Abstract
Though quantitative trace toxic metals analyses have been performed on tobacco products, little has been published on inorganic particulate constituents on and inside the products. We analyzed these constituents using scanning electron microscopy with energy dispersive x-ray spectroscopy (SEM-EDS). The nature of SEM-EDS instrumentation makes it an ideal choice for inorganic particulate analyses and yields relevant information to potential exposures during consumption of oral tobacco products, and possibly as a consequence of smoking.
Aluminum silicates, silica, and calcium compounds were common inorganic particulate constituents of tobacco products. Aluminum silicates and silica from soil were found on external leaf surfaces. Phytolithic silica, found in the lumen of the plant leaf, is of biogenic origin. Calcium oxalate was also apparently of biogenic origin.
Small mineral deposits on tobacco could have health implications. Minerals found on the surfaces of smokeless tobacco products could possibly abrade the oral mucosa and contribute to the oral inflammatory responses observed with smokeless tobacco product use. If micron and sub-micron size calcium particles on cigarette filler were transported in mainstream smoke, they could potentially induce a pulmonary irritant inflammation when inhaled. The transport of aluminum silicate and silica in smoke could potentially also contribute to chronic inflammatory disease.
Introduction
In 2010, the top five causes of death in the United States were heart disease, cancer, chronic lower respiratory diseases (asthma and chronic obstructive pulmonary disease, or COPD), cerebrovascular diseases (stroke), and unintentional injuries [1]. Tobacco use, tobacco smoke, and second hand tobacco smoke exposure are among the major modifiable risk factors for four of these five leading causes of preventable premature deaths [1]. Cigarette smoking and disease caused by exposure to secondhand smoke are estimated to cause more than 480,000 deaths annually [2].
Based on a risk assessment analysis of exposure to chemical constituents in cigarettes smoke at levels observed using the ISO smoking regimen, it has been suggested that metals contribute substantially to the health risks of smoking [3,4]. We have previously reported total concentrations of the metals in total mainstream smoke particulate matter in U.S. and counterfeit U.S. cigarettes [TPM, 5–7], using both the ISO [4] and Canada Intense [8] smoking regimens.
Little work has been done to establish the health risks to oral, pharyngeal, and other tissues of the various irritants and toxicants from smokeless tobacco products. Smokeless tobacco use (snuff and chewing tobacco) is a risk factor for heart disease, oropharyngeal cancers, and precancerous oral lesions such as leukoplakia and erythroplakia [9]. Health risks from the use of smokeless tobacco products include oral, pharyngeal and pancreatic cancers [9, 10], leukoedema [11], which may be an indication of allergic stomatitis, [12], leukoplakia and erythroplakia [13], and other pathological conditions. We have reported concentrations of toxic metals in smokeless tobacco products available in the U.S., as well as the extractability of some of these toxic substances in artificial saliva which might contribute to oral health risks [14].
We have performed toxic metals analyses in both combustible tobacco filler and in smokeless tobacco products [14, 15], but scant work has been published on characterization of inorganic particulate constituents of tobacco products whether indigenous or from environmental sources such as the soil. We analyzed inorganic particulate constituents of select cigarette filler and smokeless tobacco products using Scanning Electron Microscopy with Energy Dispersive X-Ray Spectroscopy (SEM-EDS). Exposure to inorganic particulate materials could potentially occur as an unintended consequence of smoking or from using smokeless tobacco products. The profiles of the major inorganic constituents determined on and beneath the tobacco leaf surface using SEM-EDS, provide a perspective on the inorganic substances complementary to those from trace metals product analysis performed using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Our data demonstrates that SEM-EDS enables exploration of major inorganic constituents of particles on tobacco products that is complementary with the trace toxic constituent analyses.
Experimental
Sample preparation
Commercially available cigarette and smokeless tobacco products obtained between 2010 and 2014 were evaluated for this study. The smokeless products were dried for 18 hours at 90°C, mounted on double coated conductive carbon tabs and placed in a desiccator overnight before analysis. Cigarette filler tobacco was mounted on double coated conductive carbon tabs and placed in a desiccator overnight before analysis.
