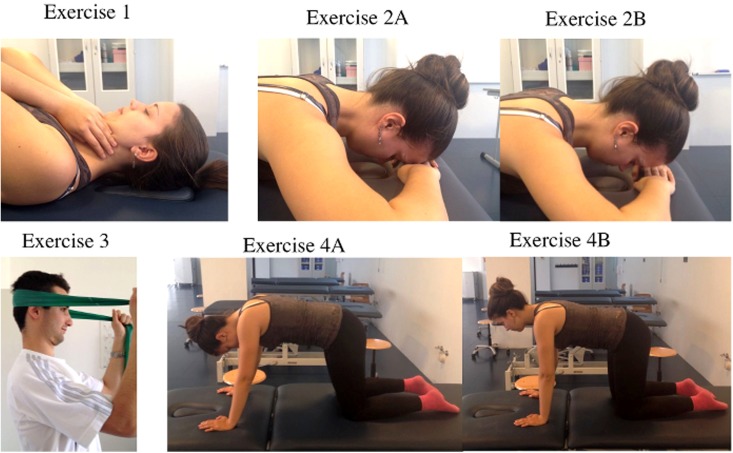
The MCTE used for this research is based on retraining the cervical muscles and included the following exercises:15 1) craniocervical flexor exercise (Figure 1, Exercise 1); 2) craniocervical extensor exercise (Figure 1, Exercises 2A‐2B); 3) co‐contraction of flexors and extensors (Figure 1, Exercise 3); and 4) a synergy exercise for retraining the strength of the deep neck flexors (Figure 1, Exercises 4A‐4B). Each of these four exercises was performed for three sets of 10‐12 repetitions, taking an approximate total duration of 10 to 20 minutes. The subjects were asked to practice the MCTE program at home once a day, 5 days a week, for 30 days.
Figure 1.
Exercises that were used in the motor control therapeutic exercise program: 1) Craniocervical flexor exercise, 2) Craniocervical extensor exercise [a=start, b=end], 3) Co‐contraction of neck flexors and extensors, 4) Synergy exercise for deep neck flexors [a=start, b=end].
MCTE in conjunction with MI (MCTE‐MI)
The subjects in the MCTE‐MI group underwent the MCTE program and also received an additional intervention based on MI. The MI intervention was explained at the end of the MCTE session and instruction lasted approximately 15 minutes. The objective of the MI program was to modify the MCTE program. The four phases of the MI intervention were performed one after the other, in order, for 4 weeks: a) kinesthetic imagery (first week); b) visual imagery (second week); c) movement observation therapy plus MI (third week); and d) exercise execution with mirror feedback (fourth week). (Table 1)
Table 1.
Phases of Motor Imagery Intervention.
| Phase | Description |
|---|---|
| 1. Kinesthetic imagery | The participants were asked to feel body sensations of the MCTE sequence without any movement |
| 2. Visual imagery | The subjects were requested to visualize mentally the MCTE sequence without any movement using the first person perspective |
| 3. MOT plus MI | The subjects viewed 4 video clips of MCTE sequences, each 15 seconds long, and had to mentally visualize performing the exercise |
| 4. Exercise execution with mirror feedback | The subjects performed the exercise in front of a mirror. |
MOT = movement observation therapy, MI = motor imagery, MCTE = motor control therapeutic exercises.
All participants (both groups) also received a booklet with written information about the indications and exercises to be practiced at home to ensure that the training program was performed properly. Each week, participants received messages by email and phone to remind and motivate them to undertake the exercise program as scheduled.
Procedure
Outcomes were obtained twice in each group, and participants were supplied with a battery of self‐report questionnaires before the intervention. The neuromotor assessment of subjects in both groups was performed before and 30 days after the intervention The measurements performed included: 1) cervical range of motion (ROM) measurements; 2) craniocervical flexion test (CCFT); 3) joint position error (JPE) test; 4) the deep neck flexor endurance test; and 5) assessment of perception of fatigue after the deep neck flexors endurance test.
Self‐report outcomes
After consenting to the study, recruited healthy subjects were given a battery of questionnaires to complete on the day of the first measurement. These included various self‐reports for sociodemographic and psychological variables, collecting information about gender, age, height, and weight, and included the validated Spanish versions of the Pain Catastrophizing Scale (PCS),27 the Tampa Scale for Kinesiophobia (TSK‐11),28 the Hospital Anxiety and Depression Scale (HADS),29,30 and the International Physical Activity Questionnaire (IPAQ).31 Each of these tools has acceptable validity and reliability.
Outcome Measures
Primary Outcome Measures
Craniocervical neuromotor control
The CCFT has been described as a neuromotor control test that evaluates the activation and isometric endurance of the deep neck flexors.16 The CCFT is performed with the subject in a supine position, with 45 º of hip flexion and 90 º of knee flexion. A feed‐back device “stabilizer” (Chattanooga Group, Inc., Hixson, TN, USA) was applied under the suboccipital region and inflated to 20 mmHg of pressure; subjects were verbally instructed to bend their heads, as if saying “yes,” to obtain a craniocervical flexion movement. A correct pattern movement of craniocervical flexion was required and had to be verified by the evaluator during the CCFT. Craniocervical flexion is described as flexion of the head over the upper cervical region without any flexion of the middle or lower cervical region. The movement was considered incorrect when activation of the sternocleidomastoid and anterior scalenus was palpable, a movement quickly took place, and/or head retraction was performed instead of craniocervical flexion. Each of these compensations were assessed by both observation and palpation. The movement was taught to each participant and practiced before the test to ensure that the craniocervical flexion was performed correctly, and the evaluator highlighted the importance of precision rather than force.32
With the CCFT, two items were measured in two phases, respectively:
Activation pressure value (APV): the highest pressure a subject could achieve and maintain for 10 seconds while properly performing the CCFT, less the baseline 20 mmHg (registered in mmHg). This first part was undertaken to determine the contractile capacity of the deep neck flexors when performing the correct movement pattern. Intra‐rater reliability for this measure was very high [ICC=0.91; 95% CI (0.85 to 0.96)].33
Highest pressure value (HPV): the highest target pressure that a subject could achieve and hold for 10 seconds, starting at a baseline of 20 mmHg and increasing by 2 mmHg at each phase, with a total of five phases and a top value 30 mmHg (target pressures of 22, 24, 26, 28, and 30 mmHg). The feed‐back device provided information to the subjects regarding the performance of the target pressure during the ten second hold, and a 30 second rest was given between phases. This second part was undertaken to determine the pressure (registered in mmHg) that the asymptomatic subject could achieve with the correct movement pattern held for ten seconds. When the subject could not perform the correct movement, the test finished and the pressure registered was the greatest pressure at which the subject performed the correct movement without substitution, which corresponded to the previous phase. The reported intra‐ and inter‐rater reliability for this test was high [ICC = 0.82, 95% CI (0.67 to 0.91)]; the minimal detectable change (MDC) was 4.70 mmHg.34
