Abstract
Background:
Ovarian hyperstimulation syndrome (OHSS) is the most serious and potentially life-threatening iatrogenic complication associated with ovarian stimulation during assisted reproductive technology protocols. The aim of this study was to evaluate the role of dopamine agonist as a preventive strategy of OHSS in women at high risk in in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment cycles.
Methods:
Seventy women at risk to develop OHSS undergoing IVF/ICSI treatment cycle were included. The study group received 0.5 mg of cabergoline for 8 days from the day of human chorionic gonadotropin administration in comparison to those who undergo no treatment for the prevention of OHSS. The reduction of the incidence of OHSS was the primary outcome.
Results:
The actual incidence of OHSS was 8.33% in the cabergoline group and 20.58% in the control group. Thus, the incidence of OHSS was significantly reduced, by almost 60%, in the cabergoline group in comparison with the control group (relative ratios: 0.4, 95% confidence interval: 0.18–0.79).
Conclusion:
Prophylactic treatment with the dopamine agonist, cabergoline, reduces the incidence of OHSS in women at high risk undergoing IVF/ICSI treatment. However, the effects of cabergoline on important outcomes, namely, live birth, miscarriage, and congenital abnormalities are still uncertain.
Keywords: Cabergoline, ovarian hyperstimulation, vascular endothelial growth factor
INTRODUCTION
Controlled ovarian stimulation (COS) is considered to be an important part of assisted reproductive techniques. However, COS imposes the risk of ovarian hyperstimulation syndrome (OHSS), a potentially life-threatening condition.[1] OHSS is a self-limiting disorder with a broad spectrum of clinical manifestations related to increased capillary permeability and fluid retention brought about by many biochemical mediators, the most important being vascular endothelial growth factor (VEGF). VEGF is a vasoactive mediator which increases capillary permeability and is expressed at a higher level in the granulosa cells.[2]
The mechanism postulated for this syndrome is increased vascular permeability and extravasation of fluid, which in turn causes hemoconcentration with reduced organ perfusion, alterations in blood coagulation and the resulting risk of thromboembolism, and leakage of fluid into the peritoneal cavity and lungs.[3] VEGF has been found to be expressed in human ovaries and it has been observed that VEGF mRNA levels increases after human chorionic gonadotropin (hCG) administration in granulosa cells and the elevated levels of the secreted proteins have been detected in serum, plasma, and peritoneal fluids in women at risk or with OHSS.[4] VEGF stimulates new blood vessel development and vascular hyperpermeability by interacting with its VEGF receptor 2 (VEGFR2).[5]
The administration of a dopamine agonist in immature rats at low doses simultaneously with hCG prevented an increase in vascular permeability and did not affect angiogenesis; the effect was due to the availability of dopamine type 2 receptors.[6] Dopamine agonists prevent the phosphorylation of VEGFR2 and reduce the in vitro and in vivo release of vasoactive angiogenic agents. As a result, vascular permeability is also reduced. Consequently, dopamine agonist at a daily dose of 0.5 mg has been supposed to be a potential new strategy to prevent OHSS and reduce its severity.[7] Cabergoline, an ergot derivative, is a potent dopamine receptor agonist on D2 receptors. Cabergoline has been shown to prevent the increase in vascular permeability by inhibiting phosphorylation of VEGFR2, and many studies have evaluated cabergoline as a preventive strategy to reduce the incidence of OHSS using varying doses and regimens.[3]
The aim of this randomized controlled study was to evaluate the efficacy and safety of a 0.5 mg of the dopamine agonist, cabergoline, in the prevention of OHSS in women at risk of developing OHSS when undergoing intracytoplasmic sperm injection (ICSI) treatment cycles.
METHODS
This study was undertaken in Istanbul University Faculty of Medicine with the approval of the local ethics committee. Seventy infertile couples undergoing ICSI at a private in vitro fertilization/ICSI and at risk of developing OHSS were included. The risk of developing OHSS was defined as follows: Estradiol (E2) level on the day of hCG >3000 pg/ml and with ≥20 follicles >12 mm. Patients with E2 ≥5000 pg/ml were excluded from the study.
