Abstract
Background
While epinephrine infusion is widely used in critical care for inotropic support, there is no direct method to detect the onset and measure the magnitude of this response. We hypothesized that surrogate measurements, such as heart rate and vascular tone, may indicate if the plasma and tissue concentrations of epinephrine and cAMP are in a range sufficient to increase myocardial contractility.
Methods
Cardiovascular responses to epinephrine infusion (0.05–0.5 mcg·kg−1·min−1) were measured in rats using arterial and left ventricular catheters. Epinephrine and cAMP levels were measured using ELISA techniques.
Results
The lowest dose of epinephrine infusion (0.05 mcg·kg−1·min−1) did not raise plasma epinephrine level and did not lead to cardiovascular response. Incremental increase in epinephrine infusion (0.1 mcg·kg−1·min−1) elevated plasma but not myocardial epinephrine levels, providing vascular, but not cardiac effects. Further increase in the infusion rate (0.2 mcg·kg−1·min−1) raised myocardial tissue epinephrine levels sufficient to increase heart rate but not contractility. Inotropic and lusitropic effects were significant at the infusion rate of 0.3 mcg·kg−1·min−1. Correlation of plasma epinephrine to hemodynamic parameters suggest that as plasma concentration increases, systemic vascular resistance falls (EC50=47 pg/ml), then HR increases (ED50=168 pg/ml), followed by a rise in contractility and lusitropy (ED50=346 pg/ml and ED50=324 pg/ml accordingly).
Conclusions
The dose response of epinephrine is distinct for vascular tone, HR and contractility. The need for higher doses to see cardiac effects is likely due to the threshold for drug accumulation in tissue. Successful inotropic support with epinephrine cannot be achieved unless the infusion is sufficient to raise the heart rate.
Keywords: epineprhine, cAMP, myocardial tissue, heart rate, vascular tone, contractility
Introduction
Cardiovascular effects of adrenergic agonists are aimed to increase tissue oxygen delivery through augmented cardiac output and tissue perfusion.[1, 2] These hemodynamic effects include an increase in myocardial contractility, heart rate, and change in vascular tone.[3–6] Increased myocardial inotropy is considered a primary therapeutic endpoint of epinephrine infusions in many clinical arenas; however, contractility is not easily measured directly in patients . While this drug has a short half-life and is, therefore, easily titrated, the lack of clear therapeutic endpoints makes dosing for this indication difficult and somewhat arbitrary. In contrast, other, not necessarily desired effects of epinephrine, such as increased heart rate or lowered vascular tone from beta-2 adrenergic agonism, are far more easily and directly quantified. Cardiac output is often used as a surrogate for contractility; however, it is also sensitive to loading conditions. Caridac output can be increased by decreasing systemic vascular resistance (SVR) or increasing preload without a change in the contractile state of the myocardium. Despite decades of experience with epinephrine and its described hemodynamic effects,[7–10] plasma level or dose dependence of inotropic, lusitropic, chronotropic, and vasodilatory responses are still vague. While it is well accepted that beta-2 dependent vasodilation occurs before heart rate (HR) rises with infused doses of epinephrine,[11] it is unclear what the myocardial deposition and intracellular sequelae are or how differentially responsive the beta-1 driven heart rate and contractility responses are to plasma concentration. By knowing the magnitude of these dose response relationships and the plasma concentrations in which they occur, one might use easily measured hemodynamic parameters, such as HR or SVR, to estimate whether the plasma drug levels are in a range sufficient to drive myocardial contractility.
We sought to determine plasma levels of epinephrine and corresponding vascular, chronotropic, inotropic and lusitropic effects over a range of infusion rates in healthy, anesthetized rats. In an effort to explain the observed phenomena, we also measured the concentration of myocardial epinephrine and cAMP, an intracellular signaling molecule responsible for modulating the force of myocardial contraction by controlling the rate of calcium release and reuptake by the sarcoplasmic reticulum.[12–14
Methods
All studies were approved by the Institutional Animal Care and Use Committee of the Steward St Elizabeth’s Medical Center, Boston, Massachusetts. Thirty six male, Sprague-Dawley rats (weight 375–475 g) were anesthetized with ketamine hydrochloride (90 mg/kg i.p.) and xylazine (10 mg/kg, i.p.) followed by intraperitoneal infusions of ketamine hydrochloride (40 mg · kg−1 · hr−1, i.p.) and xylazine (2.5 mg · kg−1 · hr−1, i.p.). The neck, thorax and inguinal areas were shaved and prepped with alcohol. Each rat was secured to a heating pad which was adjusted to maintain rectal temperature at 37 °C. The length of each experiment was 90–120 minutes.
