Abstract
Taurine is actively transported at the retinal pigment epithelial (RPE) apical membrane in an Na+- and Cl−-dependent manner. Diabetes may alter the function of the taurine transporter. Because nitric oxide (NO) is a molecule implicated in the pathogenesis of diabetes, we asked whether NO would alter the activity of the taurine transporter in cultured ARPE-19 cells. The activity of the transporter was stimulated in the presence of the NO donor 3-morpholinosydnonimine. The stimulatory effects of 3-morpholinosydnonimine were not observed during the initial 16-h treatment; however, stimulation of taurine uptake was elevated dramatically above control values with 20- and 24-h treatments. Kinetic analysis revealed that the stimulation was associated with an increase in the maximal velocity of the transporter with no significant change in the substrate affinity. The NO-induced increase in taurine uptake was inhibited by actinomycin D and cycloheximide. RT-PCR analysis and nuclear run-on assays provided evidence for upregulation of the transporter gene. This study provides the first evidence of an increase in taurine transporter gene expression in human RPE cells cultured under conditions of elevated levels of NO. cell culture
TAURINE, a β-aminosulfonic acid, is the most abundant free amino acid in the retina (38). A high concentration of taurine is essential for maintenance of the structural and functional integrity of the retina (20, 34). The precise physiological role of taurine in the retina has yet to be established, although it has been suggested to function in calcium modulation (23, 29, 34) and osmoregulation (7, 46, 50). Taurine is an unusual biological molecule. It is an amino acid that is not required for protein synthesis, it accumulates in excitable tissues, and its turnover rate is low (7–10 days). The only known metabolic role of taurine is in the synthesis of taurine-conjugated bile acids. Taurine is a zwitterion at physiological pH and, hence, carries no net charge. Because the intracellular concentration of taurine in tissues such as retina, heart, and placenta is 20–40 mM, taurine has been proposed to function as an osmolyte (39). This proposed role is intriguing, because retinal cell edema is associated with pathologies such as ischemia and reperfusion during diabetic retinopathy, macular edema, and neurodegeneration (15, 24). Taurine has been proposed also to function as an antioxidant. Taurine, in concert with zinc, protects rod outer segment membranes from ion and/or water entry occurring as a consequence of membrane lipid peroxidation (37). Recently, Keyes and Zimmerman (28) showed that taurine, in combination with retinol, protects lipids from oxidative damage and may play a key role in protecting retinal pigment epithelial (RPE) lipids during exposure to cyclic light.
In the retina, the concentration of taurine is highest in photoreceptor and RPE cells (27, 35, 55). These observations have prompted numerous investigations of transport mechanisms for taurine. Functional studies suggest that a transporter for taurine is present on the apical membrane of RPE (32, 33) and in cultured human RPE cells (30). Recently cDNAs for the human taurine transporter have been cloned, and sequence homology places it within the gene family of Na+- and Cl−-dependent neurotransmitter transporters (25, 41). The human taurine transporter cDNA encodes a protein of 619 amino acids with 12 putative transmembrane domains. The taurine transporter in various cell types, including RPE cells, is regulated by signal transduction pathways (3, 13, 26, 40), hypertonicity (50, 51), and extracellular taurine levels (19, 26).
Recently, the effect of diabetes on taurine transporter activity was investigated. In vitro studies by Stevens et al. (47) reported that high glucose levels rapidly and specifically decreased the activity and mRNA levels of the transporter in cultured RPE cells. In contrast, in vivo studies of RPE from diabetic rats showed that uptake of taurine was elevated, rather than decreased, suggesting that diabetes stimulates the activity of the taurine transporter (53). A molecule implicated in the pathogenic complications of diabetes, including diabetic retinopathy, is nitric oxide (NO) (14, 42, 48). Circulating levels of NO are elevated in diabetes mellitus (2, 29). Yilmaz and co-workers (58) reported a fivefold elevation of NO in the vitreous of patients with proliferative diabetic retinopathy compared with nondiabetic control patients. NO is produced by different isoforms of NO synthases (NOS) (57). In the retina, constitutive NOS and inducible NOS (iNOS) are present, the former in amacrine and ganglion cells and the latter in RPE and Müller cells (16, 45, 57). The cytokine-inducible form of NOS has been demonstrated also in RPE cells. There is evidence that NOS activity is increased in retinas of diabetic rats (8). Given that NO levels are increased in diabetic retinopathy (42, 58) and that the diabetic condition may affect taurine transport (47, 53), we were interested in determining the effects of NO on taurine transport in vitro. In the present study, we used the well-differentiated RPE cell line ARPE-19 (9, 10) to assess the effects of various NO donors on the activity and expression of the human taurine transporter. We demonstrate that exposure of cells to the NO donor 3-morpholinosydnonimine (SIN-1) leads to a dramatic increase in taurine transport activity. We further demonstrate that exposure of these cells to SIN-1 upregulates taurine transporter gene expression.
