Abstract
Natural products including botanicals for both therapy of clinical manifestations of atherosclerosis and reduction of atherosclerosis risk factors are topics of recent patents. Only a few recent patents are relevant to the direct anti-atherosclerotic therapy leading to regression of atherosclerotic lesions. Earlier, using a cellular model we have developed and patented several anti-atherosclerotic drugs. The AMAR (Atherosclerosis Monitoring and Atherogenicity Reduction) study was designed to estimate the effect of two-year treatment with time-released garlic-based drug Allicor on the progression of carotid atherosclerosis in 196 asymptomatic men aged 40–74 in double-blinded placebo-controlled randomized clinical study. The primary outcome was the rate of atherosclerosis progression, measured by high-resolution B-mode ul-trasonography as the increase in carotid intima-media thickness (IMT) of the far wall of common carotid arteries. The mean rate of IMT changes in Allicor-treated group (−0.022±0.007 mm per year) was significantly different (P = 0.002) from the placebo group in which there was a moderate progression of 0.015±0.008 mm at the overall mean baseline IMT of 0.931±0.009 mm. A significant correlation was found between the changes in blood serum atherogenicity (the ability of serum to induce cholesterol accumulation in cultured cells) during the study and the changes in intima-media thickness of common carotid arteries (r = 0.144, P = 0.045). Thus, the results of AMAR study demonstrate that long-term treatment with Allicor has a direct anti-atherosclerotic effect on carotid atherosclerosis and this effect is likely to be due to serum atherogenicity inhibition. The beneficial effects of other botanicals including Inflaminat (calendula, elder and violet), phytoestrogen-rich Karinat (garlic powder, extract of grape seeds, green tea leafs, hop cones, β-carotene, α-tocopherol and ascorbic acid) on atherosclerosis have also been revealed in clinical studies which enforces a view that botanicals might represent promising drugs for anti-atherosclerotic therapy.
Keywords: Anti-atherosclerotic therapy, atherosclerosis, botanicals, clinical studies, intima-media thickness, natural products, ultrasonography of carotid arteries
INTRODUCTION
Atherosclerotic lesions develop in the arterial wall and this disease remains asymptomatic until ischemia of distal organs is evident. Therapy of clinical manifestations of atherosclerosis is largely aimed at reducing symptoms or affecting hemodynamic response and often does not affect the cause or course of disease, namely the atherosclerotic lesion itself. Another approach is based on reduction of atherosclerosis risk factors, in particular high level of blood low density lipoprotein (LDL) [1–3]. Both therapies of clinical manifestations of atherosclerosis and reduction of atherosclerosis risk factors are topics of recent patents [4–14].
Of course, anti-atherosclerotic effects of statins revealed in many prospective clinical trials may be considered; however, statins were never recognized as the drugs indicated just for direct treatment or prevention of atherosclerosis [15–20]. They are used predominately in the course of hypolipidemic therapy, and the effects of treatment are estimated by success in reaching the target level of LDL cholesterol, but not the regression of atherosclerotic lesion or IMT. The last should be considered as beneficial effect, which is mainly due to pleiotropic mechanisms of action.
Atherosclerosis develops over many years, so the anti-atherosclerotic therapy should be long-term or even lifelong. Tachyphylaxis, long-term toxicity and cost amongst other issues may present problems for the use of conventional medications in long-term. Drugs based on botanicals can be a good alternative. In in vitro studies anti-atherosclerotic effects of garlic have been shown [21, 22]. The AMAR (Atherosclerosis Monitoring and Atherogenicity Reduction) study was designed to estimate the effect of two-year treatment with time-released garlic-based drug Allicor on the progression of carotid atherosclerosis in asymptomatic men in double-blinded placebo-controlled randomized clinical study.
MATERIALS AND METHODS
General Design Features
This study was named “Atherosclerosis Monitoring and Atherogenicity Reduction (AMAR)” since the principal objective was to evaluate the effect of time-released garlic powder tablets, Allicor, on the progression of IMT in the carotid arteries and on the changes in serum atherogenic potential. It was a multicenter, randomized, placebo-controlled, double-masked clinical trial of 196 asymptomatic men (40 to 74 years old) who had the evidence of early carotid atherosclerosis evaluated by B-mode ultrasound (maximum IMT of common carotid arteries 1.000–2.000 mm at first ultrasound examination). The participants included in the study had no diseases demanding continuous administration of cardiotropic, vasoactive, hypolipidemic, hypotensive, sugar-lowering drugs or diuretics. The necessity of regular (more than 2 months per year) administration of the above mentioned medications was the exclusion criterion. The primary outcome was the rate of carotid atherosclerosis progression, measured individually as the increase in mean IMT of the far wall of two arterial segments (left and right common carotid arteries). Matched random assignment was performed, and treatment groups were characterized by equal distribution by age, systolic and diastolic blood pressure, smoking history, family history, serum total cholesterol, serum atherogenicity and mean IMT from B-mode ultrasound examination at baseline, thus enabling statistical tests of effect. The AMAR study participants were randomized either to Allicor (INAT-Farma, Moscow, Russia) (150 mg twice daily) or to placebo (one tablet twice daily). Placebo and Allicor tablets looked identical. Allicor is coated tablet. The nuclei of placebo contain lactose instead of garlic powder. All participants got similar dietary and behavioral recommendations. There were three ultrasound examinations at the baseline (1 to 3 weeks apart), one examination every 3 months thereafter for the first 12 months, one examination after 18 months, and three examinations at the end of the study (1 to 3 weeks apart). The baseline data on clinical and laboratory characteristics of study participants are presented in Table 1.
Table 1.
Baseline carotid artery intima-media thickness and demographic, clinical and laboratory characteristics.
| Variable | Evaluable Allicor Recipients (n=93) | Evaluable Placebo Recipients (n=103) |
|---|---|---|
| Carotid intima-media thickness, mm | 0.929±0.015 | 0.937±0.015 |
| Age, years | 60,4±1,1 | 61,5±0,8 |
| Smoking history, n (%) | 30 (32.3) | 32 (31.1) |
| Family history, n (%) | 18 (19.4) | 17 (16.5) |
| Systolic blood pressure, mm Hg | 133.3±1.3 | 131.9±1.4 |
| Diastolic blood pressure, mm Hg | 81.8±0.8 | 81.2±0.9 |
| Total cholesterol, mMol/L | 6.14±0.12 | 6.02±0.10 |
| Triglycerides, mMol/L | 2.17±0.14 | 2.23±0.12 |
| HDL cholesterol, mMol/L | 1.07±0.03 | 1.10±0.03 |
| LDL cholesterol, mMol/L | 4.06±0.10 | 3.88±0.09 |
| Serum-induced intracellular cholesterol accumulation (serum atherogenicity *), % of control | 156.2±5.1 | 156.4±5.2 |
Serum atherogenicity was determined as described in “Subjects and Methods” section. Total intracellular cholesterol content of control cells cultured without patient’s serum addition was set for 100%. The cholesterol content of cells cultured in the presence of patient’s serum was expressed in % of control cells. The serum that induced statistically significant increase in intracellular cholesterol content as compared to control cells was defined as atherogenic.
