Abstract
Diabetic patients exhibit dysfunction of the normal wound healing process, leading to local ischemia by vascular occlusive disease as well as sustained increases in the proinflammatory cytokines and overproduction of reactive oxygen species (ROS). Of the many sources of ROS, the enzyme xanthine oxidase (XO) has been linked to overproduction of ROS in diabetic environment, and studies have shown that treatment with XO inhibitors decreases XO overactivity and XO-generated ROS. This study evaluates the role of XO in the diabetic wound and the impact of specifically inhibiting its activity on wound healing. Treatment of diabetic wounds with siXDH (xanthine dehydrogenase siRNA) decreased XDH mRNA expression by 51.6%, XO activity by 35.9%, ROS levels by 78.1%, pathologic wound burden by 31.5%, and accelerated wound healing by 7 days (23.3%). Polymerase chain reaction analysis showed that increased XO activity in wild-type wound may be due to XDH to XO conversion and/or XO phosphorylation, but not to gene transcription, whereas increased XO activity in diabetic wounds may also be from gene transcription. These results suggest that XO may be responsible for large proportion of elevated oxidative stress in the diabetic wound environment and that normalizing the metabolic activity of XO using targeted delivery of siXDH may decrease overproduction of ROS and accelerate wound healing in diabetic patients.
Complications of diabetes have an enormous public healthcare impact. In particular, the impaired cutaneous healing process characteristic of diabetic ulcers accounts for approximately $13 billion in healthcare expenditures and remains an unsolved clinical problem.1 Of the many pathophysiologic processes that have been implicated in the development of impaired wound healing, hyperglycemic-induced oxidative stress and overproduction of reactive oxygen species (ROS) have been one of the central mechanistic themes.2,3
Physiologic wound healing requires significant energy production, mainly in the form of ATP. Thus, purine metabolism plays an important role in supporting the multitude of functions required for tissue regeneration. Xanthine oxidoreductase (XOR) is a critical enzyme in the purine catabolism pathway that has been linked to overproduction of ROS in diabetes. XOR is widely distributed throughout various organs of the body and exists in two interconvertible isoforms: xanthine dehydrogenase (XDH) and xanthine oxidase (XO). Under physiologic conditions, XDH is the predominant form and is readily converted to XO either reversibly by thiol group oxidation or irreversibly by proteolytic cleavage.4 Functionally, both forms catabolize purines to urate as the rate-limiting and final step in the purine catabolism pathway. However, whereas XDH preferentially utilizes NAD+ as a reducing agent to produce NADH, XO must instead use molecular oxygen and generate ROS in the process.4
At baseline, expression of XOR is low. With increased enzymatic activity and the conversion of XOR to the XO form, a subsequent rise of ROS in plasma, hepatic, and endothelial tissues of diabetics has been previously shown.5–7 On the contrary, studies have shown that XO inhibition decreases pathologic XO activity and improves nerve and vascular function in diabetic rats and endothelial dysfunction in diabetic patients.5,6,8 However, no study to our knowledge has examined the role of XO in the diabetic wound and the impact of specifically inhibiting its activity on wound healing. In this study, we hypothesized that increased XO activity in the diabetic wound leads to elevated oxidative stress and ROS resulting in pathologic wound healing. Further, we postulate that normalizing dysfunctional metabolic activity of XO in the diabetic regenerative environment using targeted delivery of XDH siRNA (siXDH) will decrease overproduction of ROS and accelerate wound healing.
MATERIALS AND METHODS
Cell culture
NIH-3T3 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics in either low glucose (5-mM glucose) or high glucose (HG, 30 mM glucose) conditions for 2 weeks. Transfection of nonsense (NS) or siXDH (Applied Biosystems, Grand Island, NY) was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. XO activity in each culture condition was measured using the Amplex Red Xanthine/Xanthine Oxidase Assay Kit (Molecular Probes, Eugene, OR) described next.
Animals and wound healing model
Wild-type (C57Bl/6) and diabetic mice (db/db) aged 10–12 weeks were obtained from Jackson Laboratories (Bar Harbor, ME). Experiments utilized a stented excisional wound healing model described previously in full accordance with the New York University Institutional Animal Care and Use Committee.9 Briefly, after animals were anesthetized and depilated, a 6-mm punch biopsy instrument was used to create circular, full-thickness cutaneous wounds on the depilated dorsum of the mouse. To prevent wound contraction and ensure healing by secondary intention, a donut-shaped silicone splint (Grace BioLab, Bend, OR), with an external diameter of 12 mm and an internal diameter of 8 mm, was centered on the wound and affixed with interrupted 6-0 nylon sutures (Ethicon, Somerville, NJ). Semiocclusive Tegaderm dressings (3M, Flemington, NJ) were then applied over the stents to maximize wound sanitation and minimize stent removal by the animals. Animals were monitored daily for security of wound stents; any stents identified to be insecurely fastened to the mouse dorsum were reinforced with a replacement suture. At predetermined postoperative days (PODs) 1, 8, 15, 22, and 28, wounds were digitally photographed. Wound area, calibrated against the stent internal diameter, was photometrically analyzed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA), and percent wound remaining was calculated. For each group, time to wound closure (TTWC) was defined as 100% wound epithelialization and was determined to occur on the POD on which the slowest wound healed. The area under each TTWC curve was determined by GraphPad Software (GraphPad Software, Inc., La Jolla, CA). Pathologic wound burden, which reflects the degree that a pathophysiologic process retards wound healing, was calculated as the difference between the area under the curve for diabetic wounds treated with nonsense and siXDH, respectively, and that of the nonsense-treated wild-type wounds, and then expressed as percent change from the physiologic wound burden.
Topical siRNA delivery
Topical delivery of siXDH (Applied Biosystems, Grand Island, NY) to the wound healing environment was performed as previously published.10 Briefly, 20 pmol of siRNA was complexed with 0.5 uL of Lipofectamine 2000 (Invitrogen) and incorporated into a cooling (<37 °C) 0.4% (w/v) liquid agarose mixture. Based on previous studies showing optimal siRNA silencing in our wound healing model, agarose gel containing siRNA was applied on POD 1 and then every 7 days until the wound was observed to be healed. Control wounds were treated with nonsense siRNA (NS).