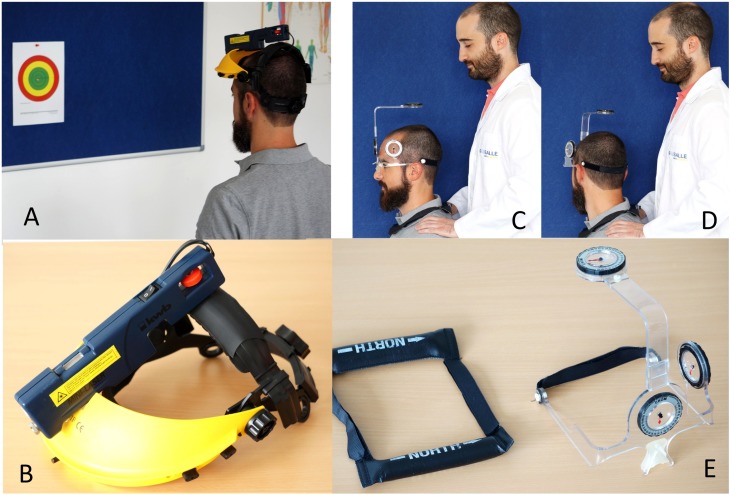
‐ Cervical kinesthetic sense. JPE tests were used (in four motions) to assess this variable. JPE is an objective measure of neck reposition sense and can quantify the alteration of neck proprioception.35,36 This measure is based on the ability to relocate the natural head posture (anatomic position) after performing several cervical movements.37 For this, a laser pointer, mounted onto a light‐weight headband, was used. The test procedure was as follows: the subjects were placed in a sitting position with the head in a resting position. A target was positioned against a wall 90 cm away from the subject's head (Figures 2A & 2B). Once the device was placed on the subject, subjects were blindfolded and asked to perform the neck movement being tested within comfortable limits and to return as accurately as possible to the starting position. The linear distance (assessed in cm) between the center and the end positions was measured and recorded. Four movements were evaluated: flexion, extension, and left and right rotations; starting each time with the patient repositioned to the center position before performing the tested movement. Regarding the reliability, the ICC for this test has been reported to range from 0.35 to 0.44, with a good agreement between days;38 and the MDC ranged from 7 to 10 mm.34
Figure 2.
Cervical kinesthetic sense testing device and target (Figure A), lightweight headband with laser pointer (Figure B), Cervical Cervical range of motion measured using the CROM (Performance Attainment Associates, Lindstrom, MN) (Figures C, D, E).
Secondary Outcome Measures
‐ Cervical ROM: Cervical ROM was measured with a cervical goniometer called CROM (Performance Attainment Associates, Lindstrom, MN). This device has three inclinometers, one in each plane of movement. A plastic support piece houses two inclinometers, which allow for the measurement of flexion, extension, and lateral flexion of the neck. The third inclinometer and magnets around the neck allow for rotation measurement39 (Figure 2C, 2D, & 2E). This device is valid and reliable for test‐retest measures [r=0.98, 95% CI (0.95 to 0.99)], with MDC for flexion of 2.2 º, extension 2.8 º, left rotation 2.1 º, right rotation 2.6 º, left lateral flexion 1.8 º, and right lateral flexion 1.6 º.40
‐Neck Flexor Muscle Endurance Test. The aim of this test was to assess neck flexor endurance, isometrically against gravity. The participants laid in a supine position with the knees and hips bent to 45 º. The test consisted of a craniocervical flexion position maintained isometrically followed by a lift of the head 2.5 cm above the plinth while the chin was maintained in the retracted position. The subjects were instructed to bend their chin and lift their head up and hold it. To check whether the subject had failed, one researcher's hand was placed under the head to monitor when the participant failed to maintain the head lift, and visual monitoring was used to establish when the chin had lost its retracted position; either event meant the test was over. The outcome of the test was time in seconds that the subject could maintain the correct craniocervical flexion position.41 The ICC value for inter‐rater reported reliability for this test ranged from 0.57 to 1.0 and the MDC was 6.4 seconds.42
‐Subjective perception of fatigue after effort. The visual analogue fatigue scale (VAFS) was used to quantify fatigue after performing the neck flexor endurance test. The VAFS consists of a 100‐mm vertical line on which the bottom represents “no fatigue” (0 mm) and the top represents “maximum fatigue” (100 mm). After the neck flexor endurance test, the subject was instructed to mark on the line the level of fatigue felt after the effort of performing the test. The researcher recorded the mark in millimeters.43
Sample Size Calculation
The necessary sample size was estimated using G*Power 3.1.7 for Windows (G*Power©, University of Dusseldorf, Germany).44 The sample size calculation was considered as a power calculation to detect between‐group differences in the primary outcome measures (craniocervical neuromotor control and cervical kinesthetic sense). To obtain 80% statistical power (1‐β error probability) with an α error level probability of 0.05, we used repeated‐measured analysis of variance (ANOVA), within‐between interaction, and a medium effect size of 0.25 to consider two groups and two measurements for primary outcomes, generating a sample size of 17 participants per group (total sample size of 34 subjects). Allowing a dropout rate of 15% and aiming to increase the statistical power of the results, the authors planned to recruit at least 40 participants to provide sufficient power to detect significant group differences.
Statistical Analysis
The Statistical Package for Social Sciences (SPSS 21, SPSS Inc., Chicago, IL, USA) software was used for statistical analysis. The normality of the variables was evaluated by the Shapiro‐Wilk test. Descriptive statistics were used to summarize the data for continuous variables and are presented as mean±standard deviation (SD), 95% confidence interval (CI), and median (interquartile interval), and categorical as absolute (number), and relative frequency (percentage). A chi‐squared test with residual analysis was used to compare categorical variables. Student's t‐test and two‐way repeated‐measures ANOVA were used to compare continuous outcome variables. The factors analyzed were group (MCTE in isolation, MCTE in conjunction with MI) and time (pre‐intervention, post‐intervention). The time x group interaction, which is the hypothesis of interest, was also analyzed. Partial eta‐squared (η2p) was calculated as a measure of effect size (strength of association) for each main effect and interaction in the ANOVAs and 0.01‐0.059 represented a small effect, 0.06‐0.139 a medium effect, and > 0.14 a large effect.45 Post hoc analysis with Bonferroni correction was performed in the case of significant ANOVA findings for multiple comparisons between variables. Effect sizes (d) were calculated according to Cohen's method, in which the magnitude of the effect was classified as small (0.20 to 0.49), medium (0.50 to 0.79), or large (0.8).46
For variables with non‐normal distributions, the Mann‐Whitney U test was utilized. The Wilcoxon signed‐rank test was used to analyze the change from the intra‐group results. The α level was set at 0.05 for all tests.