Ultrasonographic Assessment of Atherosclerosis
High-resolution B-mode ultrasonography was used for carotid arteries imaging. The protocol of ultrasound examination involved the scanning of the right and left common carotid artery and the area of the carotid sinus (bulb) as high up as possible [23]. Three fixed angles of interrogation were used (anterolateral, lateral, and posterolateral). Images were focused on the posterior wall of the artery. Ultrasonographic examinations were performed with the subject in the supine position after a rest of 15 minutes. All measurements were always done consecutively in the same session for each subject. The B-mode ultrasound system used a 7.5 MHz linear array probe. The scanning procedures were recorded by a super-VHS PAL VCR, and the videotapes were read centrally by certified operator who was blinded to treatment assignments. IMT measurements were carried out with Pro-sound computer software (R. Seltzer, USA). The measurements were always performed at 10-mm section of common carotid artery adjacent to the carotid bulb. IMT of the posterior wall was measured as the distance from the leading edge of the first echogenic (bright) line to the leading edge of the second echogenic line. The mean of three measurements (in anterolateral, lateral, and posterolateral positions) was considered to be the integral IMT estimate.
Lipid Analysis
Venous blood was taken after overnight fasting at every visit. In order to obtain serum, the blood was incubated for 1 h at 37°C and centrifuged for 15 min at 1500 g. Cholesterol and triglyceride levels were measured by commercial enzymatic kits (Boehringer Mannheim GmbH, Germany). Serum HDL cholesterol concentrations were measured after precipitation with magnesium chloride phosphotungstic acid reagent (Boehringer Mannheim GmbH, Germany). Serum LDL cholesterol was calculated by Friedewald formula.
Serum atherogenicity measurement
Serum atherogenic potential was estimated as the ability of blood serum to induce cholesterol accumulation in cell culture [24]. Human blood monocytes were isolated from the blood of healthy volunteers and matured to macrophages as described by Griffith et al. [25]. Briefly, the cells were isolated by Ficoll-Hypaque gradient centrifugation and suspended in Medium 199 (GIBCO Europe, UK) containing standard additives: 2 mM L-glutamine, 100 U/ml penicillin, 2.5 μg/ml Fungizone and 10% of fetal calf serum (FCS) (Serva, USA). Cells were seeded into sterile 48-well tissue culture plates (Nunc, Denmark) at a density of 105 cells per 1 cm2 of growth area. The cells were cultured at 37°C in a humidified CO2-incubator (95% air and 5% CO2) for 7 days. The medium was changed every other day. Then Medium 199 containing 10% of tested serum was added to cell cultures in quadruplicates, and control cells were supplied with Medium 199 containing 10% FCS. All serum samples obtained from individual patient during the whole study were analyzed in a single experiment to allow valid monitoring of changes in serum atherogenicity. Additionally, the sample of pooled serum with known atherogenic potential was tested throughout all experiments to allow obtaining reference value. After 3-h incubation cells were washed 3 times with phosphate buffered saline. Cellular lipids were extracted with a mixture of n-hexane and isopropanol (3:2, vol/vol) [26], and total cholesterol content in extract was determined enzymatically by Monotest kits (Boehringer Mannheim GmbH, Germany). The cellular protein content was determined with Folin phenol reagent [27]. Total intracellular cholesterol content of control cells cultured without patient’s serum addition was set for 100% to allow for comparison between different cell culture experiments. The patient’s serum that induced statistically significant rise in intracellular cholesterol content was defined as atherogenic.
Statistical Methods
Results were expressed in terms of means and S.E.M. Significance of differences was evaluated using SPSS 10.1.7 statistical program package (SPSS Inc., USA). Significance Baseline charac-was defined at the 0.05 level of confidence. teristics of treatment groups were compared by one-way ANOVA and unpaired two-tailed t-test for continuous variables and χ2 tests for categorical variables. The ultrasound data and the changes in lipid levels from baseline to the mean of follow-up visits were analyzed by a two-way ANCOVA and paired two-tailed t-test. Pearson’s correlation coefficient was used for correlation analysis.
Search Resources
Recent patents have been searched and downloaded from: http://www.freepatentsonline.com; http://patentscope.wipo.int/search/en/search.jsf; http://patft.uspto.gov/; http://www.google.com/patents; http://www.freshpatents.com. In addition we made a MEDLINE search with the following keywords: anti-atherosclerotic effect, atherosclerosis, botanical, LDL, natural products, plant, risk factor. treatments, ultrasound, statins.
RESULTS
Subject Enrollment, Clinical Events and Compliance
Initially a total of 257 subjects were randomized in the double-blinded study. During the study 61 subjects discontinued study medication, 26 in the Allicor group and 35 in the placebo group. Of these discontinuations, two were due to adverse events (gastrointestinal complaints, both in Allicor group). Eleven subjects died (Allicor 4, placebo 7). In Allicor-treated group, three subjects died from sudden myocardial infarction and one patient died from pulmonary thromboembolism. In placebo group, four subjects died from sudden myocardial infarction, one from fatal stroke, one from pulmonary thromboembolism and one from metastatic intestinal cancer. Twelve subjects were discontinued because they began receiving other medications due to cardiovascular and non-cardiovascular events (Allicor 4, placebo 8). Among these, there were two non-fatal strokes, non-fatal myocardial infarction and prostatic adenocarcinoma in Allicor group, and three non-fatal myocardial infarctions, three non-fatal strokes and two unstable anginas in placebo group. Seven subjects were lost to follow-up (Allicor 4, placebo 3). Twenty subjects discontinued at their own request (Allicor 8, placebo 12). Nine subjects (Allicor 4, placebo 5) were discontinued because of poor compliance with the protocol. The compliance with study drug was 92% in both groups. Thus, 196 participants (93 in Allicor group and 103 in placebo group) were evaluable by the end of the study.
Baseline Characteristics
Table 1 summarizes the baseline characteristics of 196 evaluable AMAR participants, stratified by treatment assignment. The groups did not differ significantly at baseline in carotid IMT, demographic and clinical characteristics, or laboratory values Table 1. So, there were no statistically significant differences in any of the baseline characteristics measured between the subjects who received Allicor and those receiving placebo. Thus, subsequent analyses of changes in IMT were conducted without additional parameters as covariates.
The Changes in Intima-media Thickness
IMT changes in individual patients were formally classified as progression, regression either stability on the basis of statistically significant difference of the mean of three measurements at the end of the study from the mean of three examinations at the baseline. In Allicor recipients, IMT significant increase either in one or both carotid arteries was observed in 30 (32.2%) patients, and IMT either of one or both carotid arteries decreased significantly in 44 (47.3%) patients. In 8 patients (8.6%) there were no significant IMT changes in both carotid arteries, and in the rest 11 patients (11.8%) diverse changes were observed, i.e. one carotid artery demonstrated a significant increase in IMT, and in other one IMT decreased. In placebo group, the progression was observed in 50 (48.5%) cases, and IMT decreased significantly in one or both arteries in 31 (30.1%) patients. Stable situation was observed in 11 (10.7%), and diverse changes occurred in the rest 7 (6.8%) patients. The difference in the direction of IMT changes between Allicor and placebo recipients was statistically significant (Pearson’s chi-square 9.788, P=0.020). So, spontaneous atherosclerosis progression prevailed in placebo group, and Allicor treatment yielded a beneficial impact on early atherosclerosis in carotids demonstrating the significant increase in the number of observed regressions mainly for account of diminishing the number of cases with progression.