MATERIALS AND METHODS
Materials
[1,2-3H]taurine, [2,3-3H]alanine, [2,3,4,5-3H]arginine, and [α-32P]uridine triphosphate were purchased from Amersham Pharmacia (Piscataway, NJ); [3,4-3H]glutamine and [2,3-3H]glutamate from NEN Research Products (Boston, MA); and [3H(N)]carnitine and [4,5-3H]leucine from Moravek Biochemicals (Brea, CA). TRIzol reagent and cell culture supplies were purchased from Life Technologies (Gaithersburg, MD); Ultrahyb hybridization solution from Ambion (Austin, TX); restriction enzymes and pGEM-T vector from Promega (Madison, WI); SIN-1, sodium nitroprusside (SNP), and methylene blue from Research Biochemicals International (Natick, MA); actinomycin D, cycloheximide, ascorbic acid, glutathione, β-alanine, trypan blue solution, lipopolysaccharide (LPS, Escherichia coli), and 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-BrcGMP) from Sigma Chemical (St. Louis, MO); and recombinant human interferon-γ (IFN-γ) from Biosource International (Camarillo, CA). Human RPE (ARPE-19) cells were purchased from American Type Culture Collection (Manassas, VA). Tissue-Tek OCT embedding compound was obtained from Miles Laboratories (Elkhart, IN); the polyclonal antibody against rat taurine transporter as well as the immunogenic control peptide from Alpha Diagnostic International (San Antonio, TX); and the monoclonal anti-nitrotyrosine antibody from Upstate Biologicals (Lake Placid, NY).
Animals
Three-week-old albino (ICR) mice were obtained from Harlan Sprague Dawley (Indianapolis, IN). Animals were maintained on a 12:12-h light-dark cycle and were fed the standard Purina mouse chow diet. Care and use of the animals adhered to the principles set forth in The Guiding Principles in the Care and Use of Animals (DHEW Publication No. 80-23).
Cell culture
ARPE-19 cells were cultured in 75-cm2 flasks with Dulbecco’s modified Eagle’s medium-nutrient mixture F-12 (DMEM-F-12) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were passaged by dissociation in 0.05% (wt/vol) trypsin and seeded in 24-well culture plates. In one set of experiments assessing the effects of NO donors in conjunction with elevated glucose levels, cells were exposed for 24 h to DMEM-F-12 containing 17 mM glucose or to DMEM-F-12 containing 45 mM glucose.
Uptake measurements in ARPE-19 cells
For uptake experiments, the culture medium was removed from ARPE-19 cells and the cells were subsequently washed twice with uptake buffer. The composition of the uptake buffer was 25 mM HEPES-Tris, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose, pH 7.5. For experiments dealing with the influence of Na+ and Cl− on the transport process, the uptake buffers consisted of 20 mM HEPES-Tris (pH 7.5) containing 140 mM NaCl, sodium gluconate, or N-methyl-d-glucamine chloride. Uptake was initiated by addition of 250 μl of uptake buffer containing radiolabeled substrates. Uptake measurements were done with a 15-min incubation at 37°C. At the end of the incubation, uptake was terminated by removal of the medium by aspiration followed by three washes with ice-cold uptake buffer without the radiolabeled substrates. The cells were then solubilized in 0.5 ml of 1% sodium dodecyl sulfate (SDS) in 0.2 N NaOH and transferred to scintillation vials for quantitation of radioactivity.
NO donors
To determine the effects of NO on the activity of taurine transporter in ARPE-19 cells, confluent cultures were incubated in the absence or presence of NO donors SIN-1 or SNP, and the uptake of [3H]taurine was measured. The majority of experiments were done with SIN-1, the more potent of the two NO donors in stimulating the taurine transporter. In one set of experiments, cells were incubated simultaneously with the cytokines LPS (20 ng/ml) and IFN-γ (1 μg/ml), and the uptake of [3H]taurine was measured. The recovery of the stimulation of taurine uptake was assessed by incubating ARPE-19 cells with 1 mM SIN-1 for 24 h, removing the uptake buffer, and replacing the buffer with culture medium. Cells were then maintained in the medium for 0, 2, 4, 6, and 24 h before assessment of the recovery of stimulated uptake of [3H]taurine. To test the specificity of NO donors in stimulating the activity of the taurine transporter, ARPE-19 cells were incubated with 1 mM SIN-1 for 24 h at 37°C, and then uptake of [3H]taurine and the radiolabeled compounds glutamate, glutamine, alanine, arginine, carnitine, and leucine was measured for 15 min. The capacity of antioxidants and NO scavengers to block the SIN-1-induced stimulation of taurine uptake by the taurine transporter was examined by incubating ARPE-19 cells in uptake buffer containing SIN-1 only vs. SIN-1 in the presence of ascorbate (10 mM), glutathione (10 mM), or methylene blue (1 mM). Cells were treated also with ascorbate (10 mM), glutathione (10 mM), or methylene blue (1 mM) independently to assess whether these agents had any effect of their own on taurine uptake by cultured ARPE-19 cells. Cells were treated also for 16 h with 8-BrcGMP (100 μM), a cell-permeable cGMP analog, and the uptake of [3H]taurine was measured. To determine whether cells incubated with SIN-1 were positive for nitrotyrosine, ARPE-19 cells were cultured to confluency on chamber slides. They were incubated for 2 h in the presence or absence of SIN-1. Cells were fixed in 4% paraformaldehyde for 10 min and permeabilized with acetone for 5 min. Slides were incubated overnight with the polyclonal antibody against nitrotyrosine (1:100) at 4°C. The sections were subsequently incubated overnight at 4°C with FITC-conjugated anti-mouse IgG (1:100). Sections were examined by epifluorescence using a Zeiss Axioplan 2 microscope equipped with a Spot camera and Spot software (version 2.2).
Data analysis
Each uptake experiment was performed in duplicate or triplicate and was repeated two to four times. Data analysis (analysis of variance) was performed using the NCSS statistical software package (P < 0.05 was considered significant). Kinetic analysis was done using the computer program Fig.P (version 6.0, Biosoft, Cambridge, UK). Data are presented as means ± SE.