DNA, mRNA, and protein isolation
Wounds were harvested on POD 10 and processed for nucleic acid and protein extraction.11 Total mRNA was isolated and purified from tissue homogenate and using an RNeasy RNA tissue extraction kit (Qiagen, Valencia, CA). Total DNA was isolated and purified from tissue homogenate using a DNeasy Blood & Tissue Kit (Qiagen). The quantity of mRNA and DNA was assessed using a Nanodrop-1000 Spectrophotometer (Fisher-Scientific, Waltham, MA). Tissue homogenate was place in Mammalian Protein Extraction Reagent (Fisher Scientific, Pittsburgh, PA) for cell lysis, and extracted protein was quantified using the BCA Protein Assay (Pierce, Rockford, IL).
Real-time quantitative polymerase chain reaction
Total RNA was reversed transcribed using a QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer’s instructions. Real-time RT-PCR was performed with cDNA of the samples using SYBR green (SYBR Green PCR Master Mix, Applied Biosystems) and an ABI Sequence Detection System (Applied Biosystems, Foster City, CA). All reactions were performed in triplicate. Custom-designed primers for XDH (forward sequence “CCAAGAUCCAUAUAACGGAtt”/reverse sequence “UCCGUUAUAUGGAUCUUGGaa”) and 18S were synthesized. Standard curves were generated using 18S as the internal control, with relative amounts of mRNA normalized to 18S mRNA. Values are expressed as fold increases relative to the 18S reference.
Functional XO activity assay
XO protein activity was determined using the Amplex Red Xanthine/Xanthine Oxidase Assay Kit (Molecular Probes). Briefly, tissue homogenates underwent protein extraction and quantification using a Bradford Assay. Protein samples were diluted to obtain a final concentration of 300 ug/mL and combined with a reaction mixture of Amplex Red reagent, horseradish peroxidase, xanthine substrate, and buffer solution. In the reaction mixture, superoxide spontaneously degrades to hydrogen peroxide, which in the presence of horseradish peroxidase reacts stoichiometrically with Amplex Red reagent to generate the red-fluorescent oxidation product, resorufin. Absorbance of the protein sample was measured at 560 nm with a microplate reader (Infinite, Tecan, Crailsheim, Germany) to determine levels of protein activity.
Quantification of ROS end products
The 8-hydroxy-2′-deoxyguanosine enzyme-linked immunosorbent assay (Cell Biolaboratories, San Diego, CA) was performed according to the manufacturer’s protocol. Briefly, the 8-hydroxy-2′-deoxyguanosine capture antibody was added to a 96-well plate. After blocking, wound protein was added followed by several washes and addition of the biotinylated detection antibody. Peroxidase activity was detected with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma-Aldrich, St. Louis, MO). The optical density was measured at 450 nm with a microplate reader (Infinite, Tecan).
Statistical analysis
All data are expressed as mean ± SD. To assess XDH mRNA expression or TTWC as a function of treatment group and time, a two-way analysis of variance test was performed with a Tukey-Kramer post hoc test (family error rate of α = 0.05) to determine which levels within each factor were statistically significantly different from each another. To compare XDH mRNA expression, XO activity, and ROS levels between the treatment groups, two-sample independent measure t tests (family error rate of α = 0.05) were conducted. Cohen’s d, which reflects the magnitude of the treatment effect, was calculated and reported; by convention, d > 0.8 corresponds to a large effect size.12 For all tests, a p-value less than 0.05 was considered statistically significant. Analysis was performed using Minitab 16 (Minitab Inc., State College, PA).
RESULTS
Increased XO activity in hyperglycemic fibroblasts can be reversed by XDH silencing
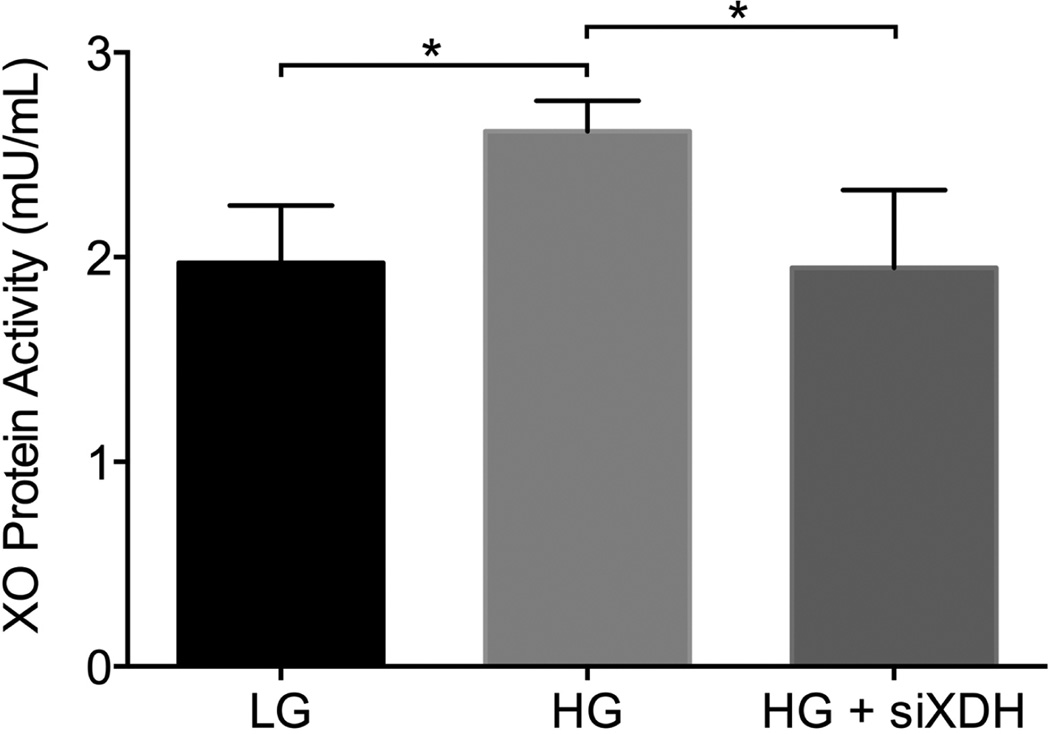
To first determine whether increased XO activity observed in diabetes was due to hyperglycemia or metabolic derangements associated with diabetes, we examined fibroblasts cultured in normal and HG conditions. We found that XO activity of hyperglycemic fibroblasts (2.615 ± 0.15 mU/mL) was significantly greater than that of normoglycemic fibroblasts (1.973 ± 0.28 mU/mL, p = 0.007; Figure 1) suggesting that hyperglycemia alone makes a significant contribution to increased enzyme activity. Furthermore, specific targeting of the XOR pathway in hyperglycemic fibroblasts using siXDH significantly decreased XO activity (1.948 ± 0.38 mU/mL) to normoglycemic levels, p = 0.017. These data suggest that hyperglycemia-induced XO overactivity may be a useful therapeutic target for normalizing purine metabolism in the diabetic milieu.