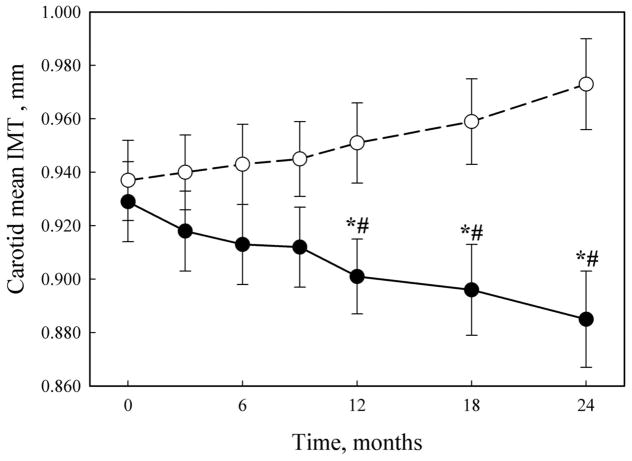
The dynamic of changes in IMT in Allicor-treated and placebo groups is demonstrated in Fig. (1). The tendency to IMT decrease in Allicor recipients was observed already after first 3 months of the study, but statistically significant difference from the baseline as well as from placebo group was achieved only after first 12 months of treatment. Upon the following examinations up to the end of the two-year study, the difference between placebo and Allicor recipients increased and remained statistically significant. For the common carotid artery, there was a moderate yearly IMT increase of 0.015±0.008 mm overall mean baseline IMT of 0.931±0.009 mm in placebo group, whereas in Allicor-treated patients the rate of IMT changes was −0.022±0.007 mm per year that was significantly different (P = 0.002) from placebo recipients. The mean rate of IMT changes in placebo group was similar in both years of the study, and in Allicor-treated patients the beneficial effect seemed to be more pronounced in the first year of treatment (−0.028±0.008 mm vs −0.016±0.007 mm for the first and the second year, respectively), but the difference did not reach statistical significance.
Fig. 1.
The dynamics of IMT changes.
Solid circles, Allicor-treated patients; open circles, placebo patients.
*, significant IMT change as compared to baseline, P < 0.05;
#, significant difference from placebo group, P < 0.05.
The beneficial effects of Allicor were also revealed upon the analysis of data from subgroups of patients who had either significant increase or reduction in IMT. So, in Allicor-treated patients with atherosclerosis progression (n=30) IMT increased by 0.029±0.011 mm, while in placebo recipients (n=50) the increase accounted for 0.070±0.016 mm (P=0.038). Similarly, the spontaneous atherosclerosis regression in placebo recipients (n=31) was characterized by significant decrease in IMT by 0.041±0.014 mm, but in Allicor-treated patients (n=44) the rate of IMT decrease over two years was 0.082±0.015 mm (P=0.049).
Effects on Serum Atherogenicity
At the baseline, the sera from 17 patients in placebo group (16.5%) did not induce significant cholesterol accumulation in cultured cells, while the sera from other 86 patients were atherogenic, i.e. induced statistically significant (1.21- to 3.91-fold) increase in intracellular cholesterol content (mean result, 166.3±5.5, % of control value). In Allicor-treated patients, 23 patients (24.7%) had non-atherogenic sera, and in other 70 patients the sera increased intracellular cholesterol by 1.22- to 3.53-fold (mean result, 172.1±5.8, % of control value).
Among patients with non-atherogenic sera at the baseline, in placebo recipients blood serum atherogenicity arrived in 11 cases during the study; in Allicor-treated patients at the end of the study serum atherogenicity was revealed in 9 cases, and in other 14 patients the sera remained non-atherogenic. The difference between Allicor and placebo recipients was statistically significant (Pearson’s chi-square 11.023, P < 0.001). Thus, Allicor treatment prevented the uprise of blood serum atherogenicity.
Among patients with initially atherogenic sera, in placebo group blood serum atherogenicity spontaneously decreased in 26 patients, did not change significantly in 28 patients, and in 32 cases there was further increase in blood serum atherogenic potential. On the opposite, in Allicor group serum atherogenicity was decreased in 39 patients by the end of the study, remained unchanged in 18 patients, and further increase in serum ability to induce intracellular cholesterol accumulation was observed only in 13 cases. Again, the difference between Allicor and placebo recipients was statistically significant (Pearson’s chi-square 11.274, P=0.004). Thus, Allicor also induced a fall in blood serum atherogenicity, if it existed at the beginning of treatment.
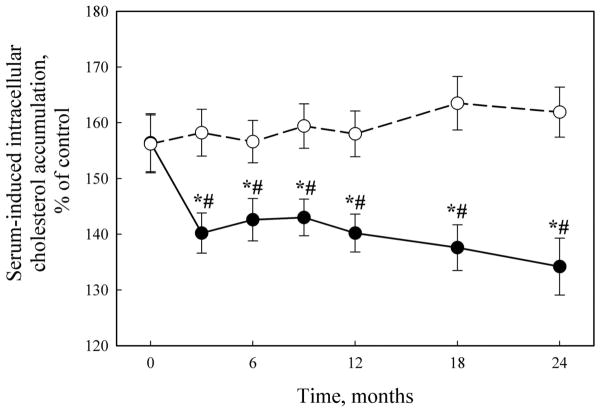
The overall dynamics of changes in serum atherogenicity is presented in Fig. (2). At the baseline, serum taken from patients was able to induce 1.56-fold increase in intracellular cholesterol content in cell culture test. In the placebo group, the mean level of serum atherogenic potential did not change significantly during two years of the study. On the opposite, in Allicor-treated patients the mean value for the ability of serum to induce intracellular lipid accumulation was significantly lowered (P = 0.016) approximately by 30% of the initial level (95% CI: 16.9, 41.0) already after first 3 months of study, and this effect was maintained during the study. General linear model analysis has demonstrated statistically significant difference in the dynamic of changes in serum atherogenicity between Allicor-treated and placebo groups (P = 0.008).
Fig. 2.
The dynamics of serum atherogenicity changes.
Solid circles, Allicor-treated patients; open circles, placebo patients.
*, significant IMT change as compared to baseline, P < 0.05;
#, significant difference from placebo group, P < 0.05.
The presence or absence of serum atherogenicity at the baseline, as well as the extent of serum-induced intracellular cholesterol accumulation at the baseline, did not correlate with the following changes in IMT. However, statistically significant correlation has been revealed between the changes in blood serum atherogenicity during the study and the changes in IMT of the common carotid arteries (r = 0.144, P = 0.045 for the total study sample). Upon the separate analysis of placebo and Allicor recipients, correlation coefficients for these two parameters did not reach statistical significance. In patients with initially non-atherogenic sera, the correlation between changes in atherogenicity and IMT was rather stronger (r = 0.342, P = 0.031), obviously due to those patients in placebo group in whom serum atherogenicity arrived during the study (r = 0.517, P = 0.034 for placebo recipients). In patients with initially atherogenic sera, the correlation between changes in atherogenicity and IMT in total group did not reach statistical significance (r = 0.147, P = 0.067), but in Allicor-treated patients in most of whom the decrease in serum atherogenicity was observed, the above parameters correlated well (r = 0.254, P = 0.034).