Instrumentation
Images were acquired with a Quanta 250 Field Emission Gun Scanning Electron Microscope (SEM) with “environmental mode (ESEM)” capabilities (FEI Co., Hillsboro, OR, USA). The SEM was equipped with an Energy Dispersive X-Ray (EDS) attachment with an 80 mm2 X-MaxN Silicon Drift Detector (SDD) with Aztec software (Oxford Instruments, Concord, MA, USA). Instrumental settings differed from sample to sample and will be described for each resulting image (see the figure captions).
Results
The utilization of low water vapor pressures to prevent sample charging in ESEM and Low Vacuum modes of the Quanta 250 made it possible to acquire images of particles on non-conductive tobacco samples without carbon coating or heavy metal complexation steps during sample preparation. The compositions of particles that were most commonly observed on tobacco product leaf surfaces were forms of silica, aluminum silicates, and calcium compounds, predominantly calcium oxalate. Two rod shaped silicates and one silicate with undetermined morphology are shown on a popular commercial chewing tobacco leaf surface in Figure 1. The elemental compositions of the three largest particles were predominantly oxygen and silicon. The small bright particle (top) and the two rod-shaped particles had 10–15% aluminum substitution on a molar basis when normalized to silicon and minor quantities of sodium and potassium. The elemental composition and the morphology of the sodium and potassium aluminum silicate rod structures were consistent with common soil halloysites [16].
Figure 1.

A SEM micrograph of soil halloysite aluminum silicate crystals on a popular commercial chewing tobacco leaf surface. The particle at the top of the image is also an aluminum silicate, though not identifiable on the basis of morphology. This image was obtained in Environmental (ESEM) mode with an accelerating voltage of 10kV, spot size 5.0, 2 torr vapor pressure in the sample chamber, with a 500µm pressure limiting aperture on a Gaseous Analytical Detector (GAD).
An SEM micrograph with an embedded EDS map of inorganic particles on a popular commercial fine cut snuff leaf surface is shown in Figure 2. An aluminum silicate particle with a width of approximately 25 µm and a smaller one with a width of approximately 3 µm are characterized using EDS in the total elemental map and in the single element maps. In addition to the aluminum silicates, the magenta, orange and yellow particles are calcium, sodium chloride and potassium chloride particles, respectively.
Figure 2.
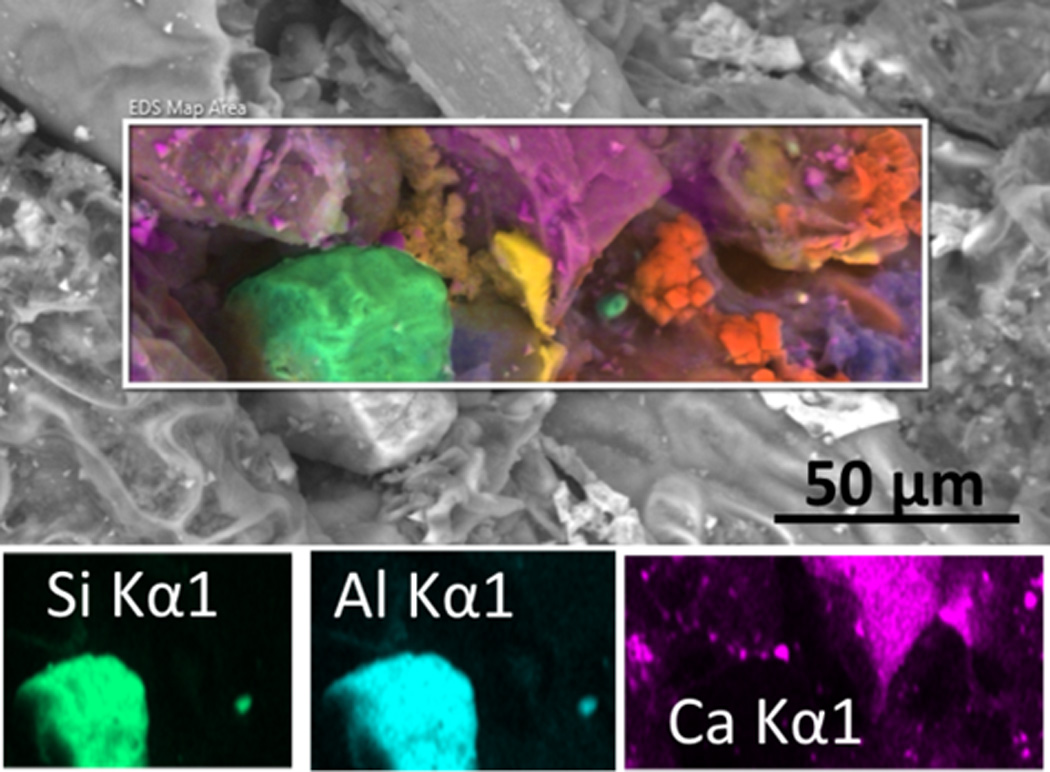
EDS color coded elemental mapping embedded in a SEM micrograph of inorganic particles on a popular commercial fine cut snuff leaf surface. Two aluminum silicate particles (green) of different sizes are visible. Also present are calcium compounds (magenta), sodium chloride (orange) and potassium chloride (yellow) particles. These images were obtained in Low Vacuum mode with an accelerating voltage of 20 kV, spot size 5.0, 0.6 torr vapor pressure in the sample chamber, with a Concentric Backscatter Detector (CBS).