Surgical Procedures
A 20 gauge endotracheal tube was inserted through a tracheotomy and the animal was placed on a ventilator (#683, Harvard rodent ventilator; Holliston, MA). Ventilation settings were calculated according to: tidal volume (Vt, ml) = 6.2×M1.01 (M = animal mass, kg), respiration rate (RR, min−1) = 53.5 × M−0.26.[15] The right femoral artery, right femoral vein and right jugular vein were cannulated for arterial blood pressure measurement, volume resuscitation, and drug infusion, respectively. Albumin (10%) in 0.9% saline was continuously infused (0.5 ml/kg/hr, i.v.) throughout the experiment to maintain blood oncotic pressure and electrolyte balance.[16] The sternum was opened and the heart exposed in order to insert a Millar™ conductance catheter (#SPR-869, 6mm, Millar catheter, MPVS-300 system; Houston, TX) into the left ventricular (LV) chamber by a transapical ventriculotomy with a 23-G needle. Each animal was rested for 15 minutes following surgical preparation prior to epinephrine infusion (0.0, 0.05, 0.1, 0.2, 0.3 or 0.5 mcg · kg−1 · min−1). Each animal received only one infusion rate of epinephrine prior to sacrificing and myocardial harvesting. Hemodynamic parameters were recorded continuously from the baseline to 15 minutes beyond the time when blood pressure, heart rate and LV contractility (max dP/dt) signals reached steady-state. At the end of each non-survival experiment, 3 ml of blood from the femoral artery was drawn for plasma epinephrine content measurement. This large volume relative to the circulating blood volume of the rat precluded serial sampling. The beating heart was then harvested for measurement of tissue epinephrine (#IB89551; Immuno-Biological Laboratories, Inc., Minneapolis, MN)[17] and cAMP (#250006; Cell Biolabs Inc, San Diego, CA)[14] levels. The hearts were quickly excised and rinsed with ice-cold saline. A transmural core (#21909-144, 6 mm Miltex biopsy punches; VWR, Radnor, PA) of tissue from the anterior wall of the left ventricle was blotted, snap frozen in liquid nitrogen and stored at −80°C.
Drug Delivery and Data Acquisition
Epinephrine was diluted in normal saline so that when infused i.v. at 0.0, 0.05, 0.1, 0.2, 0.3 or 0.5 mcg · kg−1 · min−1, the volume flow rate was always 0.5 ml/hr (Cole Parmer Syringe Pump #74900-00). These doses were chosen to evaluate the sequence of the development of hemodynamic responses as epinephrine dose increases, starting from minimal infusion rate providing no vascular effects up to a 50% increase in contractility as determined by preliminary data. The sample size (n=6 in each group) was powered to 95% to detect a 50% decrease in systemic vascular resistance (SD = 20%), and 50% increase in cardiac contractility (SD=20%) with a 5% chance of a type I error (P<0.05). Femoral arterial and central venous pressures (CVP) were continuously transduced (#TRN050; Kent Scientific, Torrington, CT), amplified (#TRN005; Kent Scientific), and digitized (PowerLab A/D converter, 8SP; Castle Hill, Australia). LV pressure and volume signals from the Millar catheter were similarly digitized at 100 Hz and stored. All data were recorded into LabChart 5.0 (AD Instruments) software for instantaneous display and post experimental analysis. HR and LV pressure were recorded from the pressure transducer of the LV Millar catheter. Mean arterial pressure (MAP) was calculated from femoral arterial pressure data. The first derivative of left ventricular pressure signal was processed for maxima and minima (max dP/dt and min dP/dt) and used as indices of myocardial contraction and relaxation, respectively. Real time stroke volume (SV) was measured as the difference in the left ventricular end-diastolic and end-systolic volumes, cardiac output (CO) was calculated as the product of the HR and the SV, and systemic vascular resistance (SVR) was calculated as: SVR=(MAP–CVP)/CO. The CVP was intermittently monitored by halting the drug infusion to the internal jugular vein with a 3-way stopcock, always for less than 10 seconds. The catheter position inside the LV was optimized at the beginning and end of each experiment by slight manipulation until the SV signal was maximized and four distinct phases of the cardiac cycle (filling, isovolumic contraction, ejection, and isovolumic relaxation) were visualized.[15] Volume calibration was obtained by collecting warm heparinized blood from rats at the end of experiments and filling cuvettes of known dimensions (#910-1048, Millar Instruments) for conductance measurements.
Ventricular Tissue Extract Preparation
Myocardial tissue was crushed in liquid nitrogen using a ceramic mortar and pestle, mixed with lysis buffer (#250006; Cell Biolabs Inc) containing phosphatase and protease inhibitor cocktails (#P2850, P5726 and P8340; Sigma, St. Louis, MO) (cocktails (#P2850, P5726 and P8340; Sigma, St. Louis, MO)[18] and homogenized (VirTishear 1700; SP scientific, Warminster, PA). Homogenized tissue was incubated in the lysis buffer at 4°C for 60 min, centrifuged at 1100 g for 15 min, and the supernatant stored at −80°C.