Figure 1.
XO activity in high glucose and low glucose fibroblast cells. XO activity of hyperglycemic fibroblasts (2.615 ± 0.15 mU/mL) was greater than that of normoglycemic fibroblasts (1.973 ± 0.28 mU/mL, p = 0.007) and decreased by treatment with siXDH (1.948 ± 0.38 mU/mL), p = 0.017, to that of normoglycemic levels. *p < 0.05.
XO activity increases following wounding and is pathologically elevated in diabetic wounds
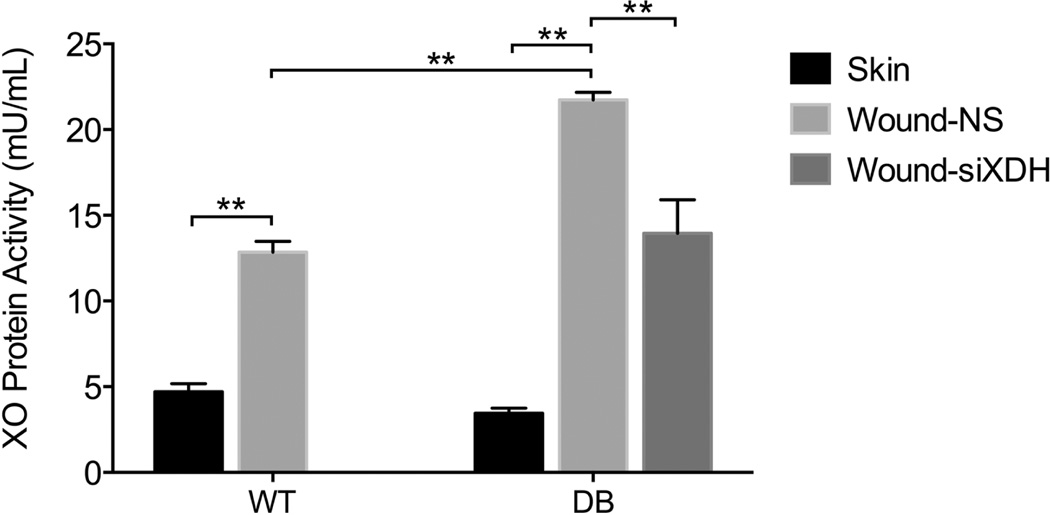
Because the wound is an ischemic environment, and low oxygen tension and diabetes are known to increase XO activity, enzyme activity was measured and compared with that of intact skin. In wild-type animals, the XO activity in the wound environment increased nearly threefold (12.86 ± 0.63 mU/mL) compared with intact skin (4.71 ± 0.46 mU/mL; p < 0.001, d = 14.8) (Figure 2), likely reflecting the increased metabolic demands of the regenerative niche. Interestingly, in diabetic animals, the XO activity in the wound environment was almost sevenfold greater (21.73 ± 0.45 mU/mL) than that of intact skin (3.45 ± 0.30 mU/mL; p < 0.001, d = 47.8) and nearly double the level of wild-type wounds (p < 0.001). These findings are consistent with XO activity in hyperglycemic fibroblasts. Therapeutic targeting of the XOR in diabetic regenerative environment using topically applied siXDH normalized XO activity in diabetic wounds (13.94 ± 1.96 mU/mL, p < 0.001) to nondiabetic levels. This suggests that specific targeting of XOR overactivity in the wound environment may have a profound impact on ROS overproduction and ultimate time to closure in this preclinical model of diabetic wound healing.
Figure 2.
XO activity in the skin and wound of wild-type and diabetic mice. In wild-type wounds, XO activity increased nearly threefold (12.86 ± 0.63 mU/mL) compared with intact skin (4.71 ± 0.46 mU/mL; p < 0.001, d = 14.8). In diabetic wounds, XO activity was nearly double the level of wild-type wounds (p < 0.001), almost sevenfold greater (21.73 ± 0.45 mU/mL) than that of intact skin (3.45 ± 0.30 mU/mL; p < 0.001, d = 47.8), and normalized by treatment with siXDH (13.94 ± 1.96 mU/mL, p < 0.001). *p < 0.001.
