Abstract
Aim:
There is sparse data on the role of noninvasive ventilation (NIV) in acute respiratory distress syndrome (ARDS) from India. Herein, we report our experience with the use of NIV in mild to moderate ARDS.
Materials and Methods:
This was a prospective observational study involving consecutive subjects of ARDS treated with NIV using an oronasal mask. Patients were monitored clinically with serial arterial blood gas analysis. The success of NIV, duration of NIV use, Intensive Care Unit stay, hospital mortality, and improvement in clinical and blood gas parameters were assessed. The success of NIV was defined as prevention of endotracheal intubation.
Results:
A total of 41 subjects (27 women, mean age: 30.9 years) were included in the study. Tropical infections followed by abdominal sepsis were the most common causes of ARDS. The use of NIV was successful in 18 (44%) subjects, while 23 subjects required intubation. The median time to intubation was 3 h. Overall, 19 (46.3%) deaths were encountered, all in those requiring invasive ventilation. The mean duration of ventilation was significantly higher in the intubated patients (7.1 vs. 2.6 days, P = 0.004). Univariate analysis revealed a lack of improvement in PaO2/FiO2 at 1 h and high baseline Acute Physiology and Chronic Health Evaluation II (APACHE II) as predictors of NIV failure.
Conclusions:
Use of NIV in mild to moderate ARDS helped in avoiding intubation in about 44% of the subjects. A baseline APACHE II score of >17 and a PaO2/FiO2 ratio <150 at 1 h predicts NIV failure.
Keywords: Acute hypoxemic respiratory failure, acute lung injury, acute respiratory distress syndrome, mechanical ventilation, noninvasive ventilation
Introduction
Acute respiratory distress syndrome (ARDS) is a clinical entity characterized by acute onset respiratory failure, diffuse pulmonary opacities, and severe hypoxemia, in the absence of obvious cardiac dysfunction.[1] Based on the severity of hypoxemia, ARDS is categorized as mild (PaO2/FiO2 ratio >200 and ≤300 mmHg), moderate (PaO2/FiO2 ratio >100 and ≤200 mmHg), and severe (PaO2/FiO2 ratio ≤100 mmHg). With increasing severity of ARDS, the mortality increases from 27% in mild to 45% in severe ARDS.[1] ARDS is further classified as extrapulmonary and pulmonary ARDS depending on the underlying cause.[2] Apart from treatment of the underlying cause, the management of ARDS involves correction of hypoxemia either by supplemental oxygen or by the provision of positive pressure ventilation.
Positive pressure ventilation can be delivered either invasively (endotracheal airway) or noninvasively (face or nasal mask). Invasive mechanical ventilation is the standard of care for the management of ARDS and has been demonstrated to reduce mortality.[3] However, invasive ventilation is associated with several complications including a higher incidence of ventilator-associated pneumonia, barotrauma, volutrauma, and others. The use of noninvasive ventilation (NIV) can potentially avoid these complications.[4] NIV has an important role in the management of acute respiratory failure, especially those secondary to acute exacerbations of chronic obstructive pulmonary disease and acute cardiogenic pulmonary edema.[5,6,7] In hypoxemic respiratory failure, NIV improves oxygenation, reduce dyspnea, unload respiratory muscle, and hence may help in avoiding invasive mechanical ventilation.[8,9]
A recent meta-analysis of six randomized controlled trials involving subjects with ARDS suggested that the use of NIV avoided intubation, but not mortality. However, in the meta-analysis, only three studies (n = 89) involved patients with ARDS and the analysis also included postsurgical subjects with atelectasis.[10] In another meta-analysis involving only subjects with ARDS, it was shown that NIV avoids intubation in approximately 50% of the patients, provided the patients are judiciously chosen.[9] There is sparse data on the role of NIV from the developing world.[7,11,12] We hypothesized that the use of NIV may help in avoiding intubation in subjects with mild to moderate ARDS. In this study, we report our experience with the use of NIV in ARDS.
Materials and Methods
Study design
This was a prospective observational study assessing the efficacy of NIV in combination with standard therapy in patients with ARDS. The study protocol was approved by the Institute's Ethics Committee and a written informed consent was obtained from all the patients or their next of kin.