RESULTS
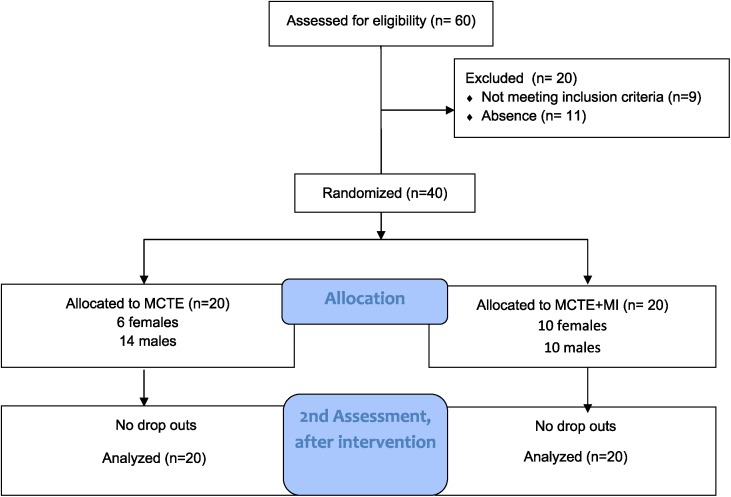
Forty healthy subjects were included in this research, and were randomly allocated in two groups of 20 subjects per group. There were no adverse events or drop‐outs reported in either group. A CONSORT flow diagram is provided in Figure 3. No statistically significant differences were present pre‐intervention between groups in demographic data and self‐reported variables (p>0.05), meaning the groups were not significantly different from each other. The demographic data and self‐reported variables are shown in Table 2. The Shapiro‐Wilk test confirmed that the data were normally distributed (p>0.05), except for age and cervical ROM.
Figure 3.
Flow diagram of recruitment and retention of subjects.
Table 2.
Summary of demographic and psychological variables. Values are mean ± SD and n (%).
| Measure | MCTE (n=20) | MCTE+MI (n=20) | P‐Value of Independent Samples χ2 Test, Mann‐Whitney U Test Or T‐Test |
|---|---|---|---|
| Age (Years) | 33.66 ± 10.47 | 33.05 ± 12.87 | 0.49† |
| Gender | 0.20** | ||
| Female | 6 (30) | 10 (50) | |
| Male | 14 (70) | 10 (50) | |
| Height (Cm) | 1.72 ± 0.72 | 1.69 ± 0.78 | 0.22* |
| Weight (Kg) | 70.60 ± 11.55 | 66.40 ± 8.42 | 0.20* |
| PCS (Points) | 6.15 ± 5.61 | 8.45 ± 7.25 | 0.27* |
| TSK‐11 (POINTS) | 18.30 ± 5.92 | 19.85 ± 6.56 | 0.44* |
| Hads (Points) | |||
| Anxiety | 2.55 ± 1.79 | 2.80 ± 1.96 | 0.68* |
| Depression | 6.05 ± 3.36 | 4.30 ± 4.03 | 0.14* |
| IPAQ (Points) | 0.44** | ||
| Slow | 4 (20) | 6 (30) | |
| Moderate | 11 (55) | 7 (35) | |
| Vigorous | 5 (25) | 7 (35) | |
MCTE = motor control therapeutic exercise; MI = motor imagery; PCS = Pain Catastrophizing Scale; TSK‐11, Tampa Scale of Kinesiophobia; HADS = Hospital Anxiety and Depression Scale; IPAQ = International Physical Activity Questionnaire;
t‐test.
χ2 test.
Mann‐Whitney U test.
Primary Outcomes
Craniocervical neuromotor control
Statistically significant differences were found for CCFT revealing differences for the group x time interaction [APV (F=9.85, p=0.003; = 0.21); HPV (F=10.18, p=0.003; = 0.21] and for the time factor [APV (F=7.54, p = 0.009; = 0.16); HPV (F=43.56, p<0.001; = 0.53)]. Pre‐post intervention differences were observed in APV and HPV in the MCTE‐MI group and between groups differences in the HPV at the post‐intervention measure, all with large effect sizes (p<0.001; Table 3).
Table 3.
Descriptive data and multiple comparisons of the primary variables.
| Measure | Group | Mean ± SD | Mean difference (95%CI); Effect size (d). | |
|---|---|---|---|---|
| Pre | Post | |||
| APV | MCTE | 5.80 ± 1.94 | 5.70 ± 1.87 | 0.10 (−0.63 to 0.83); d= 0.05 |
| MCTE+MI | 5.00 ± 1.78 | 6.50 ± 1.43 | −1.50 (−2.23 to −0.77)**; d= −0.94 | |
| Mean difference (95%CI); Effect size (d). | 0.80 (−0.39 to 1.99) d= 0.43 | −0.80 (−1.87 to 0.27) d= −0.49 | ||
| HPV | MCTE | 24.10 ± 1.77 | 24.80 ± 1.64 | −0.70 (−1.45 to 0.05); d= −0.41 |
| MCTE+MI | 23.90 ± 1.77 | 26.30 ± 1.63 | −2.40 (−3.15 to −1.66)**; d= −1.41 | |
| Mean difference (95%CI); Effect size (d). | 0.20 (−0.94 to 1.34) d= 0.11 | −1.50 (−2.55 to −0.45)** d= −0.91 | ||
| JPEFlex | MCTE | 6.64 ± 2.38 | 4.20 ± 1.70 | 2.44 (1.39 to 3.48)**; d= 1.20 |
| MCTE+MI | 5.80 ± 1.94 | 3.70 ± 1.26 | 2.10 (1.05 to 3.15)**; d= 1.31 | |
| Mean difference (95%CI); Effect size (d). | 0.84 (−0.56 to 2.23) d= 0.39 | 0.50 (−0.56 to 1.46) d= 0.34 | ||
| JPEExt | MCTE | 6.10 ± 3.11 | 4.25 ± 1.53 | 1.85 (0.45 to 3.25)*; d= 0.80 |
| MCTE+MI | 6.02 ± 2.09 | 3.03 ± 0.70 | 2.99 (1.59 to 4.39)**; d= 2.14 | |
| Mean difference (95%CI); Effect size (d). | 0.09 (−1.61 to 1.78) d= 0.03 | 1.23 (0.47 to 1.99)** d=1.09 | ||
| JPERotL | MCTE | 5.79 ± 2.86 | 4.18 ± 1.49 | 1.62 (0.34 to 2.89)*; d= 0.74 |
| MCTE+MI | 5.72 ± 2.76 | 3.43 ± 0.65 | 2.29 (1.01 to 3.57)**; d= 1.34 | |
| Mean difference (95%CI); Effect size (d). | 0.08 (−1.73 to 1.88) d=0.03 | 0.75 (0.01 to 1.49)* d=0.70 | ||
| JPERotR | MCTE | 6.35 ± 2.10 | 4.68 ± 1.68 | 1.68 (0.79 to 2.56)**; d= 0.88 |
| MCTE+MI | 5.38 ± 1.96 | 3.73 ± 1.46 | 1.65 (0.76 to 2.54)**; d= 0.97 | |
| Mean difference (95%CI); Effect size (d). | 0.98 (−0.33 to 2.28) d=0.48 | 0.95 (−0.06 to 1.96) d=0.61 | ||
MCTE = motor control therapeutic exercise; MI = motor imagery; APV = activation pressure value; HPV = highest pressure value; JPEFlex = joint position error flexion; JPEExt = joint position error extension; JPERotL = joint position error left rotation; JPERotR = joint position error right rotation.