Increased XO activity in diabetes results in ROS overproduction in the wound environment
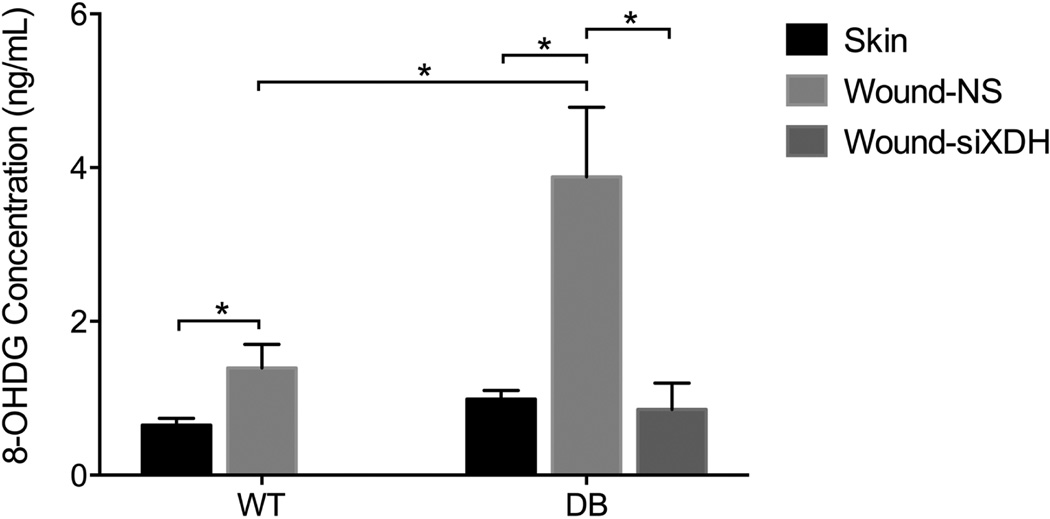
As our data suggest that XO activity is pathologically increased in the diabetic wound, and XO is known to generate ROS, we next sought to determine if XO overactivity resulted in pathologic overproduction of ROS in the wound environment. Due to its predictable and dose-dependent modification of DNA, 8-hydroxyguanosine (8-OHdG) has been shown to be a validated marker of oxidative stress and ROS levels.11 In wild-type animals, the ROS level in wounds (1.39 ± 0.31 ng/mL) was significantly greater than that of intact skin (0.65 ± 0.09 ng/mL; p = 0.0165, d = 3.24) (Figure 3). In diabetic animals, the ROS level in wounds (3.88 ± 0.91 ng/mL) was significantly greater than that of the skin (0.99 ± 0.11 ng/mL; p = 0.0055, d = 4.46) and over twofold greater than wild-type wounds (1.39 ± 0.309 ng/mL; p = 0.0109, d = 3.66).
Figure 3.
ROS levels in the skin and wound of wild-type and diabetic mice. ROS level in wild-type wounds (1.39 ± 0.31 ng/mL) was significantly greater than that of intact skin (0.65 ± 0.09 ng/mL; p = 0.0165, d = 3.24). ROS level in diabetic wounds (3.88 ± 0.91 ng/mL) was over twofold greater than wild-type wounds (1.39 ± 0.309 ng/mL; p = 0.0109, d = 3.66), significantly greater than that of the skin (0.99 Ł} 0.11 ng/mL; p = 0.0055, d = 4.46), and reduced by treatment with siXDH (0.85 ± 0.34 ng/mL, p = 0.0057). *p < 0.05.
As ROS may be generated from a variety of intracellular sources, we next examined the contribution of the XOR pathway to the observed ROS overproduction in diabetic wounds. Treatment of diabetic wounds with siXDH resulted in dramatically reduced ROS levels (0.85 ± 0.34 ng/mL, p = 0.0057) compared with nonsense-treated controls, suggesting that a significant fraction of ROS overproduction in the diabetic environment is generated from the XOR pathway of purine metabolism.
XDH mRNA expression is increased in the diabetic wound environment and can be decreased with topically applied siXDH
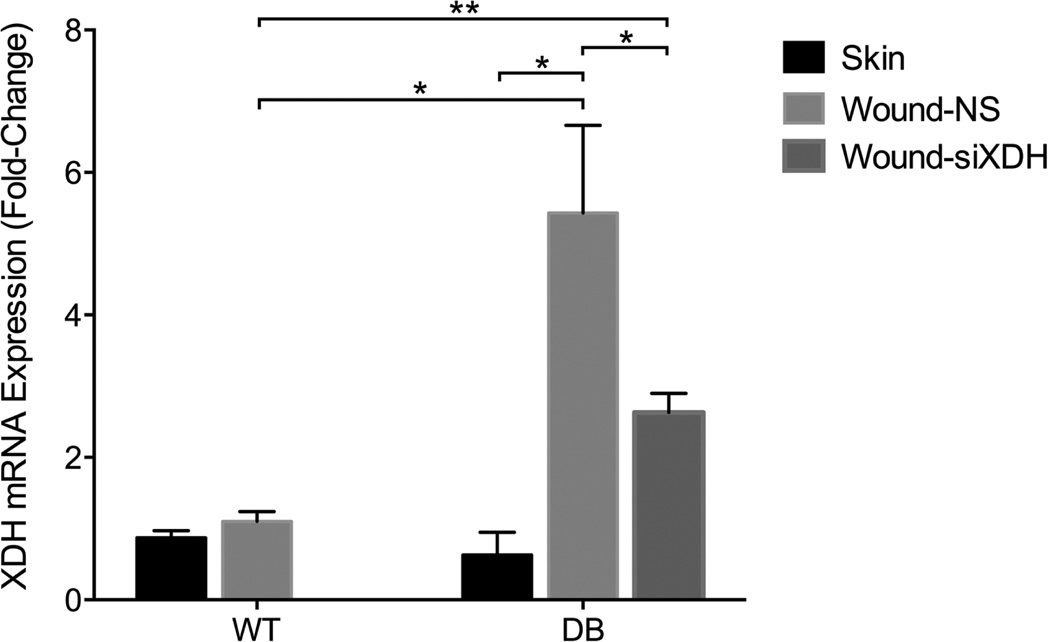
Given that XO activity is increased via multiple mechanisms including XDH to XO conversion, XDH gene transcription, and XO phosphorylation, XDH mRNA levels were measured. The XDH mRNA expression fold change was not different between the control wound and skin in the wild-type animal (Figure 4). In the diabetic animal, the XDH mRNA expression fold change in the wound environment (5.43 ± 1.23) was significantly greater than that of the skin (0.63 ± 0.32; p = 0.0028, d = 5.34) and the wild-type wound (1.10 ± 0.14; p = 0.0037, d = 4.95). However, while siXDH treatment of the diabetic wound significantly reduced XDH mRNA expression to 2.63 ± 0.27, corresponding to a 51.6% XDH mRNA knockdown efficacy, this value remained significantly greater than that of the control wild-type wound (p < 0.001). These data indicate that increased XO activity is in part due to increased XDH mRNA, and can be targeted to normalize XDH transcription and XO activity.
Figure 4.
XDH mRNA expression in the skin and wound of wild-type and diabetic mice. XDH mRNA expression fold change was not different between the control wound and skin in the wild-type animal. In the diabetic wound, XDH mRNA expression fold change (5.43 ± 1.23) was significantly greater than that of the skin (0.63 ± 0.32; p = 0.0028, d = 5.34) and the wild-type wound (1.10 ± 0.14; p = 0.0037, d = 4.95), and treatment by siXDH reduced XDH mRNA expression to 2.63 ± 0.27, significantly greater than that of the control wild-type wound. *p < 0.05; **p < 0.001.