Setting
The study was conducted between June 2011 and May 2012 in the Intensive Care Unit (ICU) of the Department of Pulmonary Medicine at the author's institute. The ICU is a 10-bedded unit catering to the care of medical-surgical critically ill patients. It comprises a team of seven physicians (two consultants and five residents specialized in Pulmonary and Intensive care). Two physicians are present round the clock and are supported by the nursing staff with a nurse to patient ratio of 1:2.
Patients
Consecutive patients aged ≥18 years with a diagnosis of ARDS were eligible for inclusion in the study. ARDS was diagnosed based on the American-European Consensus Conference: Acute onset respiratory failure of <7 days; bilateral infiltrates on chest radiograph; PaO2/FiO2 < 300; and no clinical evidence of left heart failure or left atrial size <40 mm on echocardiography.[13] Patients with any of the following were excluded: PaO2/FiO2 ≤100, age <18 years, cardiogenic pulmonary edema, more than 2 organ failure, traumatic lung injury, hemodynamic instability, chronic carbon dioxide retention (PaCO2 >50 mmHg) at presentation, severe upper gastrointestinal bleed, altered mentation with a Glasgow coma scale <8, and facial abnormality. A sample of convenience, comprising 50 subjects with a diagnosis of ARDS was planned.
Study protocol
Subjects enrolled in the study were treated with NIV using the ICU ventilators (Servo-i universal, Maquet, Getinge). NIV was started with initial inspiratory positive airway pressure (IPAP) of 6–8 cm of H2O and was gradually increased by 2 cm to achieve clinical response in the form of relief of dyspnea, respiratory rate of ≤ 30 breaths/min and a tidal volume of 6–8 ml/kg ideal body weight, or a maximum IPAP of 20 cm of H2O. Expiratory positive airway pressure (EPAP) was initiated at 3–4 cm of H2O and increased by 1 cm of H2O to achieve a SpO2 ≥ 92% or a maximum EPAP of 10 cm of H2O. During the initial 24 h of NIV, the interface was disconnected only for intake of food and to clear oral secretions. Thereafter, depending upon the response, the off NIV period was increased gradually. Patients were weaned off NIV if they were able to maintain SpO2 ≥92% on FiO2 of 30% and the respiratory rate was ≤30 breaths/min. Worsening of dyspnea, worsening of or lack of improvement in hypoxemia, persistence of respiratory rate >35 breaths/min, appearance of respiratory acidosis, circulatory shock, or altered sensorium were defined as the features of NIV failure, and the patient was qualified for endotracheal intubation. The ultimate decision to intubate was, however, left to the discretion of the ICU physician.
Study procedures
Baseline demographic and clinical characteristics including the underlying etiology for ARDS were recorded for all the study participants. All subjects were monitored for clinical (respiratory rate, blood pressure, sensorium, and others) and laboratory parameters (partial pressure of oxygen, partial pressure of carbon dioxide, arterial pH, and others). Arterial blood gases (ABG), respiratory rate, mental status, mean arterial blood pressure, and PaO2/FiO2 values were recorded at the initiation of NIV and then at 1 h, 4 h, and at the end of therapy. Liver and renal function tests, complete blood count, and Acute Physiology and Chronic Health Evaluation II (APACHE II) score[14] (at 24 h) were performed in all the subjects. Body fluid cultures were obtained before the institution of first antibiotics. The diagnosis of malaria was based on the demonstration of malarial parasite in peripheral blood film or malaria antigen by commercially available rapid diagnostic test kit (Malaria Antigen [Pf/Pan] detection test device, Mediclone Biotech Private limited, India) performed at least twice. The diagnosis of scrub typhus was made on the demonstration of positive IgM antibody against Orientia tsutsugamushi derived recombinant antigens by ELISA method (Scrub Typhus Detect™ IgM ELISA, InBios India). Presence of IgM antibody against Leptospira antigen (nine serovars; Icterohemorrhagiae, Pomona, Hebdomanis, Australis, Canicola, Grippotyphosa, Patoc, Autumnalis, and Cynopteri) detected by microscopic agglutination testing (MAT) led to a diagnosis of leptospirosis. A MAT titer of 1:100 was considered as a positive result. Regional Medical Research Centre, Port Blair, provided the Leptospira strains. H1N1 swine flu was diagnosed if influenza virus RNA was detected in the nasopharyngeal swab by reverse transcriptase polymerase chain reaction. The patients were administered standard treatment regimens for the underlying etiology. All the patients received deep venous thrombosis and stress ulcer prophylaxis as per the ICU protocol. Enteral route was the preferred route of administration of nutrition. The subjects were followed up until ICU discharge or death, whichever was earlier.