An SEM micrograph of particles on the surface of reconstituted tobacco from the blend of a popular cigarette brand is shown in Figure 3. The elongated particle is predominantly silica, similar in morphology to a halloysite, but with numerous etch pits and smaller silica particle structures on the surface [17]. The EDS analysis showed negligible aluminum substitution in this silica structure. Potassium sulfate, two aluminum silicates and a calcium compound are also observed on the surface. The high concentration of particles, and specifically of silica and aluminum silicates on reconstituted tobacco was characteristic of the reconstituted tobacco in the cigarette filler blends of multiple brands that were examined.
Figure 3.
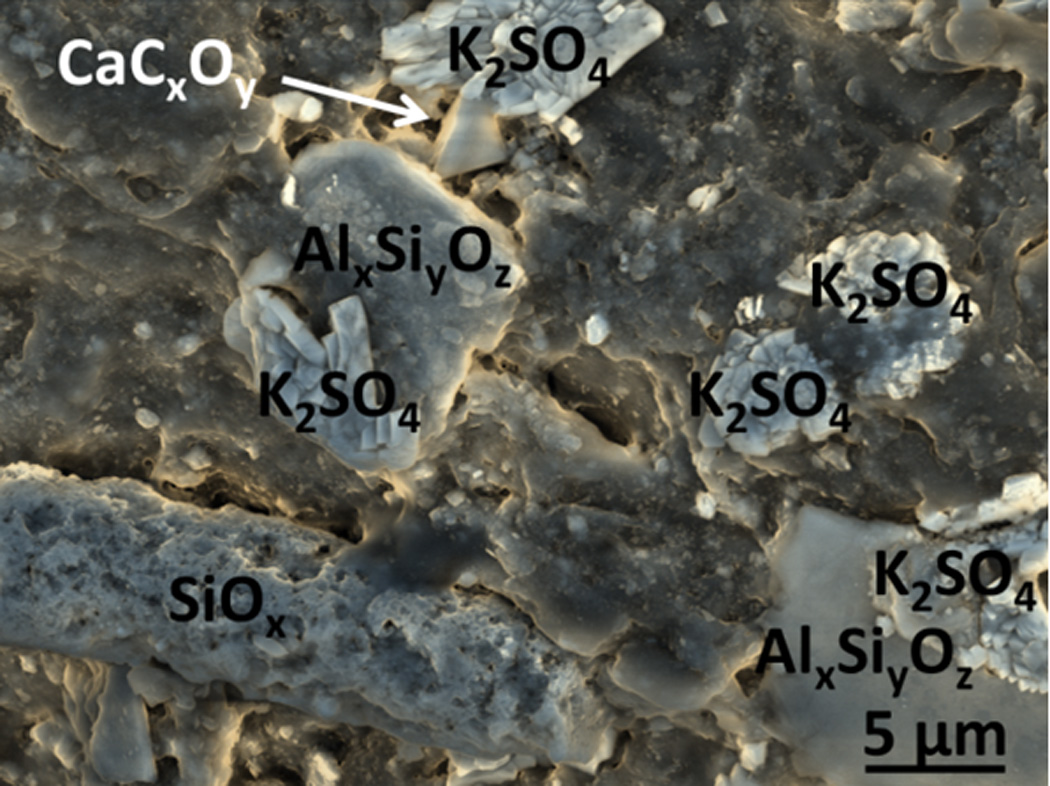
False color SEM micrograph of particles on the surface of reconstituted cigarette filler tobacco from a popular cigarette brand. Two aluminum silicate particles of different sizes are visible, along with potassium sulfate crystals, and apparently calcium oxalate. The elongated structure is a halloysite-like silica structure with numerous etch pits, but negligible aluminum. This image was obtained in Low Vacuum mode with an accelerating voltage of 10 kV, 0.6 torr vapor pressure in the sample chamber, spot size 3.0, and mixed Large Field (LFD) and Concentric Backscatter Detectors (CBS).