Therapeutic targeting of dysfunctional XO activity with topical siXDH significantly improves pathologic wound burden and diabetic wound healing
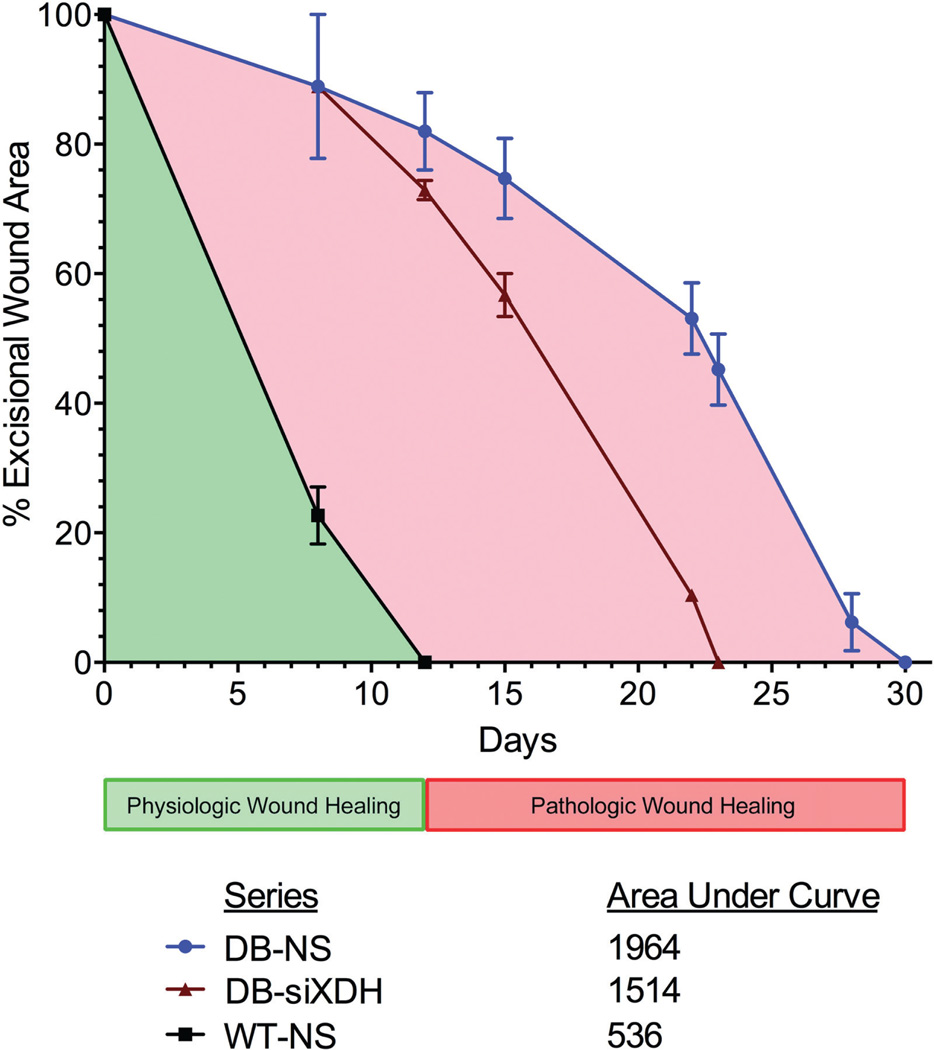
Based on the effect of topical siXDH on ROS in the diabetic wound environment, we finally sought to determine the therapeutic impact on the TTWC in our preclinical model. Compared with control diabetic wounds, which took approximately 30 days to completely heal, diabetic wounds treated with siXDH were fully healed at 23 days corresponding to a 23.3% reduction in TTWC (Figures 5 and 6). Wild-type wounds treated with NS healed at 12 days. At POD 12, the percent wound remaining of diabetic wounds treated with siXDH was 73.0 ± 1.5%, significantly less than that of diabetic wounds treated with NS (82.0 ± 6.0%; p < 0.05). At POD23, the percent wound remaining of diabetic wounds treated with NS was 45.2 ± 5.5%, significantly greater than those treated with siXDH (p = 0.0049). The pathologic wound burden in nonsense-treated diabetic wounds was decreased 31.5% by treatment with siXDH. These findings show that therapeutic targeting of XDH not only normalizes ROS of the diabetic wound to those of the wild-type wound but also that this effect is sufficient to lead to an improvement in pathologic wound burden and accelerated wound healing.
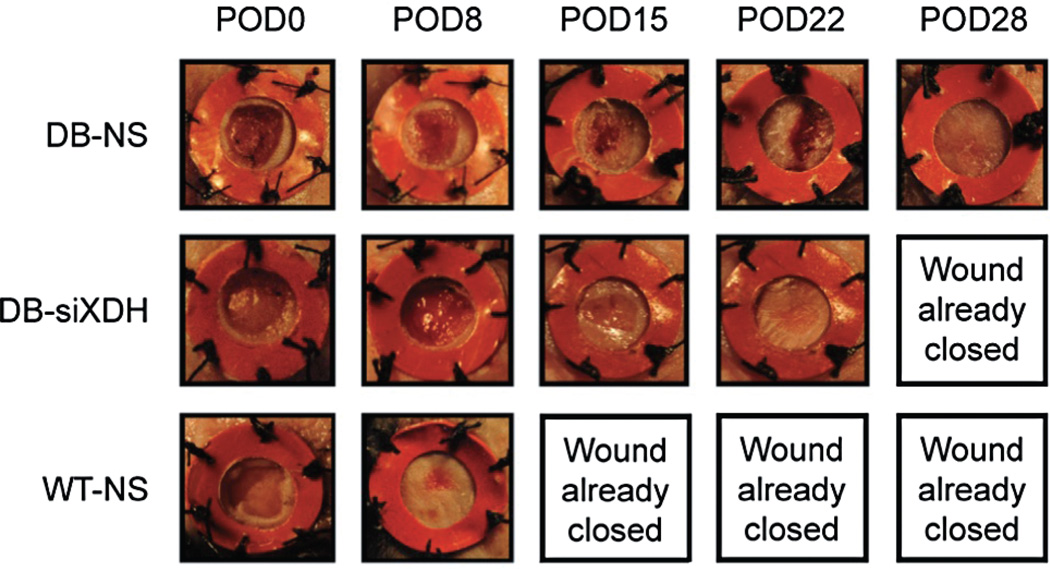
Figure 5.