Study outcomes
The primary outcome was the success of NIV defined as the number of patients in whom endotracheal intubation was avoided. The secondary outcomes were duration of NIV, ICU stay, hospital mortality, and improvement in clinical and blood gas values at 1 and 4 h. Factors predicting the success of NIV in avoiding intubation were also analyzed. Complications of NIV, such as claustrophobia, intolerance, abdominal distention, pressure sores, and nasal bridge trauma, were also noted. Changes at 1 h from baseline for PaO2/FiO2 ratio, PaCO2, pH, mean arterial pressure, and respiratory rate were calculated.
Statistical analysis
Results are presented in a descriptive fashion as number and percentage or mean ± standard deviation (SD), unless otherwise stated. The differences between means of continuous and categorical variables were analyzed using the Mann–Whitney U-test and Chi-square tests, respectively. Improvement in clinical (respiratory rate and mean arterial blood pressure) and ABG parameters (pH, PaO2, and PaCO2) was analyzed using multifactorial repeated measures analysis of variance, with Bonferroni adjustment for multiple comparisons; the within-groups factor was time (baseline, 1, and 4 h), and the between-groups factor was NIV success. For within-group analysis, univariate approach was used, as Mauchly's test of Sphericity was found to be significant (>0.05). Post-hoc analysis for calculating the difference in means within-group was done by Scheffe's test.[15] For between-groups comparison, Bonferroni method was used to adjust P value for multiple comparisons.[16] A univariate logistic regression analysis was performed to derive adjusted odds ratios and 95% confidence intervals to analyze the factors predicting NIV failure. Statistical significance was assumed at P < 0.05.
Results
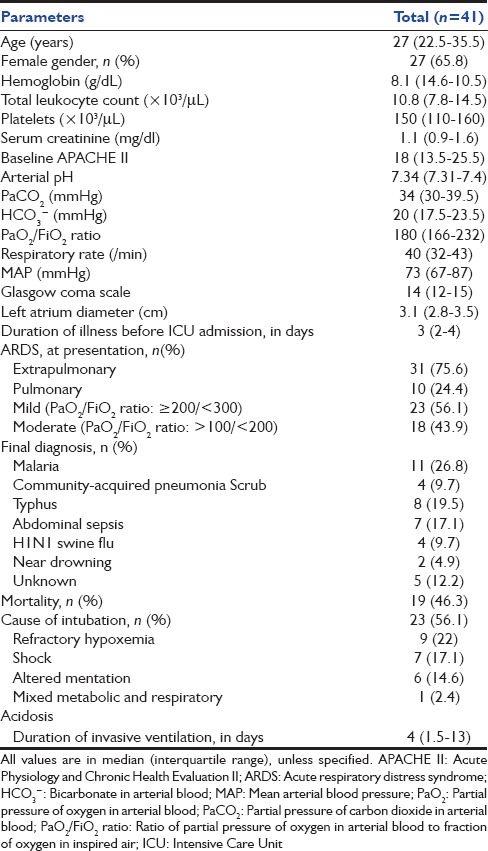
There were 250 admissions during the study period, of which 50 patients had a diagnosis of ARDS at presentation. Nine patients were excluded (cardiogenic pulmonary edema, n = 2; severe ARDS, hypotension or poor mental status, n = 7) and 41 patients were included in the study. The mean ± SD age of the study population was 30.9 ± 11.4 years; 27 (65.9%) were women. Tropical infections followed by abdominal sepsis were the most common causes of ARDS [Table 1]. The median (interquartile range [IQR]) duration of illness prior to ICU presentation was 3 (2–4) days. Extrapulmonary ARDS (n = 31, 75.6%) was encountered in the vast majority. The use of NIV was successful in 18 (44%) patients, while 23 subjects failed the initial trial of NIV and required endotracheal intubation. The median (IQR) time to intubation was 3 (1–4) h. The indications for intubation were refractory hypoxemia (n = 9; 22%), hypotension (n = 7; 17.1%), altered sensorium (n = 6; 14.6%), and uncorrected respiratory acidosis (n = 1; 2.4%). Overall, there were 19 (46.3%) deaths in the study population, all in those failing NIV.