An SEM micrograph of a cross section view of the internal space inside a cut piece of flue cured cigarette filler tobacco from a popular cigarette brand is shown in Figure 4. The large bright particle in the foreground and similar smaller particles in the background are composed of silica (SiOx). These internal particle structures are apparently silica phytoliths of tobacco biogenic origin.
Figure 4.
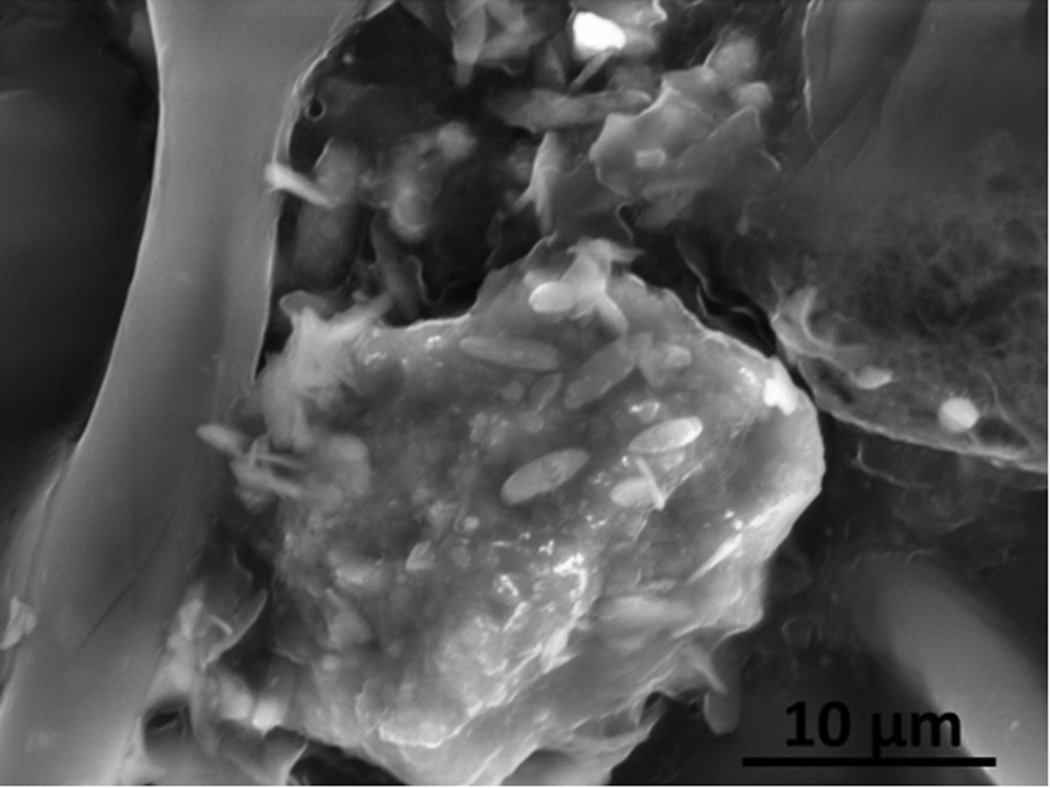
A SEM micrograph of phytolithic silica viewed in the internal cross-section of a sliced flue cured cigarette filler tobacco leaf from a popular cigarette brand. This image was obtained in Low Vacuum mode with an accelerating voltage of 20 kV, 1.0 torr water vapor pressure in the sample chamber, spot size 3.0 with mixed Large Field (LFD) and Concentric Backscatter Detectors (CBS).