Effects on Lipids Parameters
The observed changes in serum lipid levels are presented in Table 2. Serum total cholesterol levels remained stable in the Allicor-treated group, whereas in the placebo group the moderate but significant increase by 4.4% (95% CI: 2.2, 8.8) was observed by the end of the study as compared to baseline level. However, there was no significant difference between groups at any time of the study. For serum triglycerides, in both groups moderate decrease was observed that was statistically significant; in Allicor group serum triglycerides lowered by 13.4% (95% CI: 4.8, 22.4), and in placebo recipients by 15.2% (95% CI: 4.9, 26.9) by the end of the study. There was no significant difference between groups at any time of the study except for examination after 3 months of treatment. For HDL cholesterol, the similar changes were observed both in Allicor-treated and placebo groups. By the end of the study, high density lipoprotein (HDL) cholesterol levels increased by 0.27 mMol/L (95% CI: 0.07, 0.33) and 0.13 mMol/L (95% CI: 0.05, 0.30), respectively. The calculated levels of LDL cholesterol demonstrated statistically significant decrease in Allicor-treated group after 12 months of treatment, and by the end of the study it accounted for 5.2% (95% CI: 2.8, 8.9). On the opposite, in placebo group there was moderate elevation in LDL cholesterol by 9.3% by the end of the study (95% CI: 4.4, 14.2). So, there was significant difference in LDL cholesterol levels between Allicor-treated and placebo groups after 3, 18 and 24 months of the study (P<0.05 between groups).
Table 2.
The changes in lipid parameters.
| Group | Duration of the study, months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 18 | 24 | |
| Total cholesterol, mMol/L | |||||||
| Allicor | 6.14±0.12 | 6.01±0.13 | 6.01±0.13 | 6.09±0.13 | 6.05±0.13 | 6.03±0.12 | 6.01±0.14 |
| Placebo | 6.02±0.10 | 6.20±0.11* | 6.20±0.12* | 6.35±0.12* | 6.34±0.13* | 6.32±0.12* | 6.29±0.13* |
| HDL cholesterol, mMol/L | |||||||
| Allicor | 1.07±0.03 | 1.15±0.04* | 1.15±0.03* | 1.24±0.04* | 1.29±0.04* | 1.32±0.03* | 1.34±0.04* |
| Placebo | 1.10±0.03 | 1.18±0.03* | 1.24±0.04* | 1.25±0.03* | 1.34±0.04* | 1.29±0.04* | 1.23±0.04* |
| LDL cholesterol, mMol/L | |||||||
| Allicor | 4.06±0.10 | 3.85±0.11*# | 3.94±0.11 | 3.96±0.11 | 3.87±0.11* | 3.89±0.11*# | 3.85±0.12*# |
| Placebo | 3.88±0.09 | 4.16±0.10* | 4.09±0.11* | 4.23±0.11* | 4.11±0.11* | 4.19±0.10* | 4.24±0.12* |
| Triglycerides, mMol/L | |||||||
| Allicor | 2.17±0.14 | 2.18±0.13# | 1.99±0.12 | 1.91±0.11* | 1.91±0.12* | 1.87±0.13* | 1.88±0.12* |
| Placebo | 2.23±0.12 | 1.86±0.08* | 1.87±0.10* | 1.87±0.09* | 1.95±0.12* | 1.92±0.12* | 1.89±0.10* |
, significant difference from the baseline, paired test, P<0.05
, significant difference from placebo, independent samples test, P<0.05
No significant correlations between baseline IMT and its changes over two years of the study and baseline lipid parameters were found. However, some correlations were revealed between the changes in lipid parameters and IMT during the study. There was a tendency to negative correlation between HDL cholesterol changes and IMT dynamics in total group (r = −0.134, P = 0.061), and in placebo group this correlation was statistically significant (r = −0.227, P = 0.021). In those patients with initially non-atherogenic sera, HDL cholesterol changes also correlated negatively with the changes in IMT (r = −0.321, P = 0.044), but upon separation into placebo and Allicor recipients this correlation has lost significance but remained as a tendency (r = −0.458, P = 0.065 and r = −0.369, P = 0.083). In the patients with initially atherogenic sera similar tendency existed in placebo recipients (r = −0.198, P = 0.068). Additionally, in the subgroup of patients in whom serum atherogenicity did not change over the time of the study, the correlation between the increase of IMT and increase of LDL cholesterol existed (r = 0.295, P = 0.018), and the tendency to such correlation was observed in placebo recipients (r = 0.314, P = 0.070).
The present study has limitations. The limitations of this study may be due to the fact that the question on the clinical advantages of IMT regression is still disputable. The same issue should be stressed when the clinical benefits of atherogenicity reduction are discussed. Long-term follow-up studies are necessary to determine if IMT regression can provide a decrease in the incidence of major cardiovascular events, thus lowering cardiovascular morbidity and mortality.
DISCUSSION
Atherosclerosis affects most vascular beads, and noninvasive imaging of superficial arteries by ultrasound has been recognized as a surrogate measure of atherosclerosis in numerous studies. Extracoronary atherosclerotic lesion can be quickly and safely evaluated in the carotids, femoral arteries, and the abdominal aorta. The grade of atherosclerosis in extracoronary sites correlates with a greater number of standard risk factors and, more important, with greater cardiac risk [28]. Of the peripheral arterial surrogates, carotid atherosclerosis has been most closely correlated with coronary artery disease [29–32]. Peripheral arterial ultrasonography is regarded to be a sensitive tool for the detection of early atherosclerosis and may be useful in assessing response to therapy. Thickening of the intima-media of the arterial wall is the earliest detectable anatomic change in the development and progression of atherosclerosis. High-resolution B-mode ultrasonography is widely used for noninvasive quantification of carotid IMT as a measure of subclinical atherosclerosis [33]. Carotid IMT is believed to be a marker of generalized atherosclerosis and is predictive of clinical cardiovascular events [30, 32, 34–39]. So, ultrasound imaging of intima-media thickening in carotid arteries is an applicable method for atherosclerosis monitoring during Allicor long-term treatment.
The main scientific goal of the given double-masked placebo-controlled randomized study was to test the hypothesis that long-term lowering of serum atherogenicity may prevent the initial stage of atherogenesis, namely, the excessive deposition of cholesterol in the cells of the arterial wall, thus inhibiting further formation of atherosclerotis lesion [40, 41]. At the clinical level, inhibition of the processes of atherogenesis could be monitored by ultrasound examination of common carotid arteries and the measurements of IMT. For this purpose, asymptomatic men who regarded themselves as “apparently healthy” but had ultrasonographic evidence for early atherosclerosis were recruited for this study.