Table 1.
Clinical and demographic profile of the study population
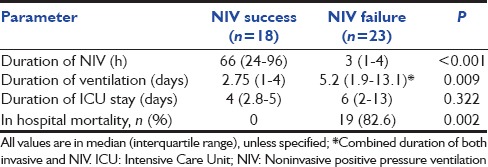
The subjects were divided into two groups namely those with the successful application of NIV and those failing NIV [Table 2]. There was no difference in terms of age, gender distribution, and duration of illness before admission to ICU between the two groups. The underlying etiology, severity (mild or moderate), and type of ARDS (pulmonary or extrapulmonary) were equally represented in the two groups. The NIV failure arm had a significantly higher APACHE II score at baseline (23.7 vs. 13.2; P < 0.0001) in comparison to NIV success. The median PaO2/FiO2 ratio at baseline was significantly higher in those requiring invasive mechanical ventilation. The duration of ventilation (noninvasive and invasive) was significantly higher in those requiring endotracheal intubation [Table 3]. There was no difference in the ICU stay between the two groups.
Table 2.
Clinical characteristics of the NIV success and NIV failure population
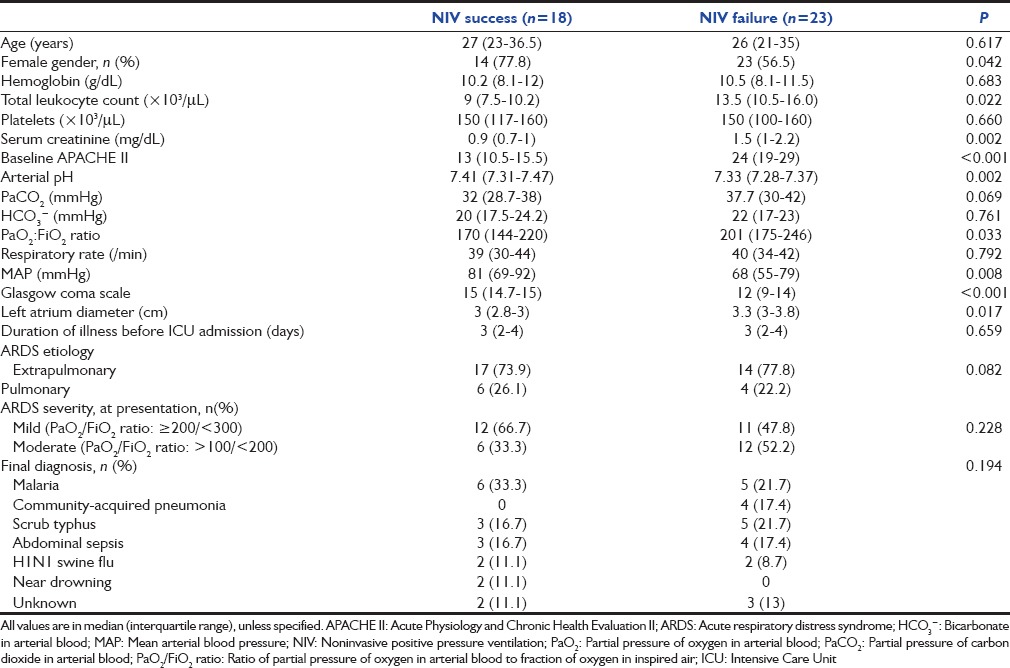
Table 3.