p<0.05
p<0.001
Cervical kinesthetic sense
The were no significant group x time interaction differences for any of the measures of JPE; however, statistically significant differences were observed for the time factor in all ROMs measured for JPE (flexion: [F=38.52, p<0.001; =0.50]; extension: [F=24.53, p<0.001; =0.39]; right rotation: [F=28.84, p<0.001; =0.43]; left rotation: [F=19.12, p<0.001; =0.33]). The post hoc analysis revealed differences between the pre‐ and post‐intervention results in both groups for all the movements (p<0.05), but not between groups. The greatest effect sizes were found in the MCTE‐MI group in all measures; the largest effect size that equates to a large effect size was for the JPE extension measure (d = 2.14). The descriptive data and multiple comparisons are presented in Table 3.
Secondary Outcomes
Cervical ROM
Statistically significant differences in cervical ROM were found in both groups when the pre‐intervention data was compared with the post‐intervention data; however, the Mann‐Whitney U‐test showed no statistically significant differences between groups. The descriptive data and multiple comparisons are presented in Table 4.
Table 4.
Descriptive data and multiple comparisons of cervical ROM outcomes.
| measure | group | Median (1 and 3 quartile) | p‐values from Wilcoxon | |
|---|---|---|---|---|
| Pre | Post | |||
| Flex | MCTE | 46.50 (42.50 & 64.63) | 51.75 (47.25 & 69.38) | 0.008** |
| MCTE+MI | 51.25 (47.88 & 64.63) | 55 (50 & 67.25) | 0.016* | |
| P‐Values From Mann‐Whitney U Test | 0.33 | 0.48 | ||
| Ext | MCTE | 75 (62.88 & 79.75) | 76 (70.25 & 81.5) | 0.016* |
| MCTE+MI | 71.25 (70 & 79) | 78.75 (72.38 & 81.75) | 0.002** | |
| P‐Values From Mann‐Whitney U Test | 0.81 | 0.39 | ||
| Rot | MCTE | 132.75 (126.63 & 139) | 135 (130.5 & 153.63) | 0.007** |
| MCTE+MI | 132.75 (119.13 & 148.88) | 147 (132.88 & 157.38) | 0.001** | |
| P‐Values From Mann‐Whitney U Test | 0.97 | 0.36 | ||
| Lat. Flex | MCTE | 89.25 (75.25 & 101.5) | 95 (78.5 & 107) | 0.004** |
| MCTE+MI | 93.25 (83.75 & 111.75) | 104.5 (90.5 & 114.75) | 0.001** | |
| P‐Values From Mann‐Whitney U Test | 0.20 | 0.20 | ||
MCTE = motor control therapeutic exercise, MI = motor imagery, Flex = flexion, Ext = extension, Rot = rotation, Lat. Flex = lateral flexion.
p < 0.05
p < 0.01
Neck flexor endurance and fatigue perception
Statistically significant differences in group x time interaction were found for only for VAFS (F=10.38, p=0.03; =0.22). Regarding pre‐ to post‐ interaction for both groups, statistically significant differences were found for the deep neck flexor endurance test (F=119.80, p<0.001; =0.75) and VAFS (F=4.2, p=0.047; = 0.1). Post hoc analysis revealed differences between pre‐ and post‐intervention in both groups but not between groups (p<0.001) for the deep flexor endurance test; however, there were differences between groups post‐intervention in the VAFS (p<0.001) and only pre‐post differences in the MCTE‐MI group (p<0.001). The effect sizes were greatest in the MCTE‐MI group in both measures, especially in the neck flexor endurance test (d = 1.50). The descriptive data and multiple comparisons are presented in Table 5.
Table 5.
Descriptive data and multiple comparisons of the secondary variables.
| Measure | Group | Mean±SD | Mean Difference (95%CI); Effect Size (d). | |
|---|---|---|---|---|
| PRE | POST | |||
| Flexors Endurance Test | MCTE | 17.90 ± 8.91 | 31.90 ± 13.35 | ‐14.00 (‐17.88 TO ‐10.12)**; D=‐1.26 |
| MCTE+MI | 21.40 ± 8.90 | 37.05 ± 12.00 | ‐15.65 (‐19.53 TO ‐11.77)**; D=‐1.50 | |
| Mean Difference (95% CI); Effect Size (D) | ‐3.50 (‐9.20 TO 2.20) D=‐0.39 | ‐5.15 (‐13.28 TO 2.98) D=‐0.41 | ||
| VAFS | MCTE | 45.50 ± 19.66 | 49.00 ± 17.21 | ‐3.50 (‐12.05 TO 5.05); D=‐0.19 |
| MCTE+MI | 46.00 ± 19.97 | 30.25 ± 12.92 | 15.75 (7.20 TO 24.30)** D = 0.79 | |
| Mean Difference (95% CI); Effect Size (D) | ‐0.50 (‐13.19 TO 12.19) D=‐0.02 | 18.75 (9.01 TO 28.49)** D= 1.25 | ||
MCTE = motor control therapeutic exercise; MI = motor imagery; VAFS = visual analog fatigue scale.
p<0.05
p<0.001
DISCUSSION
This study was designed to determine the effect of MI combined with a MCTE program on the cervical region in asymptomatic subjects. This study provides new evidence of the effects of MI and MCTE on sensorimotor variables measured in the cervical region in asymptomatic subjects. An intervention of MCTE combined with MI was effective in improving craniocervical neuromotor control and the subjective perception of fatigue after effort, while MCTE in isolation did not produce changes for these same variables.