Gross images of wounds in diabetic and wild-type mice over time. While control wild-type wounds healed at 12 days and control diabetic wounds healed at 30 days, diabetic wounds treated with siXDH healed at 23 days corresponding to a 23.3% reduction in TTWC.
Figure 6.
Time to wound closure and pathologic wound burden. At POD 12, the percent wound remaining of diabetic wounds treated with siXDH was 73.0 ± 1.5%, significantly less than that of the control diabetic wounds (82.0 ± 6.0%; p < 0.05). At POD23, the percent wound remaining of control diabetic wounds was 45.2 ± 5.5%, significantly greater than those treated with siXDH (p = 0.0049). The pathologic wound burden in nonsense-treated diabetic wounds was decreased 31.5% by treatment with siXDH.
DISCUSSION
Even after appropriate treatment, a large proportion of diabetic wounds do not heal, resulting in diabetes being the leading cause of nontraumatic lower limb amputations among adults in the US.13 To counter this dilemma, an abundance of new and diverse treatments has been created to promote wound healing, including negative pressure therapy, biologically based therapies containing growth factors, devices that correct imbalances in the wound environment, and bioengineered tissues.14 Unfortunately, none of these strategies has proven ideal for treating diabetic wounds, and an optimal therapeutic agent has yet to be found.
In diabetes, the normal wound healing sequence—clot formation, inflammation, reepithelialization, angiogenesis, granulation tissue formation, wound contracture, scar formation, and tissue remodeling—goes awry with significant derangements in the coordinated reparative process involving resident and infiltrating cell populations.15–17 Initially, diabetic wounds show impairments in both the recruitment of inflammatory cells and the bactericidal activity of neutrophils and other leukocytes.18–20 However, once infiltration is established, wounds of diabetic mice retain a high concentration of neutrophils and macrophages, leading to sustained increases in the proinflammatory cytokines IL-1 and TNF-α and thus a persistent inflammatory state.21 Together with the local ischemia caused by micro and macrovascular occlusive disease, multifactorial entities are involved in producing an impaired wound healing environment in diabetics.
In addition to stimulating immune cells, proinflammatory cytokines IL-1, TNF-α, IL-6, and IFN-γ are well documented to stimulate XOR gene transcription.22–27 Hence, among the numerous ROS-generating sources identified as contributors to this stress, XO has exhibited increased activity in diabetics as evident in the liver,5,26,28 heart,5,28 plasma,1,5,29 and endothelium.30 More specifically, XO bound to glycosaminoglycans on the luminal surface of endothelial cells plays a critical role in triggering the microvascular inflammatory response by ROS-mediated recruitment and activation of neutrophils.4,5,31 In the diabetic state, the XO is selectively released into the plasma by hepatocytes and binds to endothelial cells. In turn, this increases the concentration of XO enzyme in the vasculature, exacerbates local oxidative stress, and causes tissue damage.4,5 Although the endothelium and plasma are individual components of the wound, no study to our knowledge has examined the activity of XO in purine metabolism within the regenerative niche.
In this investigation, XO activity was initially evaluated in the wild-type wound and found to be increased 2.7-fold compared with that of wild-type skin. The wound bed is known to be an hypoxic environment, not only from trauma-induced macrocirculatory compromise but also because of the increased oxygen demand in metabolically active and proliferating cells located along the path of capillaries to the healing edge of tissue, with the circulating gradient ranging from 60 mmHg in the wound periphery to 0–10 mmHg in the center.32–34 Rees et al. discovered that XO activity is increased along the ischemic gradient of a skin flap with consistent skin necrosis in the distal end of the skin flap.35 Moreover, the group showed that allopurinol treatment decreased this elevated XO activity and reduced skin flap necrosis by 12.1 ± 1.0%. In a similar study, Im et al. showed that treatment with allopurinol reduced free radical injury and enhanced skin survival.36 Taken together, these studies indicate that an XO inhibitor can effectively decrease the harmful consequences of XO in the ischemic environment.
Given that studies show XO activity to be increased by hypoxia and diabetes independently, we hypothesized that the XO activity level in the diabetic wound, an environment that contains both a hypoxic and diabetic state, is increased when compared with a wild-type wound. In diabetic animals, we measured a 6.3-fold increase in XO activity in the wound compared with that in diabetic skin and found that this increase in activity was significantly greater than that observed in the wild-type wound, thus supporting our hypothesis. Treatment of these diabetic wounds with siXDH reduced XO activity by 35.9% of that of the wild-type wound, indicating that knockdown of XDH mRNA was an effective method of decreasing XO activity in the wound. However, it was unclear what effect this decreased XO activity would have on oxidative stress level and wound healing.
Concomitantly with this increased XO activity, ROS levels were elevated by 2.1-fold in wild-type wounds and 3.9-fold diabetic wounds compared with ROS levels in their respective skin. Furthermore, this increase was significantly greater in diabetic over wild-type wounds. While the increased ROS levels in the wild-type wound is likely from hypoxia-induced ROS, the greater increase in ROS levels seen in the diabetic wound may result from a persistent inflammatory state and ischemic oxidative stress that characterizes diabetes as mentioned earlier.2,4–8,26,29 When diabetic wounds were treated with siXDH, a 78.1% reduction in ROS levels was noted that was not different from ROS levels of either the NS-treated wild-type wound or the skin. This indicates that a decrease in XO activity is strongly correlated with a decrease in ROS levels, hence leading us to infer that siRNA-mediated knockdown is an effective method of reducing oxidative stress in the diabetic wound.