Biochemical Measurements
Arterial blood samples were centrifuged at 1100 g for 15 min and plasma stored at −20°C. Epinephrine content in blood plasma and cardiac tissue extract was evaluated using a colorimetric ELISA (#IB89551; Immuno-Biological Laboratories).[17] cAMP content in cardiac tissue extracts was measured using cAMP ELISA (# STA-501; Cell Biolabs Inc).[14] Cardiac epinephrine and cAMP levels were normalized to protein content measured by Bradford’s method with bovine serum albumin as the standard (#500-0006; Bio-Rad, Hercules, CA).[19
Statistical Analysis
Biochemical data are presented as molar or concentration normalized to protein content in the tissue extract. The effective concentration at 50% peak effect (EC50) was calculated using nonlinear regression analysis (GraphPad PRISM 5.0; GraphPad Software Inc, La Jolla, CA). One-way ANOVA analysis with Bonferroni correction for comparisons of all pairs of studied doses of epinephrine infusion were used to compare hemodynamic responses, epinephrine and cAMP levels. P value ≤ 0.05 was considered statistically significant. Nonlinear regression curve-fit was used to evaluate relationships between plasma epinephrine, HR, SVR, max dP/dt, and min dP/dt (GraphPad PRISM 5.0).
Results
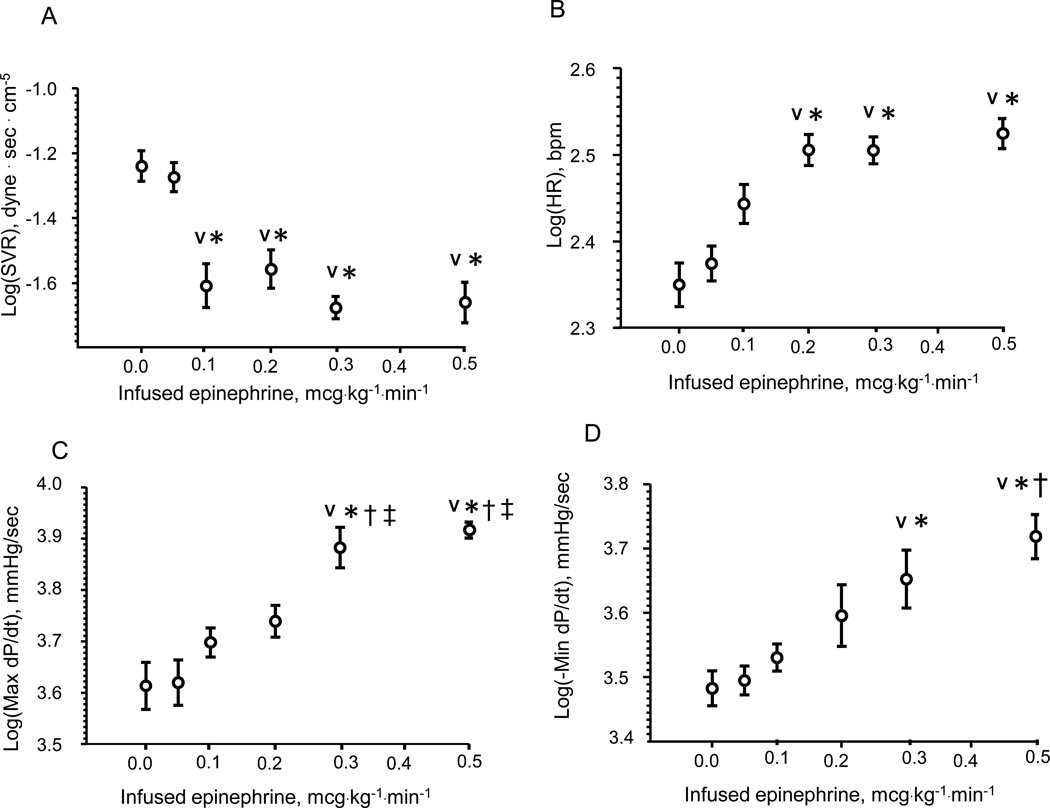
Epinephrine was given to each animal by a single, constant IV infusion at 0, 0.05, 0.1, 0.2, 0.3 or 0.5 mcg · kg−1 · min−1. The resulting SVR, HR, max dP/dt and min dP/dt are shown (Fig. 1). The lowest dose epinephrine infusion (0.05 mcg·kg−1·min−1) did not have any pharmacological effect on cardiovascular parameters. An incremental increase in dose to 0.1 mcg·kg−1·min−1 decreased SVR by 51% from 0 mcg·kg−1·min−1 (p < 0.05) without significant changes in heart rate or cardiac contractility. SVR did not fall further with higher dose rates. Further increase in the rate of epinephrine infusion (0.2 mcg·kg−1·min−1) significantly increased HR by 47% from 0 mcg·kg−1·min−1 (p < 0.05) but did not affect max dP/dt. Epinephrine infusion at 0.3 mcg·kg−1·min−1 significantly increased contractility (max dP/dt) by 82% and lusitropy (min dP/dt) by 49% from 0 mcg · kg−1 · min−1 (p < 0.05). The highest dose of epinephrine infusion (0.5 mcg·kg−1·min−1) did not further increase heart rate or max dP/dt but the increase in lusitropy was 72% from 0 mcg · kg−1 · min−1 (p < 0.05)
Figure 1.