The results of the JPE, cervical ROM, and deep neck flexor endurance tests showed no statistically significant differences between groups, but statistically significant changes were observed within each group for these variables. The reported effect sizes (d) of the differences obtained in most of these variables are larger in the combined intervention group than in the MCTE group in isolation. Previous research supports the theoretical argument regarding the changes in the variables related to the cervical region for both study groups, and MI seems to have an additional effect on sensorimotor variables, such as cervical neuromotor control, perceived fatigue, and kinesthetic sense in the normal subjects studied.
Craniocervical Neuromotor Control and Cervical Kinesthetic Sense
The main measures of this study assessed the cervical kinesthetic sense and craniocervical neuromotor control, measured by the ability to activate the deep cervical flexor muscles. These two variables serve an important proprioceptive role in the integration of sensorimotor control. Previous evidence has shown that MCTE can improve JPE,47 and the current results agree, based on the observation that both groups improved in this variable; however, MI may enhance outcomes beyond MCTE alone, since it has been studied in other investigations that MI may contribute to improving the precision of movement48,49 and integrating relevant proprioceptive information.50 It is important to note that extensive evidence supports MI practice and its effects on motor behavior and motor recovery.51‐58 MI has been useful in patients that have had a stroke, have Parkinson's disease, have sustained spinal cord injury, and those who have had an amputation. In the case of post stroke rehabilitation, MI has demonstrated changes in cerebral activation observed with neurophysiological recordings and improvements in the performance of the paretic limb, increasing functionality.52,54
At present, the recovery strategies for cervical neuromotor control are based mainly on models of motor learning using therapeutic exercise, but there is no previous evidence on the use of MI to improve craniocervical neuromotor control. The current results show promising findings about improving craniocervical neuromotor control with the combination of MI and MCTE. Consistent with these results, several authors have demonstrated that MI combined with physical practice is effective in improving motor function.51,59,60 Adding the mental rehearsal (MI) to the practice of physical exercise resulted in much better performance and reduced movement time execution when performing a specific task with the upper limb.51 Also, MI is an effective method for motor learning24,25,61,62 and the acquisition of new motor skills.24
Unlike other studies, craniocervical neuromotor control did not improve in the group that performed the MCTE alone, possibly because the MCTE program was performed at home without any supervision, which could have led to subjects performing the exercise with less precision. It is important to note that most studies of MCTE programs are conducted under the supervision of a physiotherapist. The authors believe that the combined intervention improved neuromotor control, because the MI provided more information for motor learning and sensorimotor integration and promoted retention and acquisition of motor skills,63‐66 and suggest that this may be helpful when practicing at home without supervision to achieve a better performance.
Cervical Range of Movement
Prior scientific evidence has shown that MCTE improves cervical ROM.67,68 Thus, it is assumed that the improvement observed in the cervical ROM was a result of the MCTE program performed by both groups. Therefore, MI does not produce a significant effect on ROM, and for that reason, no significant differences between groups were found in this variable. Unlike the current results, other authors have found positive effects in improving flexibility through the combination of MI and physical practice in healthy athletes.55,69 For example in a study by Guillot et al55 that included MI in flexibility training, ROM improved after training. In present study, the subjects were healthy non‐athletes; therefore, the improvements observed by Gillot et al55 may have been due to the high learning ability of the athletes in terms of MI training.70
Muscular Endurance and Fatigue Perceived
No statistically significant differences were observed between the groups for the variable neck flexor endurance, but there were differences in the pre‐ and post‐intervention results in each group, which could be due to the practice or performance of the exercise program. These results suggest that MI does not influence increases in muscular endurance. The current results differ from many studies that show changes in muscle strength and voluntary torque production after MI alone or in combination with physical activity.71‐74 When MI was compared to no intervention in those studies, an improvement of endurance was observed,74,75 while in the current study the non statistically significant improvement could be due to the exercise itself, being not enough 4 weeks of intervention to obtain the results that other studies have when combining with exercise.73,75 One of the main differences between the previous studies and the current study is the areas of the body where the intervention was focused: the current focus on the neck muscles, while previous studies focused on the muscles of the upper and lower limbs. These areas of the body have a greater cortical representation (particularly the hands and ankles) than neck muscles,76 and this might make it difficult to achieve significant differences in strengthening in present sample.
Regarding the perception of fatigue as measured by the VAFS, the results showed a decrease only for the group that used a combination of MCTE and MI. In support of this, Catalan et al75 showed that a treatment to improve motor planning based on MI was effective in reducing fatigue in patients with multiple sclerosis. Also, Rozand et al77 recently showed that MI combined with physical practice does not exacerbate neuromuscular fatigue.
Practical and Scientific Implications
This is the first study investigating the effect of MI in combination with a MCTE program for the cervical region. The results of this research are promising with regard to the sensorimotor improvements obtained, but these data should be interpreted with caution because the study was conducted with asymptomatic subjects. It is not acceptable to extrapolate the results to patients with chronic neck pain. The authors of this study believe that the investigation of this type of intervention applied to symptomatic patients could generate additional information therapeutic alternatives for those with neck pain Moreover, having found differences in asymptomatic subjects, the combination of MI with an MCTE program may be recommended as a useful preventive treatment in the fight against chronic neck pain.
Both of the intervention programs used in this research could be considered cost‐effective since there was only one supervision session, and the rest of the program was performed individually at home for 30 days, for an average of 10 to 20 minutes per session. Beinart et al84 disclosed in their systematic review that a large proportion of MI programs (focused on motor skills, performance, or strength improvement, and applied to all ages) have a total duration of 34 days and a duration of on average 17 minutes per session. Unlike the program used in the current study however, the MI programs in most other studies were conducted under professional supervision.78 The method seems to be a safe intervention, and so far, no author has reported adverse effects after its completion.79
In this study, therapeutic exercise was combined with a MI program consisting of several activities related to different mental tasks, such as kinesthetic imagery, visual imagery, movement observation therapy, and MI plus exercise execution with mirror feedback. The authors believe that the combination of these mental tasks enhances motor learning, enabling better results in the combined intervention group. This study is the first to integrate all these mental tasks with physical practice.