Semiquantitative RT-PCR analysis of the steady-state levels of mRNAs for taurine transporter and glyceraldehyde-3-phosphate dehydrogenase
Confluent cultures of ARPE-19 cells were treated with or without 1 mM SIN-1 for 24 h at 37°C, and poly(A)+ mRNA was then prepared from these cells using the TRIzol reagent. RT-PCR was carried out using primer pairs specific for human taurine transporter and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers specific for human taurine transporter were 5′-GCTAGCTGCATAGTAGTC-3′ (sense) and 5′-TGGAACACACCTCACTGC-3′ (antisense) and corresponded to nucleotide positions 872–889 and 1751–1769, respectively, of the human taurine transporter cDNA (41). The primers specific for human GAPDH were 5′-AAGGCTGAGAACGGGAAGCTTGTCATCAAT-3′ (sense) and 5′-TTCCCGTTCAGCTCAGGGATGACCTTGCCC-3′ (antisense), corresponding to nucleotide positions 241–270 and 711–740 in human GAPDH cDNA (49). Each of these RT-PCR products was subcloned in pGEM-T vector and sequenced to establish its identity. For semiquantitative RT-PCR, PCR following reverse transcription was carried out with various numbers of cycles (range 9–30). The products were size fractionated on an agarose gel and subjected to Southern hybridization with probes specific for the taurine transporter or GAPDH. These probes were generated by labeling the respective subcloned RT-PCR products with [32P]dCTP. The intensity of the hybridization signal was quantified using the STORM PhosphorImaging System (Molecular Dynamics, Sunnyvale, CA). The relationship between the intensity of the signal and the PCR cycle number was then analyzed to determine the linear range for the PCR product formation. The intensities of the signals within the linear range were used for data analysis.
Nuclear run-on transcription assay
To monitor SIN-1-induced changes in the transcription rate of the taurine transporter gene, the nuclear run-on assay was performed as described elsewhere (18) with modifications. ARPE-19 cells were incubated for 24 h with or without 1 mM SIN-1. Nuclei were isolated by homogenization with 10 strokes in a Dounce homogenizer in 2 ml of lysis buffer [10 mM Tris·HCl, pH 7.4, 3 mM CaCl2, 2 mM MgCl2, and 1% (vol/vol) NP-40] followed by centrifugation at 500 g for 5 min at 4°C. Pelleted nuclei were frozen immediately in liquid nitrogen in 200 μl of freezing buffer [50 mM Tris·HCl, pH 8.3, 40% (vol/vol) glycerol, 5 mM MgCl2, and 0.1 mM EDTA] until the assay was performed. The in vitro labeling of nascent RNA was performed in 200 μl of 2× reaction buffer {10 mM Tris·HCl, pH 8.0, 5 mM MgCl2, 0.3 M KCl, 10 mM dithiothreitol, 1 mM each ATP, GTP, and CTP, and 10 μl of [α-32P]UTP (10 mCi/ml)}. The mixture was incubated for 30 min at 30°C. RNA was isolated using the TRIzol reagent according to the manufacturer’s instructions. RNA was suspended in 50 μl of 10 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid, pH 7.4, 10 mM EDTA, and 0.2% (wt/vol) SDS, heated for 5 min at 90°C, and added directly to the hybridization solution. To prepare membranes for hybridization with radiolabeled RNA, 20 μg of plasmid DNA, containing the human taurine transporter cDNA in pGEM-T vector, were linearized with the restriction enzyme SalI. DNA was denatured by addition of 0.4 M NaOH for 30 min at room temperature and then neutralized by addition of 6× saline-sodium citrate (SSC) and placement on ice. The slot-blot apparatus was prepared, and 5 μg of plasmid DNA were applied to each slot under low vacuum. Each slot was rinsed with 500 μl of 6× SSC. Membrane strips were prehybridized for 2 h at 68°C in Ultrahyb hybridization buffer. Membranes were hybridized overnight at 68°C using 1 ml of the same buffer containing radiolabeled RNA. After hybridization, membranes were washed twice for 5 min each in 2× SSC and 0.1% SDS at 68°C and twice for 15 min each in 0.1% SSC and 0.1% SDS at 68°C and exposed to X-ray film.
Laser scanning confocal microscopic analysis of taurine transporter in cultured ARPE-19 cells and in intact RPE
Immunohistochemical methods were used to localize taurine transporter in cultured human ARPE-19 cells and in RPE of intact mouse eyes. Eyes from 3-wk-old albino mice were enucleated and frozen immediately in Tissue-Tek OCT, and 10-μm-thick cryosections were prepared. The cells were fixed with ice-cold methanol, and the cryosections were fixed with ice-cold acetone. Cells and cryosections were blocked with 10% normal goat serum. Samples were incubated with an affinity-purified polyclonal antibody against rat taurine transporter. The rabbit anti-taurine transporter antibody (Alpha Diagnostics) was prepared using a 20-mer peptide (designated Tau-11) near the carboxy-terminal cytoplasmic region of rat TAUT-1 (43). The immunogenic peptide used in production of the antibody shows 100% homology to rat and mouse and 90% to human and canine taurine transporter. The antibody has been shown to cross-react with mouse, human, rat, and cat tissues. Cells were incubated with the primary antibody for 3 h at room temperature at a dilution of 1:100; tissue cryosections were incubated for 3 h at room temperature at a dilution of 1:50 and incubated overnight at 4°C. To demonstrate the specificity of the antibody, the primary antibody was neutralized with an excess of the antigenic peptide before use for incubation with tissue sections. Additional negative controls included using buffer only and 0.1% normal rabbit serum in place of the primary antibody. After they were rinsed, all samples were incubated overnight at 4°C with an FITC-conjugated AffiniPure goat anti-rabbit IgG at a dilution of 1:100. Cells and cryosections were optically sectioned (z-series) using a Nikon Diaphot 200 Laser Scanning Confocal Imaging System (Molecular Dynamics). Images were analyzed using the Image Display 3.2 software package (Silicon Graphics, Mountain View, CA).