Hemodynamic dose response to epinephrine IV infusions in terms of systemic vascular resistance (SVR) (Panel A) heart rate (HR) (Panel B), contractility (max dP/dt) (Panel C) and lusitropy (min dP/dt) (Panel D). Each dose produces a unique hemodynamic response. The lowest dose did not elicit any hemodynamic response. SVR was significantly lowered starting at 0.1 mcg · kg −1· min −1 and heart rate increased at 0.2 mcg · kg −1· min −1 infusion rate. Max dP/dt and min dP/dt were were first increased above baseline at 0.3 mcg · kg −1· min −1. Data are presented as log-transformed to the base of 10. N=6, AVG ± SD.
V - < 0.05 – compared with 0.00 mcg · kg −1· min −1 infusion rate,
* - < 0.05 – compared with 0.05 mcg · kg −1· min −1 infusion rate,
† - < 0.05 – compared with 0.1 mcg · kg −1· min −1 infusion rate,
‡ - < 0.05 – compared with 0.2 mcg · kg −1· min −1 infusion rate.
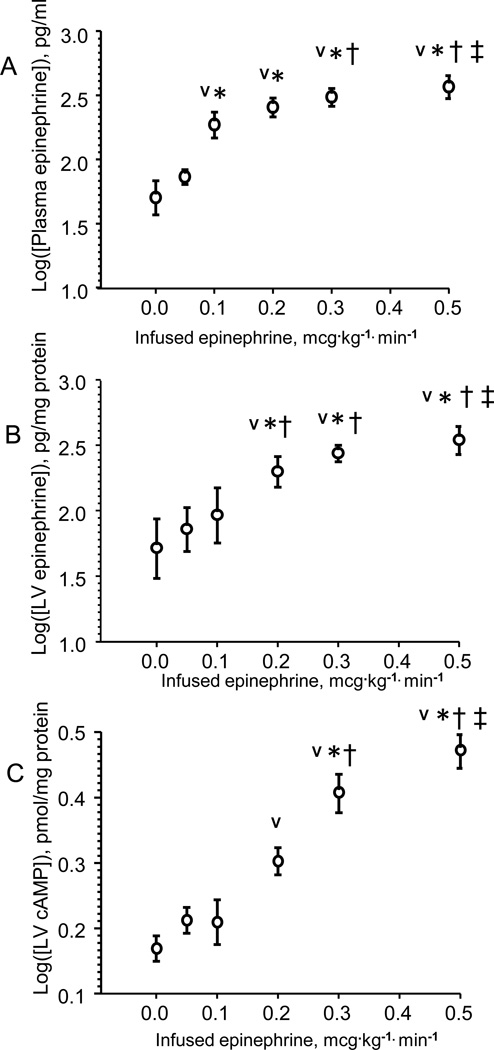
Plasma epinephrine concentration did not increase with infused dose until 0.1 mcg·kg−1·min−1 (Fig. 2A). Cardiac epinephrine and cAMP levels did not increase until the rate of infusion was raised to 0.2 mcg·kg−1·min−1 (Figs 2B–C). Thus, peripheral effects of decreased SVR are observed at the dose sufficient to raise the level of plasma epinephrine whereas central effects of increased chronotropy and inotropy are observed only at doses that allow the accumulation of the drug and upregulation of cAMP in cardiac tissue.
Figure 2.
Plasma epinephrine (Panel A), left ventricular (LV) tissue epinephrine (Panel B) and cAMP (Panel C) as a function of epinephrine infusion rate. Whereas plasma epinephrine concentration increases already at 0.1 mcg · kg −1· min −1, cardiac epinephrine and cAMP levels increase at 0.2 mcg·kg−1·min−1 infusion rate. Data are presented as log-transformed to the base of 10. N=6, AVG ± SD.
V - < 0.05 – compared with 0.00 mcg · kg −1· min −1 infusion rate,
* - < 0.05 – compared with 0.05 mcg · kg −1· min −1 infusion rate,
† - < 0.05 – compared with 0.1 mcg · kg −1· min −1 infusion rate,
‡ - < 0.05 – compared with 0.2 mcg · kg −1· min −1 infusion rate.