The obtained results clearly indicate that long-term treatment with garlic-based drug Allicor results in a significant decrease in IMT of carotid arteries as compared to measurements made at baseline either to placebo group, respectively. Overall, the regression of subclinical atherosclerosis was much more frequently observed in asymptomatic men who randomly received Allicor than in those who received placebo. This anti-atherosclerotic effect goes in parallel with serum atherogenicity lowering, and the statistically significant correlation has been revealed between the changes in blood serum atherogenicity during the study and the changes in IMT of common carotid arteries. However, those correlations revealed between IMT changes and the changes in HDL cholesterol, especially in placebo recipients, and changes in LDL cholesterol in patients who did not demonstrate significant changes in serum atherogenicity also point out to the role of lipid metabolism disturbances in early atherogenesis. As garlic-based preparations are considered to possess lipid-lowering effects [42–45], the direct anti-atherosclerotic action of garlic provided by the prevention of intracellular lipid deposition may be complemented by its effects on LDL and HDL cholesterol levels. The beneficial effects of the rise in HDL cholesterol observed in the given study, especially in placebo recipients, that are clearly associated with the spontaneous regression of early atherosclerosis, are obviously of great importance and provide the promising field for further investigations. Additionally, the rather high proportion of patients in placebo group who demonstrated spontaneous regression of early atherosclerosis may witness of the wave-form character of atherogenesis, especially at early stages, and gives the reason for future studies, since the mechanisms of this phenomenon are still obscure.
The results obtained in our study are generally in good coincidence with the data from recent double-masked placebo-controlled randomized study by Koscielny et al. [46]. It has been demonstrated that 4-year treatment with garlic-based drug Kwai inhibited the increase in the volume of atherosclerotic plaques in carotid and femoral arteries by 5–18%. The age-dependent representation of the plaque volume has shown an increase between 50 and 80 years that was diminished under garlic treatment by 6–13% related to 4 years. So, with garlic application the plaque volume in the whole collective remained practically constant within the age-span of 50–80 years.
We have compared the obtained results with the data from several studies, where the effects of different drugs on atherosclerosis progression were investigated [23, 47–54], and the changes in IMT of carotid arteries have been monitored as a primary or secondary outcome Table 3. Obviously, the decrease in IMT achieved during AMAR study is quite comparable with the results of most successful trials. However, these studies employed potent lipid-lowering agents either calcium antagonists. So, the beneficiary effects of treatment were regarded to either significant reduction of LDL cholesterol, the major risk factor for atherosclerosis development, or the reduction in arterial wall stress. In AMAR study, moderate changes in lipid levels were also observed. The reduction in serum triglycerides and elevation in HDL cholesterol both in Allicor-treated and placebo patients seem to be due to good compliance with the dietary recommendations given upon the inclusion in the study. The moderate rise in total cholesterol level in placebo group is partially due to the rise in HDL cholesterol. However, LDL cholesterol in placebo patients also increased during the study, and it may be explained by the processes of aging [55, 56]. Allicor treatment obviously prevented this rise. So, Allicor possesses mild lipid-lowering activity, like other garlic-based preparations [42–45, 57–59]. It is notable that in our study the effect on carotid atherosclerosis only in small part depended on the changes in serum lipid levels, thus enforcing the explanations quite different from convenient approaches.
Table 3.
The comparative data from clinical trials on carotid atherosclerosis regression.
| Trial | Medication | Mean annual IMT change, mm | Reference | |
|---|---|---|---|---|
| placebo | treatment | |||
| PLAC II | Pravastatin | 0.068 | 0.059 | Crouse J.R. et al., 1995 [48] |
| KAPS | Pravastatin | 0.029 | 0.010 | Salonen R. et al., 1993 [36] |
| ASAP | Simvastatin | - | −0.009 | Smilde T.J. et al., 2001 [49] |
| PREVENT | Amlodipine | 0.011 | −0.015 | Pitt B. et al., 2000 [50] |
| ASAP | Atorvastatin | - | −0.020 | Smilde T.J. et al., 2001 [49] |
| CLAS | Cholestipol, niacin | 0.010 | −0.020 | Blankenhorn D.H. et al., 1993; Hodis H.N., 1995; [51, 52] |
| MARS | Lovastatin | 0.015 | −0.028 | Blankenhorn D.H. et al., 1993; Hodis H.N., 1995 [51, 53] |
| VHAS | Verapamil | - | −0.028 | Zanchetti A. et al., 1998 [54] |
| AMAR | Allicor | 0.015 | −0.022 | This study |
There is a substantial experimental background to explain the possible mechanisms underlying a direct anti-atherosclerotic action of Allicor. The components of garlic can regulate two main intracellular enzymes responsible for cholesterol intracellular metabolism. Garlic extract stimulates cholesteryl ester hydrolase and inhibits acetyl coenzyme A : cholesterol acyl transferase, thus diminishing intracellular content of cholesteryl esters [21]. Additionally, garlic extract inhibits cellular proliferative activity and the synthesis of connective tissue matrix components [21, 22]. Allicor also possesses antioxidant activity and lowers LDL susceptibility to oxidation [22]. Allicor prevents serum-induced cholesterol accumulation in cells cultured in the presence of patient’s serum taken after single dose of Allicor administration; in other words, it reduces serum atherogenic potential [22]. In animal studies, garlic-based preparations inhibit the formation of neointimal thickening in cholesterol-fed rabbits [47]. So, it could be easily proposed that long-term Allicor treatment produced a direct antiatherosclerotic effect due to the prevention of lipid deposition and depletion of cholesterol pool already accumulated in arterial wall.
Several years ago we have demonstrated that blood sera from the majority of atherosclerotic patients, unlike sera from healthy subjects, are capable of inducing lipid accumulation in cultured cells, i.e. possess atherogenic properties [60, 61]. The serum atherogenic potential is mainly due to the presence of modified low density lipoprotein [61–63], and in certain extent to non-lipid atherogenic factors [64–66]. So, blood serum atherogenicity revealed in cell culture test may be used as the integral characteristic of intracellular lipid deposition, the primary step in atherogenesis. In our study a significant reduction of serum atherogenicity was the major phenomenon observed in Allicor-treated group that could help explaining the beneficiary effects of Allicor on the progression of carotid atherosclerosis. Certainly, intracellular lipid deposition occurred during atherogenesis may act as a trigger event followed by other pathologic changes in vascular wall, such as the enhanced synthesis of connective tissue matrix components, migration of hematogenic cells, development of inflammatory reactions and, possibly, cellular proliferation. The mechanisms of interaction of these quite different processes still remain obscure.
Garlic contains a variety of organosulfur compounds, amino acids, vitamins and minerals [67]. Some of the sulfur-containing compounds such as allicin, ajoene, S-allylcysteine, S-methylcysteine, diallyl disulfide and sulfoxides may be responsible for antiatherosclerotic activity of garlic [21, 47]. By far, a lot of garlic-based products are present on the market now. They can be classified into four groups, i.e. garlic essential oil, garlic oil macerate, garlic powder and garlic extract. The manufacturing process can markedly influence the composition of garlic product, thus sulfur-containing compounds in garlic preparations may vary greatly. As compared to other garlic preparations, dehydrated garlic powder is thought to retain the same ingredients as raw garlic, both water-soluble and organic-soluble, although the proportions and amounts of various constituents may differ significantly [68, 69]. Allicor contains just garlic powder; on the other hand, it possesses a prolonged mode of action, as its antiatherogenic effect lasts for 12–16 hours after single dose administration [22]. So, Allicor differs greatly from other garlic-based preparations and may have considerable benefits in medicinal use.