Outcome parameters of the study groups
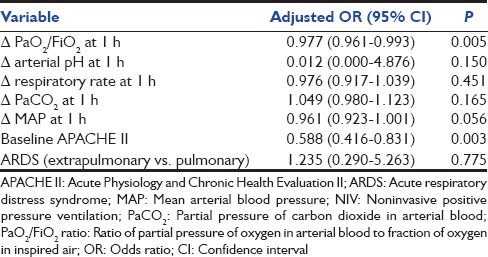
There was significant reduction in the respiratory rate in both groups at the end of 1 h and 4 h compared to baseline; however, this was not different between the two groups [Table 4]. There was an increase in PaO2/FiO2 at 1 h in those with NIV success in contrast to those with NIV failure [Table 4]. The PaO2/FiO2 value of <150 at 1 h had the best specificity in predicting NIV failure compared to other values [Table 5]. On univariate logistic regression analysis, failure of improvement in PaO2/FiO2 at 1 h and higher baseline APACHE II scores were the predictors of NIV failure [Table 6].
Table 4.
Serial clinical and arterial blood gas parameters during ICU course of the two groups
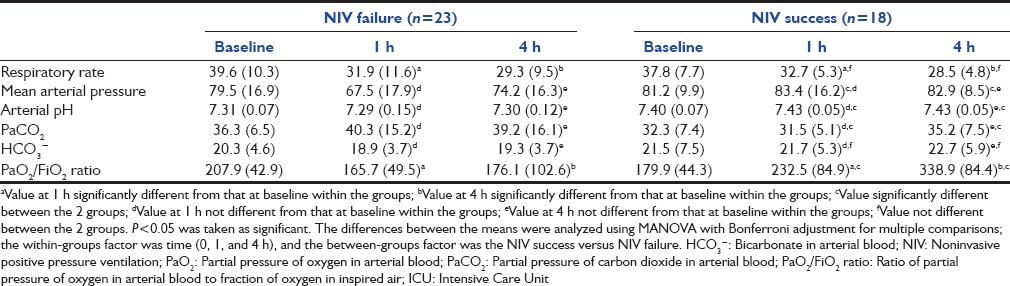
Table 5.
Impact of PaO2/FiO2 ratio at 1 h and baseline APACHE >17
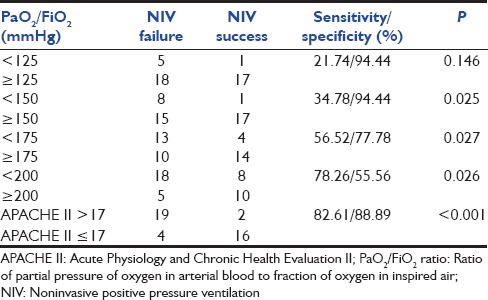
Table 6.
Factors predicting NIV failure: Univariate logistic regression analysis
Discussion
The results of this study suggest that the use of NIV helped in avoiding intubation in 44% of the subjects with mild to moderate ARDS. Baseline APACHE II score >17 and lack of improvement in PaO2/FiO2 ratio >150 after 1 h of NIV predicted NIV failure.
The successful use of NIV results in the improvement of oxygenation in subjects with ARDS. EPAP with NIV, akin to positive end-expiratory pressure opens collapsed alveoli with resultant increase in functional residual capacity and reduction of right to left shunt. This causes improvement in oxygenation, relief in dyspnea, and reduction in the respiratory muscle workload.[7,11] In a recent study describing the early use of NIV in mild ARDS, only one of the 21 patients in the NIV arm required intubation.[17] Although in a meta-analysis of thirteen studies describing the use of NIV in mild-to-moderate ARDS, NIV prevented intubation in 50% of the patients.[9] This was also observed in the current study where the use of NIV prevented intubation in 44% of the subjects.