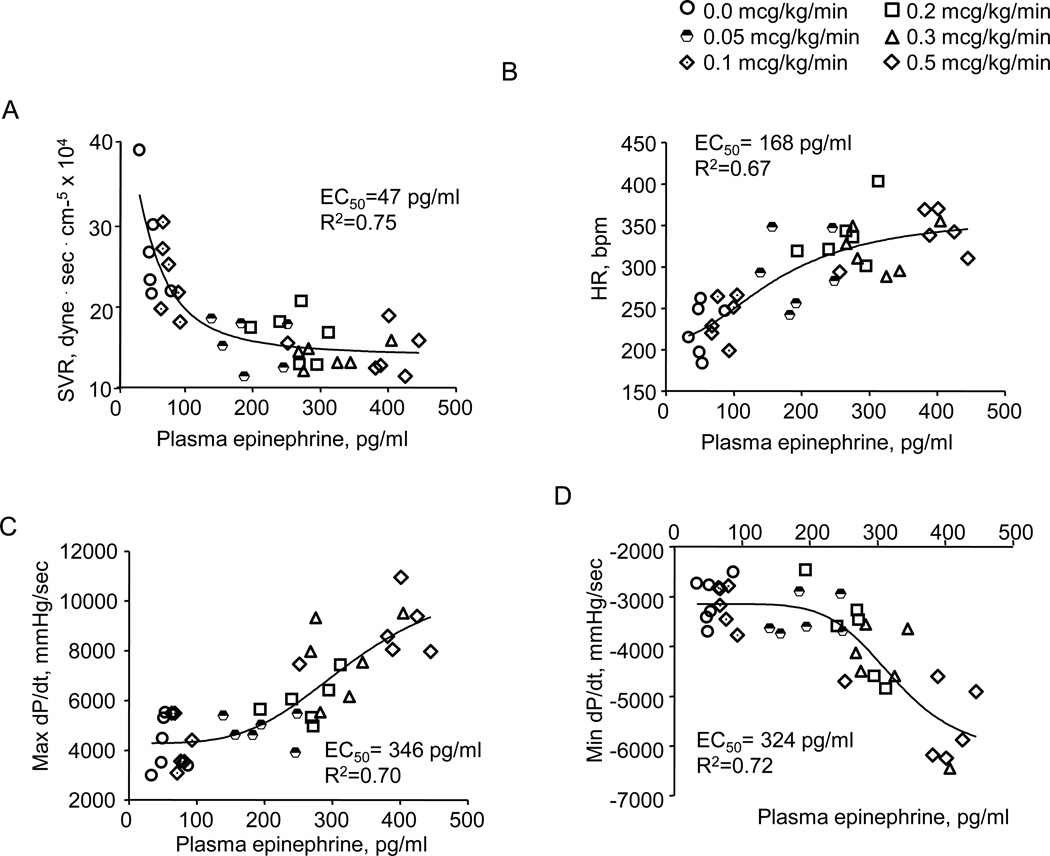
Hemodynamic responses are shown as a function of the measured plasma epinephrine concentrations (Figs. 3A–D). As plasma concentration increases, SVR falls, HR increases, contractility rises and lusitropy increases. Correlations of these data to sigmoidal curves allow estimation of the plasma concentration of epinephrine that produces 50% of the observed change in hemodynamic index (EC50). These data suggest that SVR decreases at the lowest epinephrine plasma concentrations (EC50,SVR = 47 pg/ml), followed by HR (EC50,HR=168 pg/ml), max dP/dt and min dP/dt (EC50,maxdP/dt =346 pg/ml, EC50,mindP/dt=324 pg/ml). Thus, hemodynamic response to epinephrine is limited to a loss of vascular tone at the lowest dose rates. Chronotropic effect emerges at a slightly higher dose rate, and as drug infusion is incremented further, inotropic and then lusitropic effects become manifest.
Figure 3.
Effect of plasma epinephrine content on systemic vascular resistance (SVR) (Panel A), heart rate (HR) (Panel B), max dP/dt (Panel C) and min dP/dt (Panel D). Data are fit to sigmoidal curves (Prism 5.0, Graph Pad). These correlations suggest that vascular effects occur at the lowest plasma epinephrine levels, HR increases at a significantly higher plasma level and contractile effects emerge at the highest plasma concentrations, such that the concentration where each effect half maximal (EC50) is: EC50 SVR < EC50 HR < EC50 max dP/dt and EC50 min dP/dt.
The deposition of drug and the upregulation of intracellular intermediates as a function of plasma epinephrine levels, and hemodynamic indices as a function of deposited drug or cAMP, are shown and discussed in the ONLINE Supplement.
Discussion
Epinephrine infusions are often used in intensive care and cardiac surgical applications.[20,22] Major hemodynamic responses to epinephrine have been described and include changes in vascular tone, heart rate and myocardial contractility.[3–6] While HR and SVR are easily measured, there is no technology to routinely measure contractility in patients. Therefore, animal studies may be helpful to relate these effects to plasma drug concentrations and dose infusion rates. A multitude of studies have examined vascular, chronotropic and inotropic responses;[7–10] however, these effects have not been studied simultaneously in the same animal during continuous intravenous epinphrine infusion. Analyses carried out in different animal models do not allow comparison of the relationships between infused dose rate, HR, vascular dilation, and myocardial contractility. While it may seem tempting to pool data from several studies, the hemodynamic responses are all sensitive to anesthetics, ventilation, temperature, and intravascular volume status. Therefore, the correlation of these responses to dose infusion rates needs to be performed in a single series of animals handled with uniform conditions.