Study Limitations
This study has several limitations. The first and most important is that the subjects' ability to generate motor images has not been quantified. This is a factor to take into consideration, as there are validated instruments to measure this variable in healthy subjects with restricted mobility.80‐82 It is important to consider since it may influence the efficiency of generating images.83
Secondly, variables were measured in only the short term, so it is necessary to measure the impact in the medium and long terms. Also, it could be considered a limitation that there was not a control group. It would also be interesting to include an experimental group with a supervised exercise program.
Another limitation is that our study did not measure exercise compliance, and the authors recommend that compliance be measured in future studies. Various mental tasks may produce an improvement in the acquisition of motor learning and skills and influence adherence to the program. However, this is speculation since a validated tool was not utilized to quantify the level of adherence. However, the authors did include motivation to increase compliance with the program, which is an important aspect of promoting adherence with exercise programs at home.84
Finally, although there were no statistical differences between the sexes, the MCTE group was only 30% female, while the MCTE plus MI group was 50% female. Along these lines, recent studies investigated the differences between males and females in sensorimotor cortical representation, and there is much we do not know about how the female body is represented in the brain or how it might change with different reproductive systems, hormones, or experiences.85
CONCLUSIONS
The results of the current study show that combining MI with MCTE produced statistically significant changes in sensorimotor function variables of the craniocervical region and the perception of subjective fatigue. Both interventions showed statistically significant changes in all variables measured, except for craniocervical neuromotor control and the subjective perception of fatigue after effort in the MCTE group. However, APV (craniocervical neuromotor control), JPE flexion and JPE right rotation (cervical kinesthetic sense), cervical ROM, and neck flexor muscle endurance were not significantly different between the two groups. These findings must be interpreted with caution because the study population was comprised of asymptomatic subjects. Future studies should be directed toward performing the same study protocol for patients with neck pain in order to check whether the combination of MI and MCTE is more effective than MCTE alone for ameliorating their neck pain.
REFERENCES
- 1.Fejer R Kyvik KO Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15(6):834‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asplund C Webb C Barkdull T. Neck and back pain in bicycling. Curr Sports Med Rep. 2005;4(5):271‐4. [DOI] [PubMed] [Google Scholar]
- 3.Korkia PK Tunstall‐Pedoe DS Maffulli N. An epidemiological investigation of training and injury patterns in British triathletes. Br J Sports Med. 1994;28(3):191‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villavicencio AT Hernández TD Burneikiene S Thramann J. Neck pain in multisport athletes. J Neurosurg Spine. 2007;7(4):408‐13. [DOI] [PubMed] [Google Scholar]
- 5.Weiss BD. Nontraumatic injuries in amateur long distance bicyclists. Am J Sports Med. 1985;13(3):187‐92. [DOI] [PubMed] [Google Scholar]
- 6.Wilber CA Holland GJ Madison RE Loy SF. An epidemiological analysis of overuse injuries among recreational cyclists. Int J Sports Med. 1995;16(3):201‐6. [DOI] [PubMed] [Google Scholar]
- 7.Zmurko MG Tannoury TY Tannoury CA Anderson DG. Cervical sprains #disc |herniations #minor |fractures, and other cervical injuries in the athlete. Clin Sports Med. 2003;22(3):513‐21. [DOI] [PubMed] [Google Scholar]
- 8.Bertozzi L Gardenghi I Turoni F, et al. Effect of therapeutic exercise on pain and disability in the management of chronic nonspecific neck pain: systematic review and meta‐analysis of randomized trials. Phys Ther. 2013;93(8):1026‐36. [DOI] [PubMed] [Google Scholar]
- 9.Woodhouse A Vasseljen O. Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet Disord. 2008;20(9):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falla D Jull G Hodges P. Training the cervical muscles with prescribed motor tasks does not change muscle activation during a functional activity. Man Ther. 2008;13(6):507‐12. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary S Jull G Kim M Vicenzino B. Cranio‐cervical flexor muscle impairment at maximal, moderate, and low loads is a feature of neck pain. Man Ther. 2007;12(1):34‐9. [DOI] [PubMed] [Google Scholar]
- 12.Michaelson P Michaelson M Jaric S Latash ML Sjölander P Djupsjöbacka M. Vertical posture and head stability in patients with chronic neck pain. J Rehabil Med. 2003;35(5):229‐35. [DOI] [PubMed] [Google Scholar]
- 13.Falla D O'Leary S Fagan A Jull G. Recruitment of the deep cervical flexor muscles during a postural‐correction exercise performed in sitting. Man Ther. 2007;12(2):139‐43. [DOI] [PubMed] [Google Scholar]
- 14.Jull G Kristjansson E Dall'Alba P. Impairment in the cervical flexors: a comparison of whiplash and insidious onset neck pain patients. Man Ther. 2004;9(2):89‐94. [DOI] [PubMed] [Google Scholar]
- 15.Hanney WJ Kolber MJ Cleland J a. Motor control exercise for persistent nonspecific neck pain. Phys Ther Rev. 2010;15(2):84‐91. [Google Scholar]
- 16.Jull G a O'Leary SP Falla DL. Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test. J Manipulative Physiol Ther. 2008;31(7):525‐33. [DOI] [PubMed] [Google Scholar]
- 17.Johnson S Hall J Barnett S, et al. Using graded motor imagery for complex regional pain syndrome in clinical practice: failure to improve pain. Eur J Pain. 2012;16(4):550‐61. [DOI] [PubMed] [Google Scholar]
- 18.Dickstein R Deutsch JE. Motor imagery in physical therapist practice. Phys Ther. 2007;87(7):942‐53. [DOI] [PubMed] [Google Scholar]