An EDS map of the particles on the surface of filler tobacco from a popular cigarette brand is shown in Figure 5. The large light-green particle is apparently a crystalline silica. The yellow particles are predominantly aluminum oxide. Orange colored particles are calcium-, carbon-, and oxygen-containing particles of undetermined composition.
Figure 5.
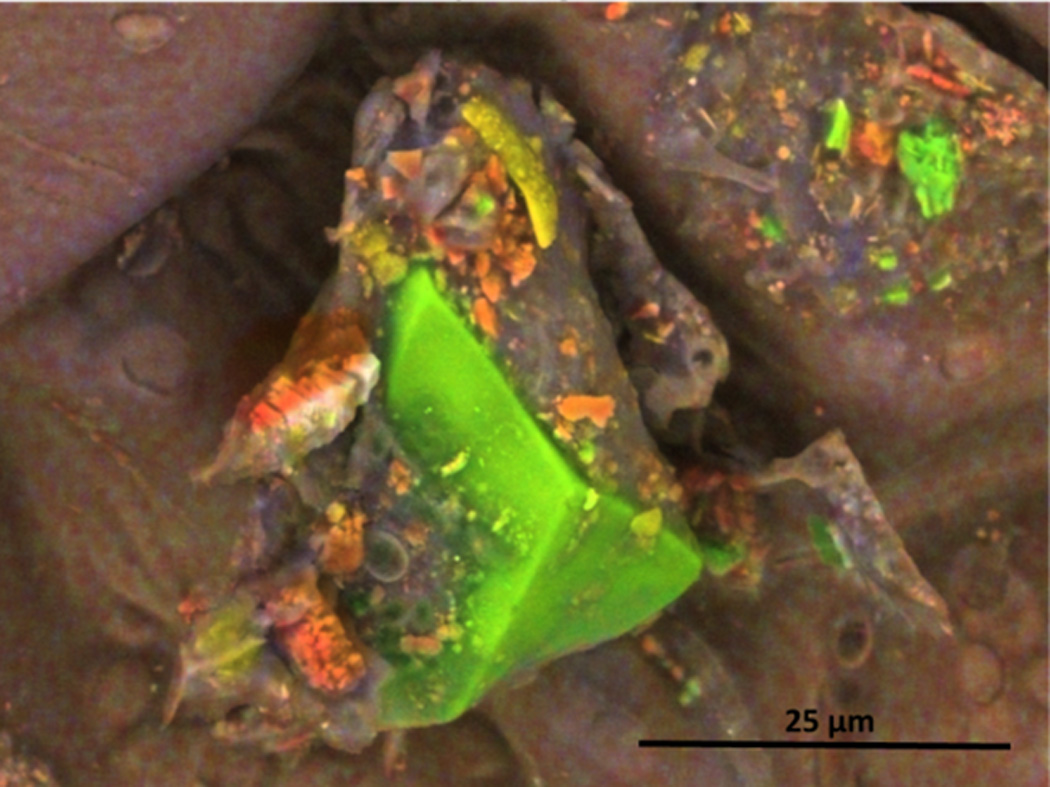
EDS color coded compositional map of particles on the surface of filler tobacco from a popular cigarette brand. The particles visible are primarily calcium, carbon, and oxygen (orange), silica (green), and aluminum and oxygen-containing particles (yellow). This image was obtained in Low Vacuum mode with an accelerating voltage of 10 kV, 0.75 torr water vapor pressure in the sample chamber, spot size 3.0 with a Concentric Backscatter Detector (CBS).
An SEM micrograph of calcium-containing particles apparently being extruded from the interior of a broken piece of burley cigarette filler tobacco from a popular cigarette brand is shown in Figure 6. The carbon to oxygen ratio obtained from the EDS analysis was more consistent with calcium oxalate than calcium carbonate, though the high carbon matrix of the underlying tobacco did not permit an exact stoichiometric ratio. Based on calcium, carbon, and oxygen content, as well as crystal morphology, they are apparently calcium oxalate [18].