We used a rat model instrumented with a left ventricular pressure-volume conductance catheter and an arterial blood pressure transducer to examine the dose-response relationships of heart rate, myocardial contractility (max dP/dt), and vascular tone to an incremental increase in the dose of infused epinephrine. In an effort to elucidate the mechanisms of these differential dose-responses, we assessed how epinephrine infusion rates relate to epinephrine plasma level, myocardial tissue deposition, second messenger upregulation and biologic effect. Bolus administration leads to rapidly falling plasma drug levels for compounds with short serum half-lives, such as epinephrine. Therefore, we performed these experiments with steady-state drug infusions from which we could sample plasma and harvest tissues for drug and intracellular intermediate level assessment. Many studies using Millar catheters to assess contractility use regression based analyses[15, 23, 24, 25] as they are deemed to be insensitive to heart loading conditions. Use of this technique requires transient occlusion of the inferior vena cava over many heart beats. This maneuver causes transient hypotension with vasoconstricting and inotropic relfexes that alter the tissue epinephrine and cAMP concentrations.[26] Therefore, we could not use regression based analyses and relied on max dP/dt signals.
Mechanism of differential dose-responses to epinephrine
Hemodynamic responses to incremental epinephrine infusion showed that vascular tone first increased at 0.1 mcg · kg−1 · min−1, HR first increased at 0.2 mcg · kg−1 · min−1, and contractility as well as lusitropy first increased at 0.3 mcg · kg−1 · min−1. (Fig. 1). Plasma and myocardial epinephrine data showed that the lowest dose (0.05 mcg · kg−1 · min−1) does not raise plasma or tissue epinephrine levels (Figs. 2A–C) explaining the absence of the pharmacological response. The increase in plasma epinephrine was observed starting at 0.1 mcg · kg−1 · min−1, but it was not until 0.2 mcg · kg−1 · min−1 when the accumulation of the drug in the myocardial tissue occurred (Fig 2B) and cardiac effects began to manifest (Fig. 1B). Of interest, the increase in myocardial epinephrine observed at 0.2 mcg · kg−1 · min−1 upregulated cAMP level by 33% which was enough to raise heart rate but not contractility (Figs. 1B–C). By increasing infused epinephrine dose to 0.3 mcg · kg−1 · min−1 cAMP was additionally upregulated by 35%, enough to significantly increase cardiac contractility (Figs. 1C and 2C).
The phenomenon of delayed myocardial tissue epinephrine accumulation with plasma concentration (Fig. 2B) may result from the balance of two processes, epinephrine delivery to the myocardium and degradation by tissue catecholamine transferase (COMT).[27, 28] The threshold for myocardial epinephrine deposition is reached when the rate of drug uptake, driven by transcapillary blood-tissue concentration gradients, exceeds the rate of degradation. Our data suggest that epinephrine did not accumulate in myocardial tissue until a plasma concentration was reached that was sufficient to induce maximal systemic vasodilation (Figs. 2A–B).
Plasma epinephrine concentration-effect curve-fits showed that distinct plasma epinephrine concentrations are required to achieve 50% of a maximal (EC50) vascular, chronotropic, inotropic and lusitropic responses (Fig. 3). These correlations suggest that EC50,SVR was 3.5 fold lower than EC50,HR, 7 fold lower than EC50,max dP/dt and EC50,min dP/dt . It is intriguing that not only do vascular and cardiac effects respond differently to plasma concentration, but that chronotropic and inotropic cardiac effects within the heart also have distinct dose-response relationships. Indeed, tachycardia becomes manifest at lower plasma concentrations than augmented contractility (Figs. 3B–C). There are several suggested mechanisms for HR to respond at lower plasma and tissue levels than contractility. First, heart rate increase may be stimulated in the absence of myocardial drug from reflexes that maintain mean arterial pressure as SVR falls. Second, differential chronotropic and inotropic effects might be explained through differential β-receptor densities in the SA node and ventricular myocytes. The ratio of high affinity β2 receptors to lower affinity β1 receptors[29, 30] is greater in the SA node and conduction system than in myocytes,[31, 32] but its physiologic role has not been fully defined. Our data suggest that this phenotypical distinction between different cells within the heart may provide different sensitivity to intracellular cAMP levels as pharmacologic response for heart rate is observed at lower myocaridal epinephrine and cAMP concentrations than for cardiac contractility. These intriguing data cannot be directly translated to humans, especially to patients with cardiac disease without further investigation.
Conclusions and Clinical Implications
A direct measure of myocardial contractility is not routinely available in clinical settings and indication of any epinephrine effect may be limited to HR, or on rare occasions when a pulmonary artery catheter is present, SVR. We demonstrate distinct hemodynamic dose responses to steady-state epinephrine infusion and suggest mechanisms for these phenomena in terms of blood and myocardial levels of the drug, and resulting myocardial cAMP upregulation. While systemic vascular dilation occurs in response to subtle increases in plasma epinephrine levels, significantly higher concentrations are required to raise myocardial epinephrine to drive cAMP upregulation and central cardiac responses of chronotropy and then inotropy and lusitropy. These data suggest that HR must rise before any inotropic effect could be exerted.