RESULTS
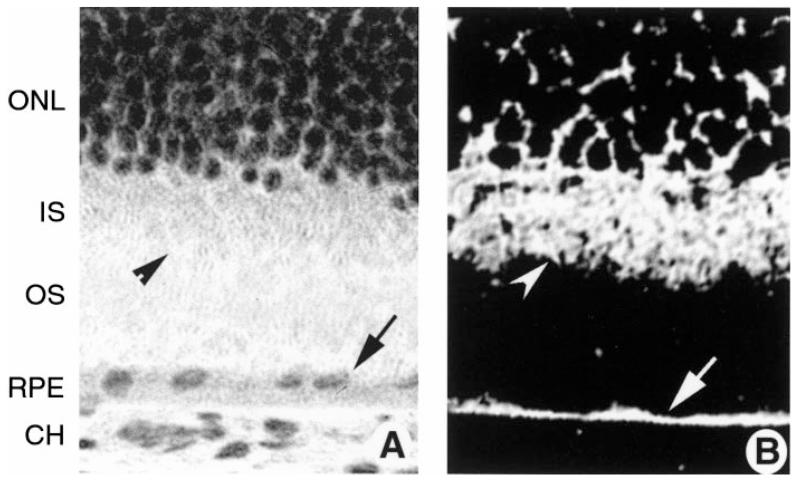
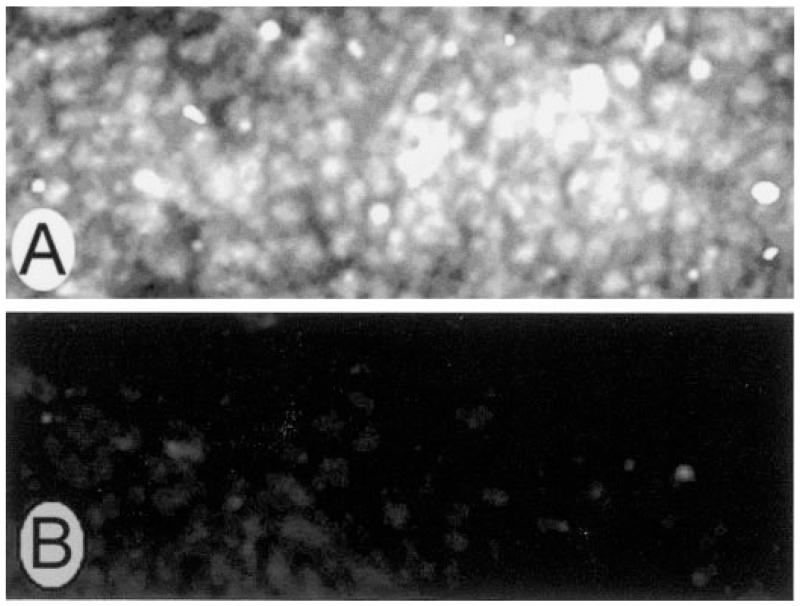
The present study focused on the role of NO in the regulation of the human taurine transporter in cultured human ARPE-19 cells. Before testing the activity of the transporter in ARPE-19 cells, we sought to establish its presence using immunohistochemical methods. ARPE-19 cells were cultured to confluency on plastic chamber supports. The cells were incubated with a commercially available, affinity-purified antibody against the taurine transporter. As shown in Fig. 1, laser scanning confocal optical sections of cells demonstrated intense labeling with the antibody. In Fig. 1A, cells were sectioned optically in the vertical plane, allowing the distribution of the protein to be viewed from the sides of the cells. There appeared to be protein distributed throughout the cell cytoplasm. In Fig. 1B, cells were scanned in a horizontal plane, allowing the cells to be viewed from above. There was an abundance of bright fluorescence across the apical surface of the cells. These data supported the use of ARPE-19 cells for study of taurine uptake activity. Incubation of cells with the antibody that had been incubated with an excess of immunogenic control peptide resulted in no positive staining (Fig. 1C). Although it is necessary to use cultured cells to study the activity of the taurine transporter, we considered it essential also to confirm the presence of the taurine transporter in RPE cells in an intact mammalian retina, inasmuch as there have been no immunohistochemical studies documenting the presence of the transporter in RPE. To this end, we used cryosections of mouse retina (Fig. 2). A hematoxylin-and-eosin-stained cryosection of the outer retina (Fig. 2A) is provided for comparison to the photomicro-graph of the immunolabeled section (Fig. 2B). The outer portion of the retina, including the outer nuclear layer of photoreceptor cell nuclei, and the inner segments of these cells are labeled. The RPE is the single layer of epithelial cells between choroid and outer segments. Figure 2B shows a cryosection of mouse retina incubated with the antibody against the taurine transporter. The taurine transporter was detected abundantly in RPE and was present throughout much of the RPE cell cytoplasm. The taurine transporter was expressed also in the photoreceptor cell inner segments, in which the endoplasmic reticulum, Golgi apparatus, and other organelles are located (Fig. 2B). Labeling was present in other regions of the retina, including the retinal ganglion cell layer and outer plexiform layer (data not shown).
Fig. 1.