Although many sources of ROS generation in diabetes have been identified, including auto-oxidation of glucose and metabolites, advanced glycation, mitochondrial respiratory chain leakage, and nitric oxide synthase and NAD(P)H oxidase,6 studies have shown that increased XO activity may play a major role.5,8 Butler et al. demonstrated in a randomized, placebo-controlled clinical study of diabetic patients that allopurinol decreased XO activity and ROS levels and normalized endothelial dysfunction.8 In a streptozotocin-induced diabetic mouse model, Rajesh et al. demonstrated that XO inhibition by allopurinol or oxypurinol reduced ROS levels by 80% to the ROS level of wild-type mice and concluded that the XO system is mostly responsible for excess superoxide generation observed in diabetic mice.28 Because treatment with siXDH normalized ROS levels in diabetic wounds to those of NS-treated wild-type wound and the skin, our findings are consistent with those of Matsumoto group.
Mechanistically, increased XO activity can occur via three different phenomena: XDH to XO conversion, gene transcription, and XO phosphorylation. In diabetes, the increased oxidative stress leads to the conversion of XDH to XO either reversibly by thiol group oxidation (reversed by reducing agents or exposure to anaerobic conditions) or irreversibly by proteolytic cleavage from proteases such as trypsin, chymotrypsin, or pancreatin.4 As previously stated, in the persistent inflammatory state of diabetes, the proinflammatory cytokines IL-1, TNF-α, IL-6 and IFN-γ are chronically elevated and stimulate XOR gene transcription.23–26 Also, the activation of p38 MAP kinase and creatine kinase 2, two kinases involved in the signal transduction during inflammatory and hypoxic states, and subsequent XO phosphorylation has been shown to be accompanied by a twofold increase in XO activity.37 To elucidate the mechanisms responsible for the increased XO activity observed in our study, XDH mRNA fold change was measured and evaluated.
In our study, the efficacy of siRNA-mediated knockdown of XDH mRNA in diabetic wounds was found to be 51.6%. There was no significant difference in the XDH mRNA fold change between the skin and wound in the wild-type animal. A significant increase in XO activity in the wild-type wound compared with skin was noted, likely from either XDH to XO conversion or XO phosphorylation, with additional experiments required for further understanding. However, increased XO activity in the diabetic wound was found to have a contaminant rise in XDH mRNA fold change by 8.6; leading us to activity increase was likely also due to increased gene transcription and is consistent with similar findings reported in the literature.22,25,28 An alternative hypothesis is that XDH up-regulation occurs secondary to HIF-1α stabilization by the hypoxic wound environment as there may be a binding sequence for HIF-1α on XOR gene.27,38–40
Clinically, diabetic wounds manifest with longer TTWC than do wounds of nondiabetic patients.18,41 In our study, control diabetic wounds healed 12 days (40.0%) slower than did wild-type wounds. This is consistent with clinical observations and previous experiments in our laboratory using the stented wound model.41 One explanation for the delayed wound healing in diabetes is that the hyperglycemia-induced increase in ROS switches the cells from providing survival signals under no or mild stress to activating apoptosis under severe stress.37 Additionally, Ceradini et al. demonstrated that endothelial progenitor cell mobilization, which is reduced by hyperglycemia-induced ROS, can be restored by lowering levels of intracellular superoxide using Mn-superoxide dismutase transgenic diabetic mice that overexpress superoxide dismutase.3 Diabetic wounds treated with siXDH healed completely 7 days faster than did control diabetic wounds, corresponding to a 31.5% decrease in pathologic wound burden. Diabetic wounds treated with siXDH showed a decreased TTWC when compared with control groups, likely due to the significantly decreased ROS level in the siXDH-treated group. However, as treating diabetic wounds with siXDH normalized ROS levels to that of wild-type wounds, TTWC was still 5 days slower, suggesting that the increased ROS in diabetic wounds is unlikely to be the only contributing factor for delayed healing. This observation contributes to the multifactorial etiology of impaired healing, and other pathophysiologic phenomena in the multifactorial etiology, such as micro and macrovascular occlusive disease, require further investigation.
There were two main reasons that we chose to manipulate purine metabolism by targeting XO activity using siRNA instead of an XO inhibitor. First, XO cannot only accept electrons from xanthine and related purines at the molybdenum site (i.e., where allopurinol acts), but the enzyme can also act as a NADH oxidase, which depends solely on the separate FAD site, and no specific inhibitor exists for this XO site.24 Second, allopurinol is not only an inhibitor but also a substrate for XO.42 Together, these reasons serve as plausible explanations for the more marked reduction in ROS levels seen in our study, which utilized gene silencing with siRNA than in the study Matsumoto et al., which used allopurinol and oxypurinol.
Lastly, the topical application of siRNA has been shown to be transient and local, alleviating concerns about prolonged action and off-target effects.10 In fact, classic xanthinuria type I, which is the genetic deficiency of XOR, is a mostly asymptomatic disease.4 Furthermore, XOR functions to catabolize purines to urate in the purine catabolism pathway within the larger purine salvage pathway, which interconverts purine-derivative nucleosides to nucleotides presumably needed during the proliferative phase of wound healing. Taken together, clinically significant complications from siXDH treatment seem unlikely, although additional studies are needed to confirm this hypothesis. Normalizing dysfunctional metabolic activity of XO in the diabetic regenerative environment using this novel therapeutic modality may offer tremendous translational potential for the treatment of diabetic wounds.
In conclusion, XO activity is increased in diabetic wounds, and XO-generated ROS is responsible for a large proportion of elevated oxidative stress in the diabetic wound environment. Treatment of diabetic wounds with siXDH decreased XDH mRNA expression by 51.6% and XO activity by 35.9%. As a result, ROS levels in the diabetic regenerative niche decreased by 78.1%, which ultimately decreased pathologic wound burden by 31.5%. PCR analysis showed that increased XO activity in wild-type wound may be due to XDH to XO conversion and/or XO phosphorylation, but not to gene transcription, whereas increased XO activity in diabetic wounds may also be from gene transcription. Although siXDH treatment accelerated wound healing by 7 days, thereby decreasing the pathologic wound burden by 31.5%, wild-type wounds still healed 5 days faster. Taken together, the results of this study support a multifactorial etiology for impaired wound healing in diabetes.