- 19.Callow N Roberts R Hardy L Jiang D Edwards MG. Performance improvements from imagery: evidence that internal visual imagery is superior to external visual imagery for slalom performance. Front Hum Neurosci. 2013;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García Carrasco D Aboitiz Cantalapiedra J. Effectiveness of motor imagery or mental practice in functional recovery after stroke: a systematic review. Neurologia. 2013;16:pii: S0213‐4853(13)00023‐6. [DOI] [PubMed] [Google Scholar]
- 21.Lotze M Halsband U. Motor imagery. J Physiol Paris. 2006;99(4‐6):386‐95. [DOI] [PubMed] [Google Scholar]
- 22.Guillot A Moschberger K Collet C. Coupling movement with imagery as a new perspective for motor imagery practice. Behav Brain Funct. 2013;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorey B Pilgramm S Bischoff M, et al. Activation of the parieto‐premotor network is associated with vivid motor imagery‐‐a parametric FMRI study. PLoS One. 2011;6(5):e20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anwar MN Tomi N Ito K. Motor imagery facilitates force field learning. Brain Res. 2011;1395:21‐9. [DOI] [PubMed] [Google Scholar]
- 25.Gentili R Papaxanthis C Pozzo T. Improvement and generalization of arm motor performance through motor imagery practice. Neuroscience. 2006;137(3):761‐72. [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF Altman DG Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726‐32. [DOI] [PubMed] [Google Scholar]
- 27.García Campayo J Rodero B Alda M Sobradiel N Montero J Moreno S. [Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia]. Med Clin (Barc). 2008;131(13):487‐92. [DOI] [PubMed] [Google Scholar]
- 28.Gómez‐Pérez L López‐Martínez AE Ruiz‐Párraga GT. Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia (TSK). J Pain. 2011;12(4):425‐35. [DOI] [PubMed] [Google Scholar]
- 29.Quintana JM Padierna A Esteban C Arostegui I Bilbao A Ruiz I. Evaluation of the psychometric characteristics of the Spanish version of the Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 2003;107(3):216‐21. [DOI] [PubMed] [Google Scholar]
- 30.Herrero MJ Blanch J Peri JM De Pablo J Pintor L Bulbena A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry. 2003;25(4):277‐83. [DOI] [PubMed] [Google Scholar]
- 31.Medina C Barquera S Janssen I. Validity and reliability of the International Physical Activity Questionnaire among adults in Mexico. Rev Panam Salud Publica. 2013;34(1):21‐8. [PubMed] [Google Scholar]
- 32.James G Doe T. The craniocervical flexion test: intra‐tester reliability in asymptomatic subjects. Physiother Res Int. 2010;15(3):144‐9. [DOI] [PubMed] [Google Scholar]
- 33.Arumugam A Mani R Raja K. Interrater reliability of the craniocervical flexion test in asymptomatic individuals‐‐a cross‐sectional study. J Manipulative Physiol Ther. 2011;34(4):247‐53. [DOI] [PubMed] [Google Scholar]
- 34.Jørgensen R Ris I Falla D Juul‐Kristensen B. Reliability, construct and discriminative validity of clinical testing in subjects with and without chronic neck pain. BMC Musculoskelet Disord. 2014;15(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revel M Andre‐Deshays C Minguet M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil. 1991;72(5):288‐91. [PubMed] [Google Scholar]
- 36.Kristjansson E Dall'Alba P Jull G. A study of five cervicocephalic relocation tests in three different subject groups. Clin Rehabil. 2003;17(7):768‐74. [DOI] [PubMed] [Google Scholar]
- 37.Treleaven J Jull G Sterling M. Dizziness and unsteadiness following whiplash injury: characteristic features and relationship with cervical joint position error. J Rehabil Med. 2003;35(1):36‐43. [DOI] [PubMed] [Google Scholar]
- 38.Kristjansson E Dall'Alba P Jull G. Cervicocephalic kinaesthesia: reliability of a new test approach. Physiother Res Int. 2001;6(4):224‐35. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher JP Bandy WD. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J Orthop Sports Phys Ther. 2008;38(10):640‐5. [DOI] [PubMed] [Google Scholar]
- 40.Audette I Dumas J‐P Côté JN De Serres SJ. Validity and between‐day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010;40(5):318‐23. [DOI] [PubMed] [Google Scholar]
- 41.Harris KD Heer DM Roy TC Santos DM Whitman JM Wainner RS. Reliability of a measurement of neck flexor muscle endurance. Phys Ther. 2005;85(12):1349‐55. [PubMed] [Google Scholar]
- 42.De Koning CHP van den Heuvel SP Staal JB Smits‐Engelsman BCM Hendriks EJM. Clinimetric evaluation of methods to measure muscle functioning in patients with non‐specific neck pain: a systematic review. BMC Musculoskelet Disord. 2008;9:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng BY Gajewski BJ Kluding PM. Reliability, responsiveness, and validity of the visual analog fatigue scale to measure exertion fatigue in people with chronic stroke: a preliminary study. Stroke Res Treat. 2010;16:pii: 412964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faul F Erdfelder E Lang A‐G Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175‐91. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Eta‐squared and partial eta‐squared in fixed factor ANOVA designs. Educ Psychol Meas. 1973;33:107‐112. [Google Scholar]
- 46.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, New Jersey: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 47.Jull G Falla D Treleaven J Hodges P Vicenzino B. Retraining cervical joint position sense: the effect of two exercise regimes. J Orthop Res. 2007;25(3):404‐12. [DOI] [PubMed] [Google Scholar]
- 48.Guillot A Desliens S Rouyer C Rogowski I. Motor imagery and tennis serve performance: the external focus efficacy. J Sports Sci Med. 2013;12(2):332‐8. [PMC free article] [PubMed] [Google Scholar]
- 49.Bernardi NF De Buglio M Trimarchi PD Chielli A Bricolo E. Mental practice promotes motor anticipation: evidence from skilled music performance. Front Hum Neurosci. 2013;7:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorey B Bischoff M Pilgramm S Stark R Munzert J Zentgraf K. The embodied nature of motor imagery: the influence of posture and perspective. Exp Brain Res. 2009;194(2):233‐43. [DOI] [PubMed] [Google Scholar]
- 51.Allami N Paulignan Y Brovelli A Boussaoud D. Visuo‐motor learning with combination of different rates of motor imagery and physical practice. Exp Brain Res. 2008;184(1):105‐13. [DOI] [PubMed] [Google Scholar]
- 52.Di Rienzo F Collet C Hoyek N Guillot A. Impact of neurologic deficits on motor imagery: a systematic review of clinical evaluations. Neuropsychol Rev. 2014;24(2):116‐47. [DOI] [PubMed] [Google Scholar]