Tropical infections followed by abdominal sepsis were the most common causes of ARDS in the current study. The predominant type of ARDS was extrapulmonary ARDS, although the type of ARDS (extrapulmonary or pulmonary) did not affect the outcome of NIV use in the current study, similar to a previous report.[18] In another study with pulmonary ARDS secondary to H1N1, NIV was successful in avoiding intubation in 48% of the subjects.[19] This suggests that irrespective of the type of ARDS (extrapulmonary or pulmonary), the use of NIV helps in avoiding intubation in roughly half of the patients.
NIV use should be judicious in patients with ARDS. Even in a highly selected group of patients with ARDS, that is, only those with mild to moderate ARDS with two or less organ system involvement and the absence of shock at presentation, NIV achieved success in only 44%. In addition, in the present study, 19 of the 23 subjects in the NIV failure group died, while there were no deaths in the NIV success group. This finding is consistent with previously reported experience where the hospital mortality was significantly higher in the NIV failure group (53.8–100%) in comparison to the NIV success group.[11,17,20] The most important reason for high mortality in ARDS patients managed with NIV, apart from refractory hypoxemia is the severe systemic illness as reflected by higher disease severity scores such as APACHE II.[21,22,23] However, one cannot rule out the possibility that overzealous use of NIV led to an inadvertent delay in invasive mechanical ventilation and might have contributed to a higher mortality in this subgroup of patients. Although the median time to intubation was 3 h, elective intubation in subjects with a PaO2/FiO2 ratio of <150 at 1 h could have resulted in lesser deaths, and delay in intubation have been associated with poor outcomes.[24,25,26]
The use of NIV was associated with a significant improvement in mean arterial blood pressure, PaCO2, and PaO2/FiO2 ratio at 1 and 4 h of NIV use in the NIV success group. In a study comprising 54 subjects with ARDS, presence of hypotension and lack of improvement in oxygenation at 1 h were associated with the failure of NIV to avoid intubation.[27] In the study by Antonelli et al., PaO2/FiO2 ratio of ≤175 predicted NIV failure, while in another study, PaO2/FiO2 ratio <200 at 1-h predicted NIV failure in subjects with ARDS. We evaluated 4 different values of PaO2/FiO2 ratio at 1 h based on previous studies [Table 5].[7,9,11,18,27] A value of PaO2/FiO2 ratio of < 150 at 1 h had the best combination of sensitivity (34.8%) and specificity (94.4%). Further, in the univariate analysis, change in PaO2/FiO2 ratio at 1 h and a high baseline APACHE II score predicted failure of NIV in avoiding intubation in subjects with ARDS. These findings suggest that patients with PaO2/FiO2 ratio of <150 after 1 h of NIV should be monitored even more closely.
Finally, the study has several limitations. The study is a single center observational study comprising a small sample size and hence the results of the current study need to be confirmed in a multicenter study with a larger sample size. The study comprised predominantly of extrapulmonary ARDS and did not include subjects with severe ARDS (PaO2/FiO2 ratio ≤100). However, a randomized trial may not be feasible in patients with moderate-to-severe ARDS as they are likely to need some form of ventilatory support (noninvasive or invasive) rather than oxygen supplementation alone.