ACKNOWLEDGMENTS
Source of Funding: The authors would like to thank The National Institute of Diabetes and Digestive and Kidney Diseases (grant number T35DK007421) and myFace for helping to fund this study.
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 3.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Banerjee A, Banerjee UC. Xanthine oxidoreductase: a journey from purine metabolism to cardiovascular excitation-contraction coupling. Crit Rev Biotechnol. 2011;31:264–280. doi: 10.3109/07388551.2010.527823. [DOI] [PubMed] [Google Scholar]
- 5.Desco MC, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 6.Inkster ME, Cotter MA, Cameron NE. Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats. Eur J Pharmacol. 2007;561:63–71. doi: 10.1016/j.ejphar.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 8.Butler R, Morris AD, Belch JJF, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 9.Galiano RD, Michaels J, 5th, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 10.Thanik VD, Greives MR, Lerman OZ, Seiser N, Dec W, Chang CC, et al. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305–1308. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen PD, Tutela JP, Thanik VD, Knobel D, Allen RJ, Jr, Chang CC, et al. Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen. 2010;18:553–559. doi: 10.1111/j.1524-475X.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. [accessed March 14, 2013];2011 National Diabetes Fact Sheet. 2011 Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 14.White R, McIntosh C. A review of the literature on topical therapies for diabetic foot ulcers. Part 2: advanced treatments. J Wound Care. 2009;18:335–341. doi: 10.12968/jowc.2009.18.8.43633. [DOI] [PubMed] [Google Scholar]
- 15.DiPietro LA, Polverini PI, Rahbe SM, Kovacs EJ. Modulation of JE/MCP-l expression in dermal wound repair. Am J Pathol. 1993;146:868–875. [PMC free article] [PubMed] [Google Scholar]
- 16.Engeihardt E, Toksoy A, Goebeler M, Debus S, Bröcker EB, Gillitzer R. Chemokines IL-8, GROalpha, MGP-t, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular. 2007;15:350–355. doi: 10.2310/6670.2007.00056. [DOI] [PubMed] [Google Scholar]
- 18.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierusz-Wysocka B, Wysocki H, Wykretowicz A, Szczepanik A, Siekierka H. Phagocytosis, bactericidal capacity, and superoxide anion production by polymorphonuclear neutrophils from patients with diabetes mellitus. Folia Haematol Int Mag Klin Morphol Blutforsch. 1985;112:658–668. [PubMed] [Google Scholar]
- 20.Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- 21.Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 22.Dupont GP, Huecksteadt TP, Marshall BC, Ryan US, Michael JR, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feve B, Bastard J. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 24.Harrison R. Structure and function of xanthine oxidase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer KD, Huecksteadt TP, Hoidal JR. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J Immunol. 1994;153:1789–1797. [PubMed] [Google Scholar]
- 26.Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardó FV, Sastre J, et al. Xanthine oxidase-induced oxidative stress causes activation of NF-κB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med. 2010;49:171–177. doi: 10.1016/j.freeradbiomed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Terada LS, Piermattei D, Shibao GN, McManaman JL, Wright RM. Hypoxia regulates xanthine dehydrogenase activity at preand post-translational levels. Arch Biochem Biophys. 1997;348:163–168. doi: 10.1006/abbi.1997.0367. [DOI] [PubMed] [Google Scholar]
- 28.Rajesh M, Mukhopadhyay P, Batkai S, Mukhopadhyay B, Patel V, Hasko G, et al. Xanthine oxidase inhibitor allopurinol attenuated the development of diabetic cardiomyopathy. J Cell Mol Med. 2009;13(8B):2330–2341. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto S, Koshiishi I, Inoguchi T, Nawata H, Utsumi H. Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice. Free Radic Res. 2003;37:767–772. doi: 10.1080/1071576031000107344. [DOI] [PubMed] [Google Scholar]
- 30.Pritsos CA. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem Biol Interact. 2000;129:195–208. doi: 10.1016/s0009-2797(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 31.Meneshian A, Bulkley GB. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- 32.Eisenbud DE. Oxygen in wound healing: nutrient, antibiotic signaling molecule, and therapeutic agent. Clin Plast Surg. 2012;39:293–310. doi: 10.1016/j.cps.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Hunt TK. Oxygen and skin wound healing. In: Rovee DT, Maibach HI, editors. The epidermis in wound healing. Boca Raton, FL: CRC Press; 2004. pp. 183–197. [Google Scholar]
- 34.Fries RB, Wallace WA, Roy S. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005;579:172–181. doi: 10.1016/j.mrfmmm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Rees R, Smith D, Li TD, Cashmer B, Garner W, Punch J, et al. The role of xanthine oxidase and xanthine dehydrogenase in skin ischemia. J Surg Res. 1994;56:162–167. doi: 10.1006/jsre.1994.1027. [DOI] [PubMed] [Google Scholar]
- 36.Im MJ, Manson PN, Bulkley GB, Hoopes JE. Effects of superoxide dismutase and allopurinol on the survival of acute island skin flaps. Ann Surg. 1985;201:357–359. doi: 10.1097/00000658-198503000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem. 2001;276:14359–14365. doi: 10.1074/jbc.M010100200. [DOI] [PubMed] [Google Scholar]
- 38.Griguer CE. Xanthine oxidase-dependent regulation of hypoxia-inducible factor in cancer cells. Cancer Res. 2006;66:2257–2263. doi: 10.1158/0008-5472.CAN-05-3364. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda E. Cellular response to tissue hypoxia and its involvement in disease progression. Pathol Int. 2005;55:603–610. doi: 10.1111/j.1440-1827.2005.01877.x. [DOI] [PubMed] [Google Scholar]
- 40.Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Regulation of HIF by the von Hippel-Lindau tumor suppressor: implications for cellular oxygen sensing. IUBMB Life. 2001;52:43–47. doi: 10.1080/15216540252774757. [DOI] [PubMed] [Google Scholar]
- 41.Tepper OM, Carr J, Allen RJ, Jr, Chang CC, Lin CD, Tanaka R, et al. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1 alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes. 2010;59:1974–1983. doi: 10.2337/db09-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriwaki Y, Yamamoto T, Higashino K. Enzymes involved in purine metabolism—a review of histochemical localization and functional implications. Histol Histopathol. 1999;14:1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]