- 53.Mulder T Zijlstra S Zijlstra W Hochstenbach J. The role of motor imagery in learning a totally novel movement. Exp Brain Res. 2004;154(2):211‐7. [DOI] [PubMed] [Google Scholar]
- 54.Stevens JA Stoykov MEP. Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil. 2003;84(7):1090‐2. [DOI] [PubMed] [Google Scholar]
- 55.Guillot A Tolleron C Collet C. Does motor imagery enhance stretching and flexibility? J Sports Sci. 2010;28(3):291‐8. [DOI] [PubMed] [Google Scholar]
- 56.Mizuguchi N Nakata H Uchida Y Kanosue K. Motor imagery and sport performance. J Phys Fit Sport Med. 2012;1(1):103‐111. [Google Scholar]
- 57.Feltz DL; Landers D. The effects of mental practice on motor skill learning and performance: A meta‐analysis. J Sport. 1997;5:25‐ 57. [Google Scholar]
- 58.Holmes P Calmels C. A neuroscientific review of imagery and observation use in sport. J Mot Behav. 2008;40(5):433‐45. [DOI] [PubMed] [Google Scholar]
- 59.Nilsen DM Gillen G Gordon AM. Use of mental practice to improve upper‐limb recovery after stroke: a systematic review. Am J Occup Ther. 2010;64(5):695‐708. [DOI] [PubMed] [Google Scholar]
- 60.Brouziyne M Molinaro C. Mental imagery combined with physical practice of approach shots for golf beginners. Percept Mot Skills. 2005;101(1):203‐11. [DOI] [PubMed] [Google Scholar]
- 61.Debarnot U Creveaux T Collet C, et al. Sleep‐related improvements in motor learning following mental practice. Brain Cogn. 2009;69(2):398‐405. [DOI] [PubMed] [Google Scholar]
- 62.Jackson PL Lafleur MF Malouin F Richards CL Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage. 2003;20(2):1171‐80. [DOI] [PubMed] [Google Scholar]
- 63.Kohl RM Ellis SD Roenker DL. Alternating actual and imagery practice: preliminary theoretical considerations. Res Q Exerc Sport. 1992;63(2):162‐70. [DOI] [PubMed] [Google Scholar]
- 64.Goss S Hall C Buckolz E Fishburne G. Imagery ability and the acquisition and retention of movements. Mem Cognit. 1986;14(6):469‐77. [DOI] [PubMed] [Google Scholar]
- 65.White A Hardy L. Use of different imagery perspectives on the learning and performance of different motor skills. Br J Psychol. 1995;86 ( Pt 2):169‐80. [DOI] [PubMed] [Google Scholar]
- 66.Hall C Bernoties L Schmidt D. Interference effects of mental imagery on a motor task. Br J Psychol. 1995;86 ( Pt 2):181‐90. [DOI] [PubMed] [Google Scholar]
- 67.Dusunceli Y Ozturk C Atamaz F Hepguler S Durmaz B. Efficacy of neck stabilization exercises for neck pain: a randomized controlled study. J Rehabil Med. 2009;41(8):626‐31. [DOI] [PubMed] [Google Scholar]
- 68.Drescher K Hardy S Maclean J Schindler M Scott K Harris SR. Efficacy of postural and neck‐stabilization exercises for persons with acute whiplash‐associated disorders: a systematic review. Physiother Can. 2008;60(3):215‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams JG Odley JL Callaghan M. Motor Imagery Boosts Proprioceptive Neuromuscular Facilitation in the Attainment and Retention of Range‐of ‐Motion at the Hip Joint. J Sports Sci Med. 2004;3(3):160‐6. [PMC free article] [PubMed] [Google Scholar]
- 70.Faubert J. Professional athletes have extraordinary skills for rapidly learning complex and neutral dynamic visual scenes. Sci Rep. 2013;3:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao WX Ranganathan VK Allexandre D Siemionow V Yue GH. Kinesthetic imagery training of forceful muscle contractions increases brain signal and muscle strength. Front Hum Neurosci. 2013;7:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lebon F Collet C Guillot A. Benefits of motor imagery training on muscle strength. J Strength Cond Res. 2010;24(6):1680‐7. [DOI] [PubMed] [Google Scholar]
- 73.Zijdewind I Toering ST Bessem B Van Der Laan O Diercks RL. Effects of imagery motor training on torque production of ankle plantar flexor muscles. Muscle Nerve. 2003;28(2):168‐73. [DOI] [PubMed] [Google Scholar]
- 74.Lebon F Guillot A Collet C. Increased muscle activation following motor imagery during the rehabilitation of the anterior cruciate ligament. Appl Psychophysiol Biofeedback. 2012;37(1):45‐51. [DOI] [PubMed] [Google Scholar]
- 75.Catalan M De Michiel A Bratina A, et al. Treatment of fatigue in multiple sclerosis patients: a neurocognitive approach. Rehabil Res Pract. 2011;2011:670537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schott GD. Penfield's homunculus: a note on cerebral cartography. J Neurol Neurosurg Psychiatry. 1993;56(4):329‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rozand V Lebon F Papaxanthis C Lepers R. Does a Mental‐Training Session Induce Neuromuscular Fatigue? Med Sci Sports Exerc. 2014;46(10):1981‐9. [DOI] [PubMed] [Google Scholar]
- 78.Schuster C Hilfiker R Amft O, et al. Best practice for motor imagery: a systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Onose G Grozea C Anghelescu A, et al. On the feasibility of using motor imagery EEG‐based brain‐computer interface in chronic tetraplegics for assistive robotic arm control: a clinical test and long‐term post‐trial follow‐up. Spinal Cord. 2012;50(8):599‐608. [DOI] [PubMed] [Google Scholar]
- 80.Williams SE Cumming J Ntoumanis N Nordin‐Bates SM Ramsey R Hall C. Further validation and development of the movement imagery questionnaire. J Sport Exerc Psychol. 2012;34(5):621‐46. [DOI] [PubMed] [Google Scholar]
- 81.Gregg M Hall C Butler A. The MIQ‐RS: A Suitable Option for Examining Movement Imagery Ability. Evid Based Complement Alternat Med. 2010;7(2):249‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malouin F Richards CL Jackson PL Lafleur MF Durand A Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther. 2007;31(1):20‐9. [DOI] [PubMed] [Google Scholar]
- 83.Martin K Moritz S Hall C. Imagery use in sport a literature review and applied model. Sport Phychologist. 1999;10:245‐248. [Google Scholar]
- 84.Beinart NA Goodchild CE Weinman JA Ayis S Godfrey EL. Individual and intervention‐related factors associated with adherence to home exercise in chronic low back pain: a systematic review. Spine J. 2013;13(12):1940‐50. [DOI] [PubMed] [Google Scholar]
- 85.Di Noto PM Newman L Wall S Einstein G. The hermunculus: what is known about the representation of the female body in the brain? Cereb Cortex. 2013;23(5):1005‐13. [DOI] [PubMed] [Google Scholar]