Figure 6.
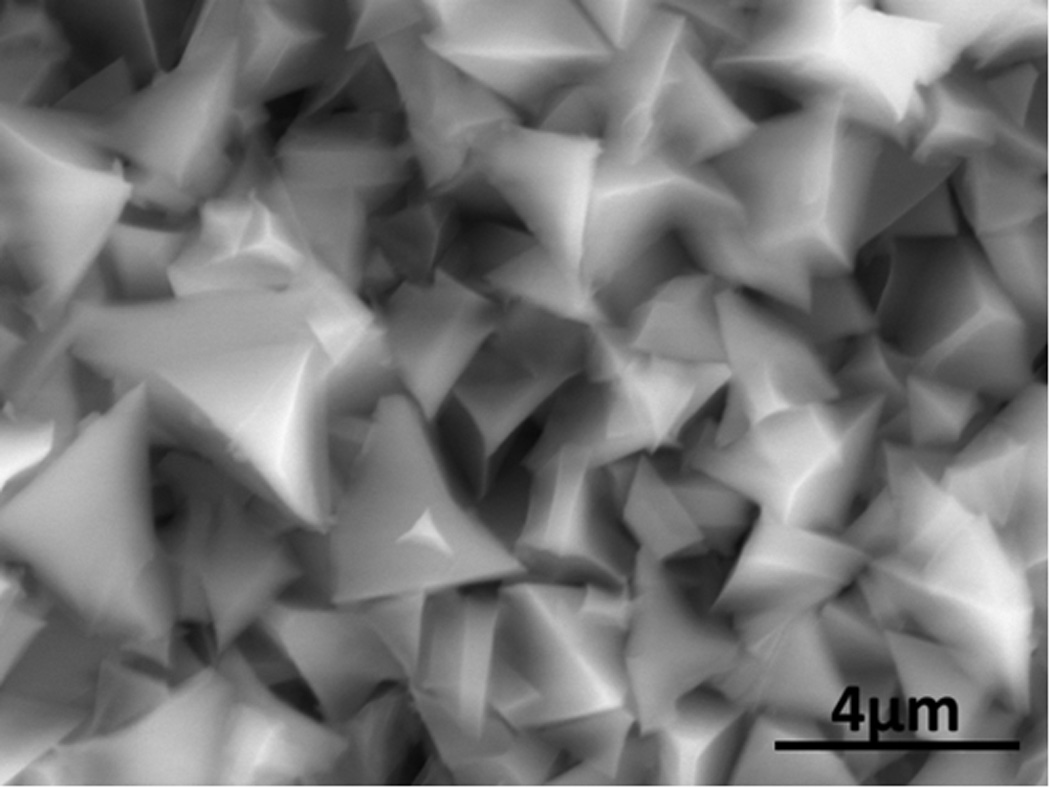
SEM micrograph of calcium containing particles along the broken edge of burley filler tobacco from a popular cigarette. These particles are apparently calcium oxalate crystals. This image was obtained in Low Vacuum mode with an accelerating voltage of 20 kV, 0.98 torr water vapor pressure in the sample chamber, spot size 3.0 with a Concentric Backscatter Detector (CBS).
Discussion
Though the living matrix of the tobacco plant is predominantly organic with carbon, nitrogen, oxygen, and hydrogen as the principal elemental constituents, the leaves are replete with inorganic substances of both environmental origin and with biogenic substances that were produced internally, and in some cases extruded from the plant leaf. The inorganic substances that were most frequently observed on multiple smokeless and cigarette tobacco product leaf surfaces and in the interiors of tobacco product leaves were silica and aluminum silicates (halloysite, kaolin, and unidentified), calcium oxalate and a few possible soil calcium carbonate compounds. The images shown here were illustrative of what was observed on all smokeless and cigarette tobacco products that were examined, regardless of brand, and including reference tobacco products.