A long mid-luteal gonadotropin-releasing hormone agonist protocol after oral contraceptive pills pretreatment, 0.1 mg triptorelin SC (Decapeptyl; Ferring, Germany) was used for pituitary down-regulation in both groups. Once pituitary down-regulation had been confirmed, controlled ovarian hyperstimulation was started using fixed dose of human menopausal gonadotropin, 150–225 IU (Menogon 75 IU, IM injections, Ferring, Germany), for 5 days; the dose was then adjusted according to response. When three leading follicles reached 18 mm, final oocyte maturation was triggered with a single dose recombinant hCG of 250 mcg (Ovidrel; Merck Serono SA). On the day of hCG administration, couples were allocated by a series of computer generated random numbers into two groups: The cabergoline group (Group I; n = 36), received 0.5 mg daily for 8 days and the non-cabergoline group (Group II; n = 34), did not receive cabergoline. Transvaginal guided oocyte retrieval was performed 36 h later. Ultrasound guided the transfer of 1–2 embryos was performed 72 h later. The luteal phase was supported with vaginal progesterone gel (Crinone 8%, 90 mg, Merck Serono SA), once daily up to the day of a pregnancy test.
Hematocrit, albumin, the presence of ascitis, measuring the perpendicular diameter of free fluid in the pouch of Douglas and the ovarian volume were reported in both groups on the day of hCG and 4 days later. The outcome measure was a reduction of the overall incidence of OHSS according to Golan's classification.[8]
Statistical analysis was performed using SPSS (Version 17, SPSS Inc., Chicago, IL, USA). Dichotomous outcomes were expressed as percentages and relative ratios (RR) with 95% confidence interval [CI]. Continuous outcomes were expressed as mean ± standard deviation, Chi-square test, and Student's t-test were performed to evaluate the statistical differences between the variables. A P <0.05 was considered statistically significant. All outcomes were calculated according to intention to treat analysis.
RESULTS
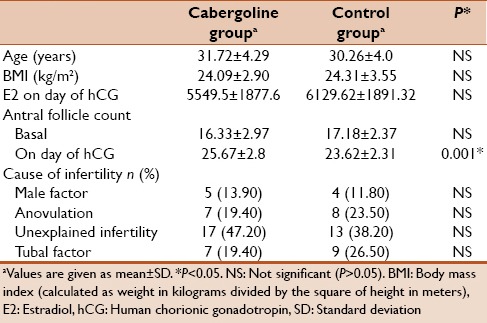
Seventy patients were randomized: 36 women to the cabergoline group and 34 women to the control group. There was no evidence of a statistically significant difference between both groups as regards their age, body mass index, E2 on the day of hCG, basal antral follicle count, and cause of infertility [Table 1].
Table 1.
Demographic criteria of included women in both groups
The actual incidence of OHSS was 8.33% in the cabergoline group and 20.58% in the control group. Thus, the incidence of OHSS was significantly reduced, by almost 60%, in the cabergoline group in comparison with the control group (RR: 0.4, 95% CI: 0.18–0.79). OHSS was stratified into mild, moderate, and severe according to Golan's classification.[8] The incidence of severe OHSS was significantly lower in the cabergoline group compared to the control group (odds ratio [OR]: 13.4, 95% CI: 0.71–2.58). The incidence of both mild and moderate OHSS was similar in the cabergoline group compared to controls (P > 0.05) [Table 2].
Table 2.
Clinical outcomes in cabergoline and control groups
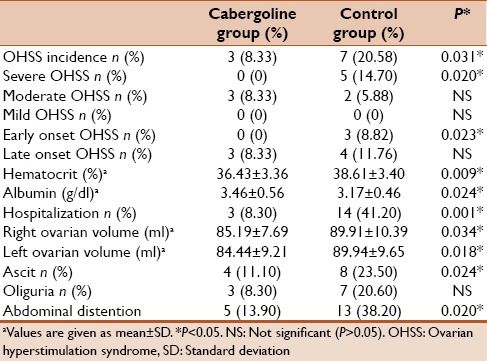
As regards the onset of OHSS, we could stratify the OHSS cases into an early (≤7–9 days from the day of hCG administration) and a late (≥10 days) onset. While cabergoline markedly reduced early onset OHSS, there was no statistical evidence of a reduction of late onset OHSS in comparison with the control group. There was evidence of a statistically significant increase in the signs of OHSS on the day of embryo transfer in the form of hematocrit, ascitic fluid collection, abdominal distention, and ovarian volume in the control group in comparison with the cabergoline group. The hospitalization rate was significantly lower in the cabergoline group in comparison with the control group [Table 2].