On the whole, the results of our study demonstrate that long-term treatment with time-released garlic-based drug Allicor provides a direct anti-atherosclerotic effect on carotid atherosclerosis. Being the remedy of natural origin, Allicor is safe with the respect to adverse effects and allows even perpetual administration, which may be quite necessary for prevention and treatment of subclinical atherosclerosis.
Unfortunately natural products possessing anti-atherosclerotic therapeutic potential are not prescribed by medical practitioners as anti-atherosclerotic agents. However the potential of these substances allows considering them as mainline additional supplements or prescriptions [70]. Patents that protect exactly natural products as anti-atherosclerotic agents have recently been published [71, 72].
In particular, pomegranate juice reduced carotid IMT in men and women, thus demonstrating direct anti-atherosclerotic effect [73]. Our pilot studies demonstrated that two other drugs based on natural products, Inflamint and Karinat, may also be used for the treatment of asymptomatic atherosclerosis. Both drugs have anti-atherosclerotic effects on cellular level revealed in cell culture. In addition, Inflaminat possesses anti-inflammatory properties [74] and Karinat is enriched with phytoestrogens and antioxidant vitamins. These additional features allow recommending Inflaminat to patients predisposed to inflammatory pathologies, while Karinat designed for peri- and postmenopausal women. Both drugs have demonstrated anti-atherosclerotic effects in clinical studies. Karinat was patented as a drug for prevention of cardiovascular diseases and cancer [75]. In addition, Karinat was patented as a drug for the treatment of precancerous diseases [76, 77]. Inflaminat is patented for prevention and treatment of atherosclerosis [74].
In a pilot study of Inflaminat using a protocol similar to the AMAR study Inflaminat demonstrated atherosclerosis regression effects and statistically significant difference from the baseline as well as from placebo group Table 4. A randomized double-blinded placebo-controlled pilot clinical study on atherosclerotic effect of Karinat was performed in healthy peri- and postmenopausal women to understand the risks and benefits of phytoestrogen therapy in relation to atherosclerosis progression. The primary endpoint was the annual rate of changes in common carotid artery IMT, and the secondary endpoint was the dynamics of climacteric syndrome, that is monitored only in perimenopausal women. Table 5 demonstrates the effect of Karinat treatment on the dynamics of carotid atherosclerosis in posmenopausal women. In the placebo group an increase in the average IMT of more than 100 μm per year was observed. Thus, the rate of the natural history of atherosclerosis in postmenopausal women is extremely high: the average increase in IMT is 13% per year, and growth of atherosclerotic plaques of 40% per year.
Table 4.
Carotid IMT changes in 1-year inflaminat pilot study (unpublished data).
| Karinat | Placebo | p** | |
|---|---|---|---|
| Number of participants | 81 | 77 | - |
| IMT change, μm | −62±48 * (−91; −32) p=0.002 |
42±75 (−9; 93) p=0.109 |
0.002 |
significant differences, p<0.05, Wilcoxon’s signed ranked test;
statistical significance of differences was estimated by Mann-Whitney U-test.
Table 5.
Carotid IMT changes in 1-year karinat pilot study on postmenopausal women (unpublished data).
| Inflaminat | P | Placebo | P | |
|---|---|---|---|---|
| Number of participants | 80 | - | 77 | - |
| IMT change, μm | +6 (85) | NS | +111 (91) | P < 0.02 |
| Plaques, scores | +0.21 (0.59) | 0.009 | +0.31 (0.55) | < 0.001 |
CONCLUSIONS
Two-year treatment with Allicor (garlic powder) has a direct anti-atherosclerotic effect on carotid atherosclerosis in asymptomatic men.
Botanicals can be considered as promising drugs for anti-atherosclerotic therapy.
CURRENT & FUTURE DEVELOPMENTS
Currently, the direct anti-atherosclerotic therapy does not exist. We believe that this problem can be solved by non-pharmaceutical products based on natural ingredients. The growing interest in non-pharmaceutical products is explained by the limitations of medication. Toxicity and side effects of drugs are particularly important in atherosclerosis as an anti-atherosclerotic therapy should be long-term or even lifelong. Recent publications and patents allow us to consider anti-atherosclerotic natural products as an alternative to drugs [70–77]. Detection of anti-atherosclerotic effects of natural products is a promising example of future success in the fight against atherosclerosis.
Supplementary Material
Acknowledgments
This work was supported by the Russian Ministry of Education and Science.
Footnotes
Send Orders of Reprints at reprints@benthamscience.net
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A. Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab. 2012;61:126–34. doi: 10.1159/000342077. [DOI] [PubMed] [Google Scholar]
- 2.Giordano P, Scicchitano P, Locorotondo M, Mandurino C, Ricci G, Carbonara S, et al. Carotenoids and cardiovascular risk. Curr Pharm Des. 2012;18:5577–89. doi: 10.2174/138161212803307527. [DOI] [PubMed] [Google Scholar]
- 3.Sposito AR, Aguiar Filho GB, Aarão AR, Sousa FT, Bertolami MC. Statins in acute coronary syndromes. Arq Bras Cardiol. 2011;97:350–6. doi: 10.1590/s0066-782x2011001300012. [DOI] [PubMed] [Google Scholar]
- 4.Wong NCW, Tucker JEL, Hansen HC, Chiacchia FS, McCaffrey D. Flavanoids and isoflavanoids for the prevention and treatment of cardiovascular diseases. 8242130. US. 2012
- 5.Fan CT, Lai CS, Lin MJ. Pharmaceutically acceptable salts of aporphine derivatives and carboxyl group-containing agents and methods for preparing the same. 8202995. US. 2012
- 6.Hansen HC, Chopade SP, Citineni JR, Short RP, Yiannikouros GP. Methods of preparing quinazolinone derivatives. 8114995. US. 2012
- 7.Wong NCW, Tucker JEL, Hansen HC, Chiacchia FS, McCaffrey D. Stilbenes and chalcones for the prevention and treatment of cardiovascular diseases. 7846915. US. 2010
- 8.Kumar A. Composition and manufacturing processes of a toxicity free botanical drug for curative treatment of chronic diseases. 092712. WO. 2011
- 9.Tripp ML, Babish JG, Bland JS, Hall Amy J, Konda V, Pacioretty LM. Anti-inflammatory botanical products for the treatment of metabolic syndrome and diabetes. 20070281045. US. 2007
- 10.Leonard A, Saltzman D, Mueller M. Method and use of cold-pressed botanic seed oils for lowering blood pressure and LDL cholesterol. 20080260645. US. 2008
- 11.Bhagat U. Optimized nutritional formulations, methods for selection of tailored diets therefrom, and methods of use thereof. 051591. WO. 2012