Laser scanning confocal microscopic immunolocalization of taurine transporter in cultured human ARPE-19 cells and mouse retina. ARPE-19 cells were grown to confluency on laminin-coated chamber slides and processed for immunohistochemistry using a primary antibody against taurine transporter followed by an FITC-labeled secondary antibody. A: optical section of cells taken in a vertical plane (x,z). Bright band of fluorescence is indicative of positive detection of the antibody in cultured cells. Top and bottom arrows at left of A point to apical and basal surfaces of cells. B: optical section of cells taken in a horizontal plane (x,y) shows taurine transporter on apical retinal pigment epithelial (RPE) surface. C: horizontal section of cells incubated with peptide that had been preincubated with antibody against taurine transporter (negative control) shows no positive staining. Original magnifications ×600.
Fig. 2.
Laser scanning confocal microscopic immunolocalization of taurine transporter in intact mouse retinal tissue. Eyes of ICR albino mice were frozen in OCT embedding medium, and cryosections were prepared and subjected to immunohistochemical detection of taurine transporter using a commercially available antibody. A: hematoxylin-and-eosin-stained cryosection of outer portion of normal mouse retina showing outer layers of the retina. B: immunolocalization of taurine transporter. Arrow points to intense labeling of the RPE; arrow-head points to labeling of inner segments (IS). Magnification ×400. ONL, outer nuclear layer; OS, outer segments; CH, choroid.
Stimulation of human taurine transporter by NO donors
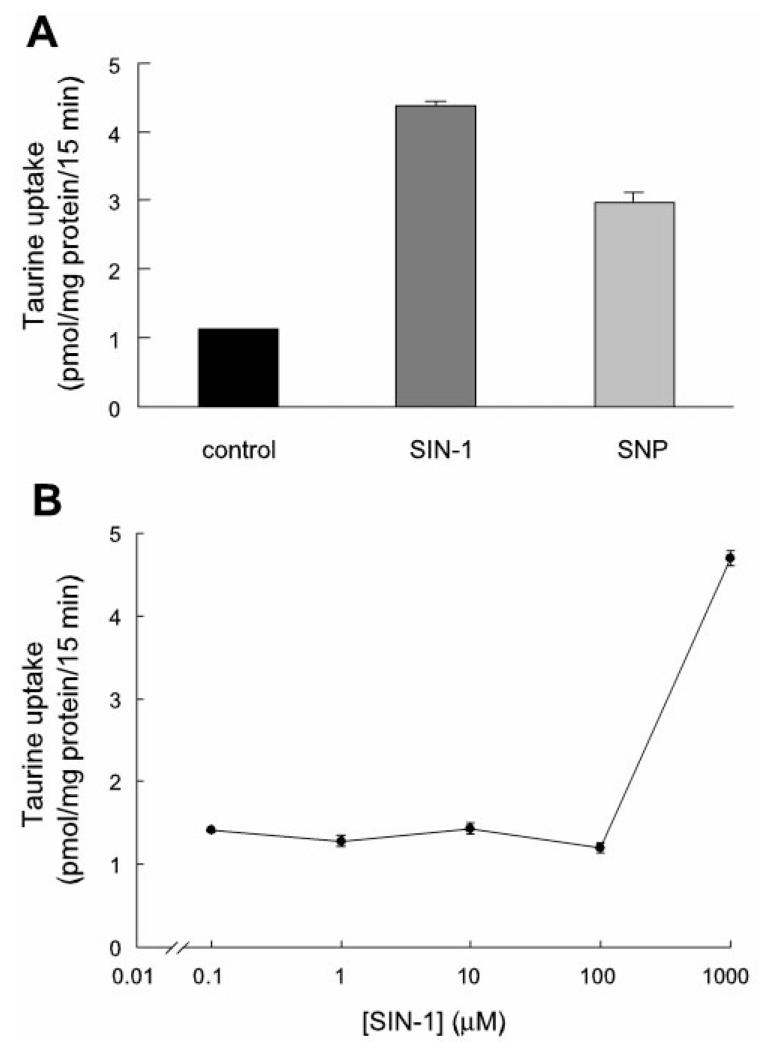
Figure 3A shows the effect of the NO donors SIN-1 and SNP on the uptake of 80 nM [3H]taurine by the taurine transporter in human ARPE-19 cells. Both NO donors, at 1 mM and when incubated with cells for 24 h, stimulated the uptake process. SIN-1 was a more potent stimulator of taurine uptake than SNP. Exposure of ARPE-19 cells to SIN-1 led to a 3.5-fold stimulation of taurine uptake. Exposure of cells to 1 mM SNP under similar conditions resulted in an ~2.5-fold stimulation of taurine uptake. Figure 3B shows the dose-response relationship for SIN-1. SIN-1 was ineffective up to 100 μM. The stimulation was seen only at 1 mM. The effect was observed with freshly prepared SIN-1. Incubation of cells with “spent” SIN-1 (i.e., SIN-1 that had been prepared 48 h before incubation) showed a markedly reduced stimulatory effect (data not shown).
Fig. 3.
Effects of NO donors on uptake of taurine in cultured ARPE-19 cells. A: uptake of [3H]taurine (80 nM) in the absence (control) or presence of 1 mM 3-morpholinosydnonimine (SIN-1) or sodium nitroprusside (SNP) for 24 h. B: dose-response relationship for effect of SIN-1 on uptake of 80 nM [3H]taurine (treatment time 24 h). Values are means ± SE for 4 determinations from 2 independent experiments.