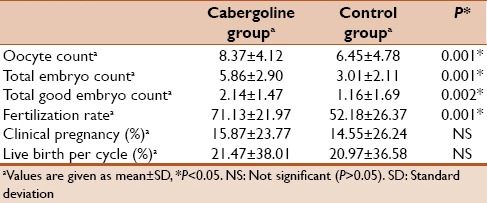
Total number embryos, number of total good quality embryos, and number of fertilization rate significantly higher in the cabergoline group in comparison with the control group (P < 0.05). However, we observed that, clinical pregnancy and rate live births per cycle no showed differences statistically significant when compared to control group (P > 0.05) [Table 3].
Table 3.
Epidemiological data, controlled ovarian hyperstimulation data and assisted reproduction treatment outcome of the study groups
CONCLUSIONS
Ovarian hyperstimulation syndrome, primarily a systemic disease results from vasoactive products released by the ovaries hyperstimulated with gonadotropins. It is more frequently seen when a strong ovarian response occurs, characterized by the development of a large number of follicles, high E2 values, and enlarged ovaries.[2] Of the various pathophysiological mechanisms implicated for the causation of OHSS, it is the angiogenic molecule, VEGF, which has been found to be the biggest mediator of this potentially dreadful complication. It has been proven that VEGF stimulates new blood vessel development and vascular hyperpermeability by interacting with its VEGFR2.[9]
Various forms of pharmacologic tools have been used for several years by many workers for the prevention and treatment of this complication in assisted reproductive technology (ART). The newer drugs, mainly the dopamine agonists in the light of the new pathogenetic and pharmacological evidence, should definitely be considered for prevention of OHSS. This prospective randomized controlled trial (RCT) has demonstrated that the use of a 0.5 mg dose of cabergoline in high-risk women reduces the risk of OHSS development. We assumed that 0.5 mg of cabergoline could be safely and effectively reduce the incidence of OHSS in women undergoing ICSI treatment.
Different studies have evaluated the use of cabergoline as a preventive strategy for OHSS with a varying dose from 0.25 mg up to 1.0 mg. Recently, a systematic review and meta-analysis of eight randomized controlled studies (n = 858 women), including the present study, comparing cabergoline versus placebo, albumin or no treatment for the prevention of severe OHSS were published. There was evidence of a statistically significant reduction in the incidence of severe OHSS in the cabergoline group (RR: 0.38, 95% CI: 0.29–0.51) which agrees with our results.[10] The studies used different regimens, 0.5 mg tablet of cabergoline for 12 days beginning on the day after oocyte retrieval;[11] 0.5 mg tablet of cabergoline for 8 days beginning on the day after hCG injection;[12] 0.5 mg tablet of cabergoline for 3 weeks beginning on the day after oocyte retrieval;[13] 0.5 mg tablet of cabergoline for 2 days, repeating 1-week later, beginning on the day after hCG injection;[14] 0.25 mg tablet of cabergoline for 8 days beginning on the day after hCG injection;[7] 0.5 mg tablet of cabergoline for 7 days beginning on the day after hCG injection;[15] and 0.5 mg tablet of cabergoline for 7 days beginning on the day after oocyte retrieval.[16] There is no statistical evidence of the timing of cabergoline administration (day of hCG injection versus after oocyte retrieval) on final oocyte maturation, fertilization rate or clinical outcome for the prevention of OHSS in high-risk patients. Both treatment modalities effectively decrease the incidence of severe OHSS.[17]
Ovarian hyperstimulation syndrome can also be divided into “early” and “late” depending on the time of onset, which helps in predicting the prognosis. The manifestations occurring within 9 days after the triggering dose of hCG reflects the excessive ovarian response and the precipitating effect of exogenously administered hCG for the final follicular maturation. On the other hand, OHSS presenting after this duration reflects the stimulation secondary to endogenous hCG from an early pregnancy. Thus, late OHSS is likely to last longer and of greater severity.[18] A human proof-of-concept study showed that the cabergoline reduces early onset OHSS by reducing ovarian vascular permeability in women at risk of OHSS.[19] Randomized clinical trials later confirmed that different commercial dopamine D2 receptor agonist exert similar effects. Paradoxically, these drugs impede the onset of early onset OHSS in 50% of women at risk of the syndrome but did not prevent late onset OHSS, which agrees with our results.[20]