- 12.Niazi SK. Compositions and methods for reducing or controlling blood cholesterol, lipoproteins, triglycerides, and sugar and preventing or treating cardiovascular diseases. 20040253327. US. 2004
- 13.Managoli NB. Herbal composition for treatment of hyperlipidemia and the inhibition of myocardial infarction. 8067043. US. 2011
- 14.Rangel O, Angel J. Angina pectoris and ischemic heart disease and synergistic phytoceutical composition for same. 8231913. US. 2012
- 15.Montecucco F, Quercioli A, Mirabelli-Badenier M, Viviani GL, Mach F. Statins in the treatment of acute ischemic stroke. Curr Pharm Biotechnol. 2012;13:68–76. doi: 10.2174/138920112798868737. [DOI] [PubMed] [Google Scholar]
- 16.Adameova A, Xu YJ, Duhamel TA, Tappia PS, Shan L, Dhalla NS. Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des. 2009;15:3094–107. doi: 10.2174/138161209789058048. [DOI] [PubMed] [Google Scholar]
- 17.Sipahi I, Tuzcu EM. Candidate mechanisms for regression of coronary atherosclerosis with high-dose statins: Insight from intravascular ultrasonography trials. Am J Cardiovasc Drugs. 2008;8:365–71. doi: 10.2165/0129784-200808060-00003. [DOI] [PubMed] [Google Scholar]
- 18.Riboldi P, Gerosa M, Meroni PL. Statins and autoimmune diseases. Lupus. 2005;14:765–8. doi: 10.1191/0961203305lu2217oa. [DOI] [PubMed] [Google Scholar]
- 19.Toth PP. New insights in the treatment of dyslipidemia: A focus on cardiovascular event reduction and the anti-atherosclerotic effects of atorvastatin. Curr Atheroscler Rep. 2005;7:335–43. doi: 10.1007/s11883-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 20.Ganesh SK, Nass CM, Blumenthal RS. Anti-atherosclerotic effects of statins: Lessons from prevention trials. J Cardiovasc Risk. 2003;10(3):155–9. doi: 10.1097/01.hjr.0000072574.13775.6d. [DOI] [PubMed] [Google Scholar]
- 21.Orekhov AN, Tertov VV. In vitro effect of garlic powder extract on lipid content in normal and atherosclerotic human aortic cells. Lipids. 1997;32:1055–60. doi: 10.1007/s11745-997-0136-7. [DOI] [PubMed] [Google Scholar]
- 22.Orekhov AN, Tertov VV, Sobenin IA, Pivovarova EM. Direct anti-atherosclerosis-related effects of garlic. Ann Med. 1995;27:63–5. doi: 10.3109/07853899509031938. [DOI] [PubMed] [Google Scholar]
- 23.Salonen R, Nyyssonen K, Porkkala E, Rummukainen J, Belder R, Park JS, et al. Kuopio Atherosclerosis Prevention Study (KAPS) A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92:1758–64. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 24.Tertov VV, Orekhov AN, Nikitina NA, Perova NV, Lyakishev AA, Serebrennikov SG, et al. Peritoneal macrophages: a model for detecting atherogenic potential in patients’ blood serum. Ann Med. 1989;21:455–459. doi: 10.3109/07853898909149238. [DOI] [PubMed] [Google Scholar]
- 25.Adams DO. Macrophages. Methods Enzymol. 1979;58:494–505. doi: 10.1016/s0076-6879(79)58164-6. [DOI] [PubMed] [Google Scholar]
- 26.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90:420–6. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 28.Simon A, Giral P, Levenson J. Extracoronary atherosclerotic plaque at multiple sites and total coronary calcification deposit in asymptomatic men. Association with coronary risk profile. Circulation. 1995;92:1414–21. doi: 10.1161/01.cir.92.6.1414. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JR, Schwartz CJ. Relationship between arterial disease at different sites. Br Med J. 1962;1:1293–301. doi: 10.1136/bmj.1.5288.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craven TE, Ryu JE, Espeland MA, Kahl FR, McKinney WM, Toole JF, et al. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation. 1990;82:1230–42. doi: 10.1161/01.cir.82.4.1230. [DOI] [PubMed] [Google Scholar]
- 31.Geroulakos G, O’Gorman DJ, Kalodiki E, Sheridan DJ, Nicolaides AN. The carotid intima-media thickness as a marker of the presence of severe symptomatic coronary artery disease. Eur Heart J. 1994;15:781–5. doi: 10.1093/oxfordjournals.eurheartj.a060585. [DOI] [PubMed] [Google Scholar]
- 32.Geroulakos G, O’Gorman D, Nicolaides A, Sheridan D, Elkeles R, Shaper AG. Carotid intima-media thickness: correlation with the British Regional Heart Study risk score. J Intern Med. 1994;235:431–3. doi: 10.1111/j.1365-2796.1994.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 33.Crouse JR, 3rd, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92:1141–7. doi: 10.1161/01.cir.92.5.1141. [DOI] [PubMed] [Google Scholar]
- 34.Blankenhorn DH, Hodis HN George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14:177–92. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- 35.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 36.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87:1156–65. [PubMed] [Google Scholar]
- 37.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 38.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 39.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 40.Tertov VV, Orekhov AN, Ryong LH, Smirnov VN. Intracellular cholesterol accumulation is accompanied by enhanced proliferative activity of human aortic intimal cells. Tissue Cell. 1988;20:849–54. doi: 10.1016/0040-8166(88)90026-2. [DOI] [PubMed] [Google Scholar]
- 41.Orekhov AN, Tertov VV, Kudryashov SA, Smirnov VN. Trigger-like stimulation of cholesterol accumulation and DNA and extracellular matrix synthesis induced by atherogenic serum or low density lipoprotein in cultured cells. Circ Res. 1990;66:311–20. doi: 10.1161/01.res.66.2.311. [DOI] [PubMed] [Google Scholar]
- 42.Harenberg J, Giese C, Zimmermann R. Effect of dried garlic on blood coagulation, fibrinolysis, platelet aggregation and serum cholesterol levels in patients with hyperlipoproteinemia. Atherosclerosis. 1988;74:247–9. doi: 10.1016/0021-9150(88)90244-4. [DOI] [PubMed] [Google Scholar]
- 43.Lau B, Lam F, Wang-Chen R. Effect of odor-modified garlic preparation on blood lipids. Nutr Res. 1987;7:139–49. [Google Scholar]
- 44.Neil HA, Silagy CA, Lancaster T, Hodgeman J, Vos K, Moore JW, et al. Garlic powder in the treatment of moderate hyperlipidaemia: A controlled trial and meta-analysis. J R Coll Physicians Lond. 1996;30:329–34. [PMC free article] [PubMed] [Google Scholar]
- 45.Silagy C, Neil A. Garlic as a lipid lowering agent - a meta-analysis. J R Coll Physicians Lond. 1994;28:39–45. [PMC free article] [PubMed] [Google Scholar]
- 46.Koscielny J, Klüssendorf D, Latza R, Schmitt R, Radtke H, Siegel G, et al. The antiatherosclerotic effect of Allium sativum. Atherosclerosis. 1999;144:237–49. doi: 10.1016/s0021-9150(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 47.Campbell JH, Efendy JL, Smith NJ, Campbell GR. Molecular basis by which garlic suppresses atherosclerosis. J Nutr. 2001;131:1006S–1009S. doi: 10.1093/jn/131.3.1006S. [DOI] [PubMed] [Google Scholar]