Supplementary Material
Acknowledgements
This study was supported by the American Heart Association (09SDG2060320) to Mark A. Lovich, the Society for Cardiovascular Anesthesiologists Starter Grant to Mark A. Lovich, the Department of Anesthesiology and Pain Medicine, Steward St. Elizabeth’s Medical Center, Brighton.
References
- 1.Salak N, Pajk W, Knotzer H, Hofstötter H, Schwarz B, Mayr A, Labeck B, Kafka R, Ulmer H, Mutz N, Hasibeder W. Effects of epinephrine on intestinal oxygen supply and mucosal tissue oxygen tension in pigs. Crit Care Med. 2001;29:367–373. doi: 10.1097/00003246-200102000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Schwarte LA, Schwartges I, Schober P, Scheeren TW, Fournell A, Picker O. Sevoflurane and propofol anaesthesia differentially modulate the effects of epinephrine and norepinephrine on microcirculatory gastric mucosal oxygenation. Br J Anaesth. 2010;105:421–428. doi: 10.1093/bja/aeq215. [DOI] [PubMed] [Google Scholar]
- 3.Spencer MP, Denison AB, Jr, Green HD. The Direct Renal Vascular Effects of Epinephrine and Norepinephrine before and after Adrenergic Blockade. Circ Res. 1954;2:537–540. doi: 10.1161/01.res.2.6.537. [DOI] [PubMed] [Google Scholar]
- 4.Chilian WM, Layne SM, Eastham CL, Marcus ML. Effects of epinephrine on coronary microvascular diameters. Circ Res. 1987;61(5 Pt 2):1147–1153. [PubMed] [Google Scholar]
- 5.Latorre G, Cardenas V, Lopez. O. Mechanism of the effect of constant infusion of epinephrine on blood pressure, heart rate and arterial hematocrit in normal and sympathectomized-splanchnicectomized dogs. Arch Int Physiol Biochim. 1960;68:785–792. doi: 10.3109/13813456009075170. [DOI] [PubMed] [Google Scholar]
- 6.Furnival CM, Linden RJ, Snow HM. The inotropic and chronotropic effects of catecholamines on the dog heart. J Physiol. 1971;214(1):15–28. doi: 10.1113/jphysiol.1971.sp009416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratton JR, Pfeifer MA, Ritchie JL, Halter JB. Hemodynamic effects of epinephrine: concentration-effect study in humans. J Appl Physiol. 1985;58:1199–1206. doi: 10.1152/jappl.1985.58.4.1199. [DOI] [PubMed] [Google Scholar]
- 8.Tarnow J, Müller RK. Cardiovascular effect of low-dose epinephrine infusions in relation to the extent of preoperative beta-adrenoceptor blockade. Anesthesiology. 1991;74:1035–1043. doi: 10.1097/00000542-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bersten AD, Rutten AJ, Summersides G, Ilsley AH. Epinephrine infusion in sheep: systemic and renal hemodynamic effects. Crit Care Med. 1994;22:994–1001. doi: 10.1097/00003246-199406000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Nachar RA, Booth EA, Friedlich P, Borzage M, Soleymani S, Wider MD, Seri I. Dose-dependent hemodynamic and metabolic effects of vasoactive medications in normotensive, anesthetized neonatal piglets. Pediatr Res. 2011;70:473–479. doi: 10.1203/PDR.0b013e31822e178e. [DOI] [PubMed] [Google Scholar]
- 11.Goodman A, Rall T, Neis A, Taylot P. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 8th ed. New York: Pergamon Press; 1990. p. 187. [Google Scholar]
- 12.Keely SL. Prostaglandin E1 Activation of Heart cAMP-dependent Protein Kinase: Apparent Dissociation of Protein Kinase Activation from Increases in Phosphorylase Activity and Contractile Force. Mol Pharmacol. 1979;15(2):235–245. [PubMed] [Google Scholar]
- 13.Kaumann AJ. Adrenaline and noradrenaline increase force of human ventricle through both beta1 and beta2-adrenoreceptors. Biomed Biochim Acta. 1987;46(8–9):S411–S416. [PubMed] [Google Scholar]
- 14.Tanigawa T, Yano M, Kohno M, Yamamoto T, Hisaoka T, Ono K, Ueyama T, Kobayashi S, Hisamatsu Y, Ohkusa T, Matsuzaki M. Mechanism of preserved positive lusitropy by cAMP-dependent drugs in heart failure. Am J Physiol Heart Circ Physiology. 2000;278:H313–H320. doi: 10.1152/ajpheart.2000.278.2.H313. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure–volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horstick G, Lauterbach M, Kempf T, Ossendorf M, Kopacz L, Heimann A, Lehr HA, Bhakdi S, Horstick M, Meyer J, Kempski O. Plasma protein loss during surgery: beneficial effects of albumin substitution. Shock. 2001;16:9–14. doi: 10.1097/00024382-200116010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Westermann J, Hubl W, Kaiser N, Salewski L. Simple, rapid and sensitive determination of epinephrine and norepinephrine in urine and plasma by non-competitive enzyme immunoassay, compared with HPLC method. Clin Lab. 2002;48:61–71. [PubMed] [Google Scholar]