The safety of cabergoline use during infertility treatment, especially the ART outcome had been a concern. The function of VEGF during final oocyte maturation in humans is still unknown. To avoid the detrimental effect on follicular growth, final oocyte maturation, fertilization rate or subsequent clinical outcome due to blockage of the VEGF system too early, cabergoline is suggested to be administered immediately after oocyte retrieval by some investigators.[17] However, Alvarez et al. in their study have presented that endometrial angiogenesis was not greatly altered, by the dose of cabergoline employed since neither implantation nor the overall ART outcome was affected.[19] The results of a Cochrane meta-analysis showed that no significant difference in the clinical pregnancy rate (OR: 0.94, 95% CI: 0.56–1.59; 2 RCTs, 230 women), miscarriage rate (OR: 0.31, 95% CI: 0.03–3.07; 1 RCT, 163 women) or any other adverse effects of the treatment (OR: 2.07, 95% CI: 0.56–7.70; 1 RCT, 67 women) between the cabergoline group and the control group.[21] A recently meta-analysis showed probably has no clinically relevant negative impact on clinical pregnancy (RR: 1.02, 95% CI: 0.78–1.34, 4 studies, 561 women) or on the number of retrieved oocytes (mean differences 1.15, 95% CI: 0.76–3.07, 5 studies, 628 women). However, the estimates were imprecise for distinguishing between substantial harm, no effect, and substantial benefit considering live birth (RR: 1.03, 95% CI: 0.71–1.48, 1 study, 200 women), and miscarriage (RR: 0.69, 95% CI: 0.27–1.76, 3 studies, 194 pregnant women). No studies reported congenital abnormalities.[10] In our study, we observed that clinical pregnancy and rate live births per cycle no showed differences statistically significant when compared to control group.
In conclusion, the dopamine agonist, cabergoline, reduces the incidence of OHSS when used by women who are at high risk for that complication while undergoing COS. Although the estimates were imprecise, cabergoline probably does not have a clinically relevant impact on clinical pregnancy rates or on the number of retrieved oocytes. However, the effects of cabergoline on important outcomes, namely, live birth, miscarriage, and congenital abnormalities are still uncertain. Large, well-designed, and well-executed randomized controlled trials that involve more clinical endpoints are necessary to further evaluate the role of cabergoline in OHSS prevention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nastri CO, Ferriani RA, Rocha IA, Martins WP. Ovarian hyperstimulation syndrome: Pathophysiology and prevention. J Assist Reprod Genet. 2010;27:121–8. doi: 10.1007/s10815-010-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naredi N, Talwar P, Sandeep K. VEGF antagonist for the prevention of ovarian hyperstimulation syndrome: Current status. Med J Armed Forces India. 2014;70:58–63. doi: 10.1016/j.mjafi.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares SR, Gómez R, Simón C, García-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008;14:321–33. doi: 10.1093/humupd/dmn008. [DOI] [PubMed] [Google Scholar]
- 4.Wang TH, Horng SG, Chang CL, Wu HM, Tsai YJ, Wang HS, et al. Human chorionic gonadotropin-induced ovarian hyperstimulation syndrome is associated with up-regulation of vascular endothelial growth factor. J Clin Endocrinol Metab. 2002;87:3300–8. doi: 10.1210/jcem.87.7.8651. [DOI] [PubMed] [Google Scholar]