To determine whether incubation of cells with NO donors affected Na+ or Cl− dependency of the taurine transporter, cells were incubated for 24 h with or without SIN-1, and the uptake of [3H]taurine was measured in the presence of NaCl in the absence of Na+ but in the presence of Cl− (N-methyl-d-glucose chloride) or in the presence of Na+ but the absence of Cl− (sodium gluconate). The inhibition of taurine uptake by β-alanine, a known competitive inhibitor of taurine uptake (54), was also assessed in cells exposed to SIN-1. As shown in Table 1, taurine uptake was observed only in the presence of NaCl. In the absence of Na+ or Cl−, the uptake was reduced to 5% of the control. SIN-1 stimulated the uptake only in the presence of NaCl but did not stimulate taurine uptake in the absence of Na+ or Cl−. β-Alanine inhibited taurine uptake in the presence and absence of SIN-1. These data suggest that NO did not affect the Na+/Cl− dependency of the taurine transporter, nor did it alter the inhibitory effect of β-alanine on taurine uptake. We also analyzed the Na+- and Cl−-activation kinetics of taurine uptake in control cells and in cells treated with SIN-1 (data not shown). The activation of taurine uptake with increasing concentrations of Na+ (the concentration of Cl− was kept constant at 140 mM) exhibited a sigmoidal relationship in control and SIN-1-treated cells. In both cases, the Hill coefficient was 2 (1.7 ± 0.6 and 2.2 ± 0.1 in control and SIN-1-treated cells, respectively). The activation of taurine uptake with increasing concentrations of Cl− (the concentration of Na+ was kept constant at 140 mM) exhibited a hyperbolic relationship in control and SIN-1-treated cells. In both cases, the Hill coefficient was 1 (0.96 ± 0.10 and 0.93 ± 0.07 in control and SIN-1-treated cells, respectively). Thus the Na+-Cl−-taurine stoichiometry remained 2:1:1 in control cells and in cells treated with SIN-1.
Table 1. Influence of SIN-1 on Na+ and Cl− dependency of taurine uptake.
| Uptake, fmol·mg protein−1 ·15 min−1 |
||
|---|---|---|
| Buffer | −SIN-1 (control) |
+SIN-1 |
| NaCl (140 mM) | 2,036.6 ± 54.9 | 6,898.4 ± 94.2 |
| NaCl (140 mM) + β-alanine (2.5 mM) | 94.6 ± 1.3 | 166.8 ± 5.9 |
| NMDG-Cl (140 mM) | 27.9 ± 1.6 | 23.4 ± 1.7 |
| Sodium gluconate (140 mM) | 49.3 ± 7.2 | 31.2 ± 3.4 |
Values are means ± SE. ARPE-19 cells were treated with or without 1 mM 3-morpholinosydnonimine (SIN-1) for 24 h, and uptake of [3H]taurine (80 nM) was measured. Uptake buffer consisted of 20 mM HEPES-Tris (pH 7.5) containing 140 mM NaCl, sodium gluconate, or N-methyl-d-glucamine (NMDG) chloride. Inhibition of taurine transport in the presence of β-alanine (in NaCl buffer) was also measured.
To determine whether stimulation of taurine uptake by SIN-1 was influenced by exposure of cells to high glucose, ARPE-19 cells were incubated for 24 h in medium containing high (45 mM) or normal (17 mM) glucose present in the DMEM-F-12 culture medium (9, 10). For each glucose concentration, cells were incubated with or without 1 mM SIN-1. Uptake of [3H]taurine (80 nM) in cells grown in high or low glucose without SIN-1 was 1.89 ± 0.08 and 1.80 ± 0.06 pmol·mg protein−1·15 min−1, respectively. Uptake of [3H]taurine in cells grown in high or low glucose in the presence of SIN-1 was 3.59 ± 0.11 and 3.53 ± 0.10 pmol·mg protein−1·15 min−1, respectively. These data suggest that exposure of cells for 24 h to high levels of glucose does not alter the SIN-1-induced stimulation of taurine uptake by ARPE-19 cells. To determine whether induction of iNOS activity with cytokines can replicate the results achieved by the addition of the NO donor molecules, RPE cells were incubated for 48 h with the cytokines LPS (20 ng/ml) and IFN-γ (1 μg/ml). It has been shown in cultured RPE cells that incubation with these cytokines induces the synthesis of iNOS and production of NO (45). Uptake of [3H]taurine (80 nM) in cells grown in the presence of both of these cytokines was 2.50 ± 0.11 pmol·mg protein−1·15 min−1, which was ~50% greater than uptake in control cells (1.73 ± 0.06 pmol·mg protein−1·15 min−1). These data suggest that exposure of RPE cells to cytokines known to induce NOS can stimulate taurine uptake in a manner similar to exposure directly to NO donors.