- 48.Crouse JR, 3rd, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, et al. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II) Am J Cardiol. 1995;75:455–9. doi: 10.1016/s0002-9149(99)80580-3. [DOI] [PubMed] [Google Scholar]
- 49.Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): A prospective, randomised, double-blind trial. Lancet. 2001;357:577–81. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 50.Pitt B, Byington RP, Furberg CD, Hunninghake DB, Mancini GB, Miller ME, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102:1503–10. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- 51.Hodis HN. Reversibility of atherosclerosis-evolving perspectives from two arterial imaging clinical trials: the cholesterol lowering atherosclerosis regression study and the monitored atherosclerosis regression study. J Cardiovasc Pharmacol. 1995;25(Suppl 4):S25–S31. [PubMed] [Google Scholar]
- 52.Blankenhorn DH, Selzer RH, Crawford DW, Barth JD, Liu CR, Liu CH, et al. Beneficial effects of colestipolniacin therapy on the common carotid artery. Two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation. 1993;88:20–8. doi: 10.1161/01.cir.88.1.20. [DOI] [PubMed] [Google Scholar]
- 53.Blankenhorn DH, Azen SP, Kramsch DM, Mack WJ, Cashin-Hemphill L, Hodis HN, et al. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). The MARS Research Group. Ann Intern Med. 1993;119:969–76. doi: 10.7326/0003-4819-119-10-199311150-00002. [DOI] [PubMed] [Google Scholar]
- 54.Zanchetti A, Rosei EA, Dal Palù C, Leonetti G, Magnani B, Pessina A. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. J Hypertens. 1998;16:1667–76. doi: 10.1097/00004872-199816110-00014. [DOI] [PubMed] [Google Scholar]
- 55.Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153:1065–73. [PubMed] [Google Scholar]
- 56.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Twenty-year dynamics of serum cholesterol levels in the middle-aged population of eastern Finland. Ann Intern Med. 1996;125:713–22. doi: 10.7326/0003-4819-125-9-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 57.Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta-analysis. Ann Intern Med. 1993;119:599–605. doi: 10.7326/0003-4819-119-7_part_1-199310010-00009. [DOI] [PubMed] [Google Scholar]
- 58.Steiner M, Khan AH, Holbert D, Lin RI. A double-blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am J Clin Nutr. 1996;64:866–70. doi: 10.1093/ajcn/64.6.866. [DOI] [PubMed] [Google Scholar]
- 59.Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131:989S–993S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 60.Chazov EI, Tertov VV, Orekhov AN, Lyakishev AA, Perova NV, Kurdanov KA, et al. Atherogenicity of blood serum from patients with coronary heart disease. Lancet. 1986;2:595–8. doi: 10.1016/s0140-6736(86)92426-8. [DOI] [PubMed] [Google Scholar]
- 61.Tertov VV, Orekhov AN, Martsenyuk ON, Perova NV, Smirnov VN. Low-density lipoproteins isolated from the blood of patients with coronary heart disease induce the accumulation of lipids in human aortic cells. Exp Mol Pathol. 1989;50:337–47. doi: 10.1016/0014-4800(89)90043-9. [DOI] [PubMed] [Google Scholar]
- 62.Tertov VV, Sobenin IA, Gabbasov ZA, Popov EG, Jaakkola O, Solakivi T, et al. Multiple-modified desialylated low density lipo-proteins that cause intracellular lipid accumulation. Isolation, fractionation and characterization. Lab Invest. 1992;67:665–75. [PubMed] [Google Scholar]
- 63.Tertov VV, Sobenin IA, Orekhov AN. Characterization of desialylated low-density lipoproteins which cause intracellular lipid accumulation. Int J Tissue React. 1992;14:155–62. [PubMed] [Google Scholar]
- 64.Orekhov AN, Tertov VV, Pokrovsky SN, Adamova IYu, Martsenyuk ON, Lyakishev AA, et al. Blood serum atherogenicity associated with coronary atherosclerosis. Evidence for nonlipid factor providing atherogenicity of low-density lipoproteins and an approach to its elimination. Circ Res. 1988;62:421–9. doi: 10.1161/01.res.62.3.421. [DOI] [PubMed] [Google Scholar]
- 65.Orekhov AN, Tertov VV, Kabakov AE, Adamova IYu, Pokrovsky SN, Smirnov VN. Autoantibodies against modified low density lipoprotein. Nonlipid factor of blood plasma that stimulates foam cell formation. Arterioscler Thromb. 1991;11:316–26. doi: 10.1161/01.atv.11.2.316. [DOI] [PubMed] [Google Scholar]
- 66.Kacharava AG, Tertov VV, Orekhov AN. Autoantibodies against low-density lipoprotein and atherogenic potential of blood. Ann Med. 1993;25:551–5. [PubMed] [Google Scholar]
- 67.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–9. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 68.Iberl B, Winkler G, Müller B, Knobloch K. Quantitative determination of allicin and alliin from garlic by HPLC. Planta Med. 1990;56:320–6. doi: 10.1055/s-2006-960969. [DOI] [PubMed] [Google Scholar]
- 69.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 70.Slevin M, Ahmed N, Wang Q, McDowell G, Badimon L. Unique vascular protective properties of natural products: supplements or future main-line drugs with significant anti-atherosclerotic potential? Vasc Cell. 2012;4:9. doi: 10.1186/2045-824X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aviram M, Dornfeld L. Methods of using pomegranate extracts for causing regression in lesions due to arteriosclerosis in humans. 8221806. US. 2012
- 72.Belcaro G, Burki C, Ferrari V. Combination of proanthocyanidins and centella asiatica for the treatment of atherosclerosis. 20120164244. US. 2012
- 73.Davidson MH, Maki KC, Dicklin MR, Feinstein SB, Witchger M, Bell M, McGuire DK, Provost JC, Liker H, Aviram M. Effects of consumption of pomegranate juice on carotid intima-media thickness in men and women at moderate risk for coronary heart disease. Am J Cardiol. 2009;104:936–42. doi: 10.1016/j.amjcard.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 74.Orekhov AN, Sobenin IA, Kirichenko TV, Myasoedova VA, Melnichenko AA, Orekhova VA. Method of prevention and treatment of atherosclerosis. 02455016. RU. 2012
- 75.Orekhov AN. Agent “Carinat” for prophylaxis of cardiovascular and oncological diseases. 02129874. RU. 1999
- 76.Bespalov VG, Barash N Ju, Ivanova OA, Krzhivitskij PI, Semiglazov VF, Aleksandrov VA, Orekhov AN, Sobenin IA. Method for treating mastopathy. 02226400. RU. 2004 [PubMed]
- 77.Bespalov VG, Aleksandrov VA, Shcherbakov AM, Kalinovskij VP, Novik VI, Chepik OF, Orekhov AN, Sobenin IA. Method for treating the cases of chronic atrophic gastritis. 02219940. RU. 2003
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.