- 18.Kakarla SK, Fannin JC, Keshavarzian S, Katta A, Paturi S, Nalabotu SK, Wu M, Rice KM, Manzoor K, Walker EM, Jr, Blough ER. Chronic acetaminophen attenuates age-associated increases in cardiac ROS and apoptosis in the Fischer Brown Norway rat. Basic Res Cardiol. 2010;105:535–544. doi: 10.1007/s00395-010-0094-3. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Günnicker M, Brinkmann M, Donovan TJ, Freund U, Schieffer M, Reidemeister JC. The efficacy of amrinone or adrenaline on low cardiac output following cardiopulmonary bypass in patients with coronary artery disease undergoing preoperative beta-blockade. Thorac Cardiovasc Surg. 1995;43(3):153–160. doi: 10.1055/s-2007-1013790. [DOI] [PubMed] [Google Scholar]
- 21.Tempe DK, Virmani S. Pharmacologic support of circulation in patients undergoing cardiac surgery. J Indian Med Assoc. 1999;97(10):411–418. [PubMed] [Google Scholar]
- 22.Annane D, Vignon P, Renault A, Bollaert PE, Charpentier C, Martin C, Troché G, Ricard JD, Nitenberg G, Papazian L, Azoulay E, Bellissant E CATS Study Group. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007;370:676–684. doi: 10.1016/S0140-6736(07)61344-0. [DOI] [PubMed] [Google Scholar]
- 23.Kass DA, Maughan WL. From 'Emax' to pressure-volume relations: a broader view. Circulation. 1988;77(6):1203–1212. doi: 10.1161/01.cir.77.6.1203. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Shishido T, Kawada T, Miyano H, Miyashita H, Inagaki M, Sugimachi M, Sunagawa K. ESPVR of in situ rat left ventricle shows contractility-dependent curvilinearity. Am J Physiol. 1998;274(5 Pt 2):H1429–H1434. doi: 10.1152/ajpheart.1998.274.5.H1429. [DOI] [PubMed] [Google Scholar]
- 25.Champion HC, Georgakopoulos D, Haldar S, Wang L, Wang Y, Kass DA. Robust adenoviral and adeno-associated viral gene transfer to the in vivo murine heart: application to study of phospholamban physiology. Circulation. 2003;108(22):2790–2797. doi: 10.1161/01.CIR.0000096487.88897.9B. [DOI] [PubMed] [Google Scholar]
- 26.Dobson JG., Jr Protein kinase regulation of cardiac phosphorylase activity and contreactility. Am J Physiol. 1978 May;234(5):H638–H645. doi: 10.1152/ajpheart.1978.234.5.H638. [DOI] [PubMed] [Google Scholar]
- 27.Labrosse EH, Axelrod J, Kety SS. O-Methylation, the principal route of metabolism of epinephrine in man. Science. 1958;128:593–604. doi: 10.1126/science.128.3324.593. [DOI] [PubMed] [Google Scholar]
- 28.Gennser G, Rosengren E, von Studnitz W. Distribution of noradrenaline and of monoamine oxidase and catechol-O-methyltransferase activity in human heart. Experientia. 1973;29:20–22. doi: 10.1007/BF01913226. [DOI] [PubMed] [Google Scholar]
- 29.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 30.Weitl N, Seifert RJ. Distinct interactions of human beta1- and beta2-adrenoceptors with isoproterenol, epinephrine, norepinephrine, and dopamine. Pharmacol Exp Ther. 2008;327:760–769. doi: 10.1124/jpet.108.143412. [DOI] [PubMed] [Google Scholar]
- 31.Beau SL, Hand DE, Schuessler RB, Bromberg BI, Kwon B, Boineau JP, Saffitz JE. Relative densities of muscarinic cholinergic and beta-adrenergic receptors in the canine sinoatrial node and their relation to sites of pacemaker activity. Circ Res. 1995;77:957–963. doi: 10.1161/01.res.77.5.957. [DOI] [PubMed] [Google Scholar]
- 32.Rodefeld MD, Beau SL, Schuessler RB, Boineau JP, Saffitz JE. Beta-adrenergic and muscarinic cholinergic receptor densities in the human sinoatrial node: identification of a high beta 2-adrenergic receptor density. J Cardiovasc Electrophysiol. 1996;7(11):1039–1049. doi: 10.1111/j.1540-8167.1996.tb00479.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.