It is uncertain whether aluminum silicate particles on smokeless tobacco products pose any significant health risk, since little work has been done with regard to oral exposures to these compounds, though it is possible that they might contribute to abrasion of the oral mucosa. Calcium oxalate crystals form inside the vacuoles of specialized idioblast cells [18]. They are very commonly produced by plants [19]. Such crystals are very water insoluble, and there is no indication in the literature that calcium oxalate crystals have high toxicity potential. However, it has been proposed that these crystals are used by various plants in a defensive capacity, so they may cause or contribute to abrasion of the oral mucosa, permitting irritants or toxins to more readily penetrate epidermal surfaces. [19–21]. There is no empirical evidence that abrasion by calcium oxalate crystals or other abrasives on tobacco surfaces mediates the inflammation of oral epidermal tissues from use of smokeless tobacco products. Davis et al., however, described combinations of mechanical and chemical irritants as common causes of oral irritant contact stomatitis. Davis et al. specifically stated that a diverse group of irritant reactions may appear in the oral cavity as a consequence of exposure to the primary irritant, nicotine, and that the localized nature of many of the epidermal pathologies to the area of immediate exposure implies that chemical and/or mechanical irritation from the tobacco is indeed responsible for the inflammatory response [22]. In addition, increased absorption of toxic constituents in tobacco due to abrasion of the skin while handling tobacco leaves may contribute to the contact dermatitis reported in tobacco workers [23].
If particles composed predominantly of silica, aluminum silicates, and calcium compounds from tobacco leaves were inhaled during smoking, the health risk from particulate inhalation are potentially more severe. Langer et al. reported that the mineral content in cigarette smoke consisted principally of potassium chloride, potassium sulfate, potassium carbonate, calcium carbonate, and “quartz” [24] (silica), several of which we have reported here as present on tobacco leaf surfaces and in the interiors of the leaves. Indeed, aluminum silicates accumulate in the lungs of smokers and appear in the brown pigmented “smokers’ macrophages” associated with Respiratory Bronchiolitis-Interstitial Lung Disease, a Chronic Obstructive Pulmonary Disease observed in some smokers generally after smoking for seven to seventy-five pack years [25, 26].
This work highlights a microscopic study of inorganic particulates on tobacco surfaces and those commonly found on and inside of tobacco products. We have shown examples of calcium compounds, aluminum silicates, and silica on the surfaces of tobacco products and beneath the surfaces of tobacco leaves. Further studies on examining mainstream smoke particulate matter are on-going to determine whether particles such as these, and especially silica or aluminum silicates transfer to mainstream cigarette smoke as Brody and Craighead suggested [26]. Such investigations could help identify potential causative agents in inflammatory and obstructive diseases that are consequences of tobacco smoking.
Acknowledgements
This study was partially funded by an interagency agreement by the U.S. Food and Drug Administration Center for Tobacco Products. We gratefully acknowledge the assistance of Stephen Stanfill, Ph.D. with classification of cigarette tobacco types.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death — United States, 2008–2010. Morbidity and Mortality Weekly Report. 2014;63:369–374. [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [last accessed 4 November 2014]. p. 2.p. 17. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/exec-summary.pdf. [Google Scholar]
- 3.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobacco Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Organization for Standardization. Routine analytical cigarette-smoking machine — Definitions and standard conditions. ISO 3308. 2012:1–23. [Google Scholar]