Time course of stimulation of taurine uptake by SIN-1
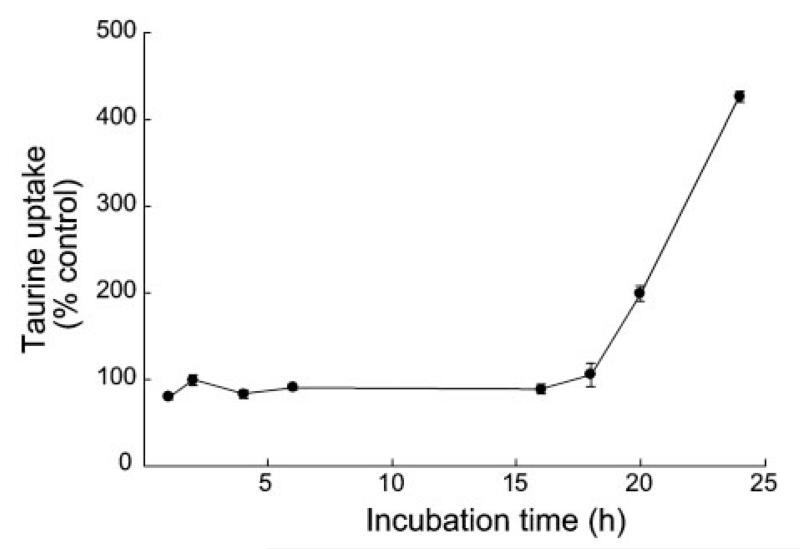
After determining that SIN-1 stimulated the uptake of taurine in ARPE-19 cells after 24 h of incubation, we performed a time-course study. Figure 4 shows the data for exposure of cells for various lengths of time to 1 mM SIN-1. During the initial 16 h of exposure to SIN-1, the uptake of taurine was similar to that of cells not exposed to the NO donor; however, by 18 h the uptake of taurine in SIN-1-treated cells increased and was dramatically elevated above control values by 20 and 24 h.
Fig. 4.
Time course of stimulation of taurine uptake by SIN-1. ARPE-19 cells were exposed to 1 mM SIN-1 for various lengths of time, and uptake of [3H]taurine (80 nM) was determined. Parallel experiments were carried out with cells cultured similarly, but in the absence of SIN-1. Results are given as percentage of taurine uptake measured in corresponding control cells not treated with SIN-1. Values are means ± SE for 4 determinations from 2 independent experiments.
Inhibition of NO-induced stimulation of taurine transporter by antioxidants and NO scavengers
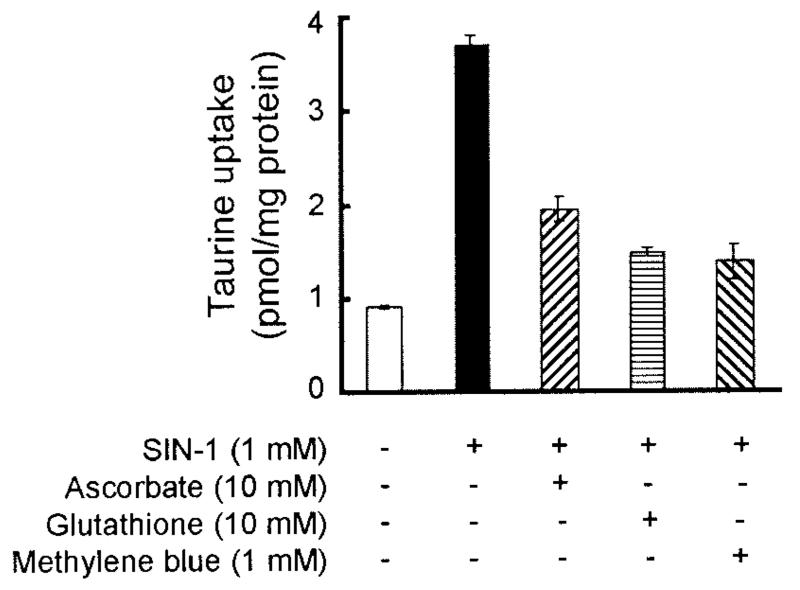
To determine whether the stimulation of taurine uptake by SIN-1 could be inhibited by antioxidants and NO scavengers, ARPE-19 cells were incubated for 24 h with or without 1 mM SIN-1 in the presence or absence of antioxidants, ascorbate (10 mM) or glutathione (10 mM), or with the NO scavenger methylene blue (1 mM). The uptake of [3H]taurine (80 nM) was then measured for 15 min in these cells. As shown in Fig. 5, uptake of taurine was threefold higher in cells treated with SIN-1 alone than in cells treated similarly but in the absence of SIN-1. When cells were incubated simultaneously with SIN-1 and ascorbate or SIN-1 and glutathione, the SIN-1 stimulation of taurine uptake was markedly attenuated. Similarly, methylene blue also attenuated the stimulatory effect of SIN-1 on taurine uptake. These antioxidants and NO scavengers by themselves had no noticeable effect on taurine transporter activity (data not shown). Incubation of ARPE-19 cells with SIN-1 for 2 h followed by immunohistochemical detection of nitrotyrosine revealed the presence of higher levels of nitrotyrosine in cells treated with SIN-1 (Fig. 6A) than in cells not exposed to SIN-1 (Fig. 6B). A positive nitrotyrosine reaction is considered an indicator of NO production. These data further support the belief that the SIN-1-induced stimulation of taurine transporter in ARPE-19 cells is mediated through NO. In experiments in which ARPE-19 cells were exposed to 8-BrcGMP (100 μM), the uptake of taurine (80 nM) was stimulated 23% (2.67 ± 0.10 pmol/mg protein) compared with control cells (2.17 ± 0.14 pmol/mg protein), suggesting that the influence of NO on taurine transporter is mediated, at least in part, by cGMP.
Fig. 5.
Effects of antioxidants and nitric oxide (NO) scavengers on SIN-1-induced stimulation of taurine uptake in ARPE-19 cells. Confluent cells were treated with or without 1 mM SIN-1 for 24 h. Cells were incubated at the same time in the presence or absence of ascorbate, glutathione, or methylene blue. Uptake of [3H]taurine (80 nM) was measured for 15 min. Values are means ± SE for 3 determinations from 2 independent experiments.
Fig. 6.