Conclusion
Judicious use of NIV in subjects with mild to moderate ARDS helped in avoiding intubation in 44% of the subjects in the current study. A baseline APACHE II score of >17 and a PaO2/FiO2 ratio <150 at 1 h predicted failure of NIV in preventing intubation in the study population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest. 2006;130:724–9. doi: 10.1378/chest.130.3.724. [DOI] [PubMed] [Google Scholar]
- 3.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Brochard L, Mancebo J, Elliott MW. Noninvasive ventilation for acute respiratory failure. Eur Respir J. 2002;19:712–21. doi: 10.1183/09031936.02.00295502. [DOI] [PubMed] [Google Scholar]
- 5.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–22. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Gupta R, Aggarwal AN, Gupta D. Noninvasive positive pressure ventilation in acute respiratory failure due to COPD vs other causes: Effectiveness and predictors of failure in a respiratory ICU in North India. Int J Chron Obstruct Pulmon Dis. 2008;3:737–43. doi: 10.2147/copd.s3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Agarwal R, Aggarwal AN, Gupta D, Jindal SK. A survey of noninvasive ventilation practices in a respiratory ICU of North India. Respir Care. 2012;57:1145–53. doi: 10.4187/respcare.01541. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Non-invasive ventilation in acute cardiogenic pulmonary oedema. Postgrad Med J. 2005;81:637–43. doi: 10.1136/pgmj.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: A proportion meta-analysis. Respir Care. 2010;55:1653–60. [PubMed] [Google Scholar]
- 10.Luo J, Wang MY, Zhu H, Liang BM, Liu D, Peng XY, et al. Can non-invasive positive pressure ventilation prevent endotracheal intubation in acute lung injury/acute respiratory distress syndrome? A meta-analysis. Respirology. 2014;19:1149–57. doi: 10.1111/resp.12383. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Handa A, Aggarwal AN, Gupta D, Behera D. Outcomes of noninvasive ventilation in acute hypoxemic respiratory failure in a respiratory intensive care unit in north India. Respir Care. 2009;54:1679–87. [PubMed] [Google Scholar]
- 12.Agarwal R, Nath A, Gupta D. Noninvasive ventilation in Plasmodium vivax related ALI/ARDS. Intern Med. 2007;46:2007–11. doi: 10.2169/internalmedicine.46.0401. [DOI] [PubMed] [Google Scholar]
- 13.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 15.Klockars AJ, Hancock GR. Scheffé's more powerful F-protected post-hoc procedure. J Educ Behav Stat. 2000;25:13–9. [Google Scholar]
- 16.Chan YH. Biostatistics 301. Repeated measurement analysis. Singapore Med J. 2004;45:354–68. [PubMed] [Google Scholar]
- 17.Zhan Q, Sun B, Liang L, Yan X, Zhang L, Yang J, et al. Early use of noninvasive positive pressure ventilation for acute lung injury: A multicenter randomized controlled trial. Crit Care Med. 2012;40:455–60. doi: 10.1097/CCM.0b013e318232d75e. [DOI] [PubMed] [Google Scholar]
- 18.Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35:18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 19.Nicolini A, Tonveronachi E, Navalesi P, Antonelli M, Valentini I, Melotti RM, et al. Effectiveness and predictors of success of noninvasive ventilation during H1N1 pandemics: A multicenter study. Minerva Anestesiol. 2012;78:1333–40. [PubMed] [Google Scholar]
- 20.BouAkl I, Bou-Khalil P, Kanazi G, Ayoub C, El-Khatib M. Weaning from mechanical ventilation. Curr Opin Anaesthesiol. 2012;25:42–7. doi: 10.1097/ACO.0b013e32834e6430. [DOI] [PubMed] [Google Scholar]
- 21.Cheung TM, Yam LY, So LK, Lau AC, Poon E, Kong BM, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845–50. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida Y, Takeda S, Akada S, Hongo T, Tanaka K, Sakamoto A. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth. 2008;22:201–6. doi: 10.1007/s00540-008-0637-z. [DOI] [PubMed] [Google Scholar]
- 23.Rocker GM, Mackenzie MG, Williams B, Logan PM. Noninvasive positive pressure ventilation: Successful outcome in patients with acute lung injury/ARDS. Chest. 1999;115:173–7. doi: 10.1378/chest.115.1.173. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosino N. Noninvasive mechanical ventilation in acute respiratory failure. Monaldi Arch Chest Dis. 1996;51:514–8. [PubMed] [Google Scholar]
- 25.Ambrosino N. Noninvasive mechanical ventilation in acute respiratory failure. Eur Respir J. 1996;9:795–807. doi: 10.1183/09031936.96.09040795. [DOI] [PubMed] [Google Scholar]
- 26.Wood KA, Lewis L, Von Harz B, Kollef MH. The use of noninvasive positive pressure ventilation in the emergency department: Results of a randomized clinical trial. Chest. 1998;113:1339–46. doi: 10.1378/chest.113.5.1339. [DOI] [PubMed] [Google Scholar]
- 27.Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: Observational cohort study. Crit Care. 2006;10:R79. doi: 10.1186/cc4923. [DOI] [PMC free article] [PubMed] [Google Scholar]