- 5.Pappas RS, Polzin GM, Zhang L, Watson CH, Paschal DC, Ashley DL. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food and Chemical Toxicology. 2006;44:714–723. doi: 10.1016/j.fct.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Pappas RS, Polzin GM, Watson CH, Ashley DL. Cadmium, lead, and thallium in smoke particulate from counterfeit cigarettes compared to authentic U.S. brands. Food and Chemical Toxicology. 2007;45:202–209. doi: 10.1016/j.fct.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Pappas RS, Fresquez MR, Martone N, Watson CH. Toxic metal concentrations in mainstream smoke from cigarettes available in the U.S. Journal of Analytical Toxicology. 2014;38:204–211. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond D, Wiebel F, Kozlowski LT, Borland R, Cummings KM, O’Connor RJ, McNeill A, Connolly GN, Arnott D, Fong GT. Revising the machine smoking regime for cigarette emissions: implications for tobacco control policy. Tobacco Control. 2007;16:8–14. doi: 10.1136/tc.2005.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department Of Health and Human Services. The Health Consequences of Using Smokeless Tobacco. A Report of the Advisory Committee to the Surgeon General. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 1986. [last accessed 4 November 2014]. pp. 29–136. http://profiles.nlm.nih.gov/ps/access/NNBBFC.pdf. [Google Scholar]
- 10.International Agency for Research on Cancer. Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, France: International Agency for Research on Cancer; 2007. pp. 1–370. [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson J, Björnberg G, Curvall M. Oral mucosal changes and nicotine disposition of Swedish smokeless tobacco products: a comparative study. Journal of Oral Pathology & Medicine. 1994;23:161–167. doi: 10.1111/j.1600-0714.1994.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 12.Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: A review of the condition. Journal of Periodontology. 1998;69:620–631. doi: 10.1902/jop.1998.69.6.620. [DOI] [PubMed] [Google Scholar]
- 13.Grady D, Greene J, Daniels TE, Ernster VL, Robertson PB, Hauck W, Greenspan D, Silverman S. Oral mucosal lesions found in smokeless tobacco users. Journal of the American Dental Association. 1990;121:117–123. doi: 10.14219/jada.archive.1990.0139. [DOI] [PubMed] [Google Scholar]
- 14.Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaska iqmik. Journal of Analytical Toxicology. 2008;32:281–291. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 15.Fresquez MR, Pappas RS, Watson CH. Establishment of Toxic Metal Reference Range in Tobacco from U.S. Cigarettes. Journal of Analytical Toxicology. 2013;37:298–304. doi: 10.1093/jat/bkt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilkes RJ, Suddhiprakarn A, Armitage TM. Scanning electron microscope morphology of deeply weathered granite. Clays and Clay Minerals. 1980;28:29–34. [Google Scholar]
- 17.Jeong GY, Kim SJ. Boxwork fabric of halloysite-rich kaolin formed by weathering of anorthosite in the Sancheong area, Korea. Clays and Clay Minerals. 1993;41:56–65. [Google Scholar]
- 18.Bouropoulos N, Weiner S, Addadi L. Calcium Oxalate Crystals in Tomato and Tobacco Plants: Morphology and in Vitro Interactions of Crystal-Associated Macromolecules. Chemistry – A European Journal. 2001;7:1881–1888. doi: 10.1002/1521-3765(20010504)7:9<1881::aid-chem1881>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Stephens WE. Whewellite and its key role in living systems. Geology Today. 2012;28:180–185. [Google Scholar]
- 20.Doege SJ, Korth K. The role of natural calcium oxalate crystals in plant defense against chewing insects. Inquiry. 4:88–94. doi: 10.1104/pp.106.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korth K, Doege SJ, Park S-H, Goggin FL, Wang Q, Gomez SK, Liu G, Jia L, Nakata PA. Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiology. 2006;141:188–195. doi: 10.1104/pp.106.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: A review of the condition. Journal of Periodontology. 1998;69:620–631. doi: 10.1902/jop.1998.69.6.620. [DOI] [PubMed] [Google Scholar]
- 23.Abraham NF, Feldman SR, Vallejos Q, Whalley LE, Brooks T, Cabral G, Earp P, Fleischer AB, Jr, Quandt SA, Arcury TA. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40–43. doi: 10.1111/j.1600-0536.2007.01148.x. [DOI] [PubMed] [Google Scholar]
- 24.Langer AM, Nolan RP, Bowes DR, Shirey SB. Inorganic particles found in cigarette tobacco, cigarette ash, and cigarette smoke. In: Wehner AF, Felton D-L, editors. Biological Interaction of Inhaled Mineral Fibers and Cigarette Smoke. Columbus, OH USA: Battelle; 1989. pp. 421–439. [Google Scholar]
- 25.Voelkel NF. In: Chronic Obstructive Lung Disease. MacNee W, editor. USA: People’s Medical Publishing House; 2008. pp. 175–176. www.pmph-usa.com, [Google Scholar]
- 26.Brody AR, Craighead JE. Cytoplasmic inclusions in pulmonary macrophages of cigarette smokers. Laboratory Investigation. 1975;32:125–132. [PubMed] [Google Scholar]