- 5.Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–30. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 6.Ata B, Seyhan A, Orhaner S, Urman B. High dose cabergoline in management of ovarian hyperstimulation syndrome. Fertil Steril. 2009;92:1168.e1–4. doi: 10.1016/j.fertnstert.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Shaltout A, Shohyab A, Youssef MA. Can dopamine agonist at a low dose reduce ovarian hyperstimulation syndrome in women at risk undergoing ICSI treatment cycles? A randomized controlled study. Eur J Obstet Gynecol Reprod Biol. 2012;165:254–8. doi: 10.1016/j.ejogrb.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: An update review. Obstet Gynecol Surv. 1989;44:430–40. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Naredi N, Karunakaran S. Calcium gluconate infusion is as effective as the vascular endothelial growth factor antagonist cabergoline for the prevention of ovarian hyperstimulation syndrome. J Hum Reprod Sci. 2013;6:248–52. doi: 10.4103/0974-1208.126293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitao VM, Moroni RM, Seko LM, Nastri CO, Martins WP. Cabergoline for the prevention of ovarian hyperstimulation syndrome: Systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2014;101:664–75. doi: 10.1016/j.fertnstert.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi S, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxy of ovarian hyperstimulation syndrome. Reprod Biomed Online. 2010;20:S41. [Google Scholar]
- 12.Amir H, Kovalski DY, Amit A, Azem F. Can dopamine agonist cabergoline reduce ovarian hyperstimulation syndrome in ART treatment cycles? A prospective randomized study. Fertil Steril. 2011;96:S84. [Google Scholar]
- 13.Carizza C, Abdelmassih V, Abdelmassih S, Ravizzini P, Salgueiro L, Salgueiro PT, et al. Cabergoline reduces the early onset of ovarian hyperstimulation syndrome: A prospective randomized study. Reprod Biomed Online. 2008;17:751–5. doi: 10.1016/s1472-6483(10)60401-4. [DOI] [PubMed] [Google Scholar]
- 14.Edeen AM, Alhelou YM. Can cabergoline prevent ovarian hyperstimulation syndrome in PCO patients undergoing gonadotropin stimulation? Comparative study with prednisolone. Hum Reprod. 2009;24:i61. [Google Scholar]
- 15.Sohrabvand F, Ansaripour S, Bagheri M, Shariat M, Jafarabadi M. Cabergoline versus coasting in the prevention of ovarian hyperstimulation syndrome and assisted reproductive technologies outcome in high risk patients. Int J Fertil Steril. 2009;3:35–40. [Google Scholar]
- 16.Tehraninejad ES, Hafezi M, Arabipoor A, Aziminekoo E, Chehrazi M, Bahmanabadi A. Comparison of cabergoline and intravenous albumin in the prevention of ovarian hyperstimulation syndrome: A randomized clinical trial. J Assist Reprod Genet. 2012;29:259–64. doi: 10.1007/s10815-011-9708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seow KM, Lin YH, Bai CH, Chen HJ, Hsieh BC, Huang LW, et al. Clinical outcome according to timing of cabergoline initiation for prevention of OHSS: A randomized controlled trial. Reprod Biomed Online. 2013;26:562–8. doi: 10.1016/j.rbmo.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Ng E, Leader A, Claman P, Domingo M, Spence JE. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome in an in-vitro fertilization programme. Hum Reprod. 1995;10:807–10. doi: 10.1093/oxfordjournals.humrep.a136043. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez C, Alonso-Muriel I, García G, Crespo J, Bellver J, Simón C, et al. Implantation is apparently unaffected by the dopamine agonist cabergoline when administered to prevent ovarian hyperstimulation syndrome in women undergoing assisted reproduction treatment: A pilot study. Hum Reprod. 2007;22:3210–4. doi: 10.1093/humrep/dem315. [DOI] [PubMed] [Google Scholar]
- 20.Ferrero H, García-Pascual CM, Gómez R, Delgado-Rosas F, Cauli O, Simón C, et al. Dopamine receptor 2 activation inhibits ovarian vascular endothelial growth factor secretion in vitro: Implications for treatment of ovarian hyperstimulation syndrome with dopamine receptor 2 agonists. Fertil Steril. 2014;101:1411–8. doi: 10.1016/j.fertnstert.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Tang H, Hunter T, Hu Y, Zhai SD, Sheng X, Hart RJ. Cabergoline for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev. 2012;2:CD008605. doi: 10.1002/14651858.CD008605.pub2. [DOI] [PubMed] [Google Scholar]