Immunodetection of nitrotyrosine in ARPE-19 cells exposed to SIN-1. ARPE-19 cells were grown to confluency on laminin-coated chamber slides and then exposed to 1 mM SIN-1 for 2 h. Cells were processed for immunohistochemistry using a primary antibody against nitrotyrosine followed by an FITC-labeled secondary antibody. A: SIN-1 treated cells. B: control cells. Magnification ×600.
Specificity of NO-induced stimulation of taurine transporter
The NO-induced stimulation of [3H]taurine uptake in ARPE-19 cells was not a nonspecific effect, because the uptake of other nutrients, glutamine, glutamate, alanine, arginine, carnitine, and leucine, was not affected under identical experimental conditions (Table 2). Because the uptake of taurine was actually stimulated in the presence of SIN-1, while uptake of other compounds was not affected, it is not likely that SIN-1 damaged the RPE cells. Total protein levels measured in cells (n = 8) exposed to SIN-1 (0.21 ± 0.02 mg) did not differ significantly from protein levels in cells not exposed to SIN-1 (0.23 ± 0.02, P > 0.5). Moreover, when cells were cultured in the presence or absence of SIN-1 for 24 h and subjected to the trypan blue exclusion assay (0.2% wt/vol) to assess cell viability, there was no significant difference in the number of cells excluding the dye (data not shown).
Table 2. Specificity of SIN-1-induced stimulation of taurine uptake.
| Uptake, pmol·mg protein−1·15 min−1 |
||
|---|---|---|
| Substrate | Control | SIN-1 |
| Taurine (80 nM) | 2.58 ± 0.088(100) | 10.65 ± 0.053(413) |
| Glutamine (50 nM) | 5.64 ± 0.388(100) | 6.07 ± 0.269(108) |
| Glutamate (100 nM) | 13.75 ± 0.152(100) | 13.93 ± 0.583(101) |
| Carnitine (30 nM) | 0.41 ± 0.024(100) | 0.37 ± 0.032(90) |
| Leucine (30 nM) | 1.75 ± 0.085(100) | 2.08 ± 0.006(118) |
| Alanine (30 nM) | 2.22 ± 0.059(100) | 2.44 ± 0.086(110) |
| Arginine (30 nM) | 2.30 ± 0.101(100) | 2.08 ± 0.090(90.4) |
Values are means ± SE; values in parentheses are percentages of respective control uptake. ARPE-19 cells were treated with or without 1 mM SIN-1 for 24 h, and then uptake of [3H]taurine and other radiolabeled amino acids was measured for 15 min.
Kinetic analysis of NO-induced stimulation of taurine transporter activity
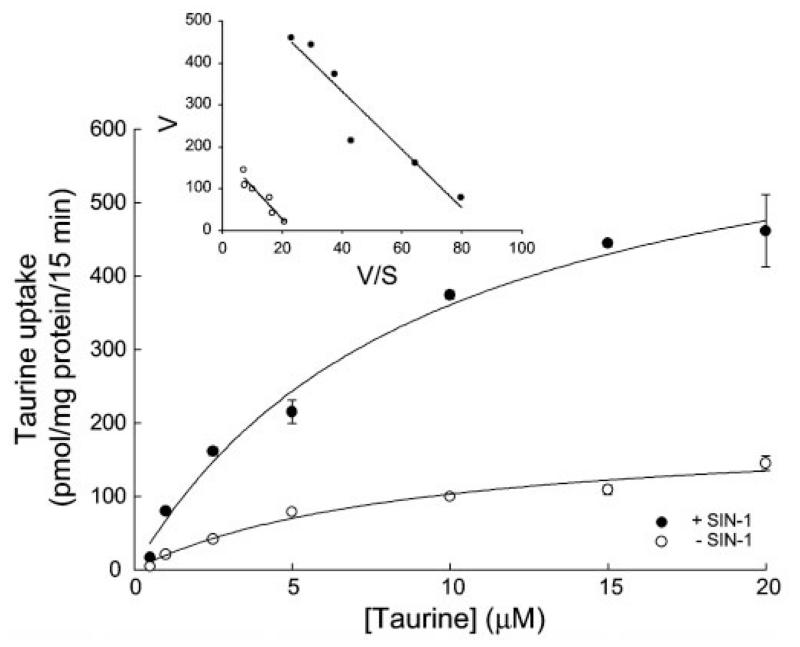
We then analyzed the kinetics of taurine transporter in control cells and in cells treated for 24 h with 1 mM SIN-1 (Fig. 7). The analysis showed that the increase in the transport activity of the taurine transporter observed in SIN-1-treated ARPE-19 cells compared with control cells was primarily associated with an increase in the maximal velocity of the transporter with no significant change in the substrate affinity. The maximal velocity of taurine uptake was 3.5-fold greater in SIN-1-treated cells than in control cells (697.8 ± 61.9 vs. 194.3 ± 25.6 pmol·mg protein−1·15 min−1). The Michaelis-Menten constant for taurine remained almost the same in SIN-1-treated and control cells (9.4 ± 1.9 vs. 8.9 ± 2.7 μM).
Fig. 7.
Kinetic analysis of taurine uptake in control and SIN-1-treated ARPE-19 cells. Confluent cells were treated with or without 1 mM SIN-1 for 24 h. Uptake of taurine was measured for 15 min over a taurine concentration range of 0.5–20 μM. Values are means ± SE for 3 determinations from 3 independent experiments. Results are presented as plots describing the relationship between taurine concentration and taurine uptake rate and also as Eadie-Hofstee plots: V/S vs. V, where V is taurine uptake (in pmol·mg protein−1·15 min−1) and S is taurine concentration (in μM).
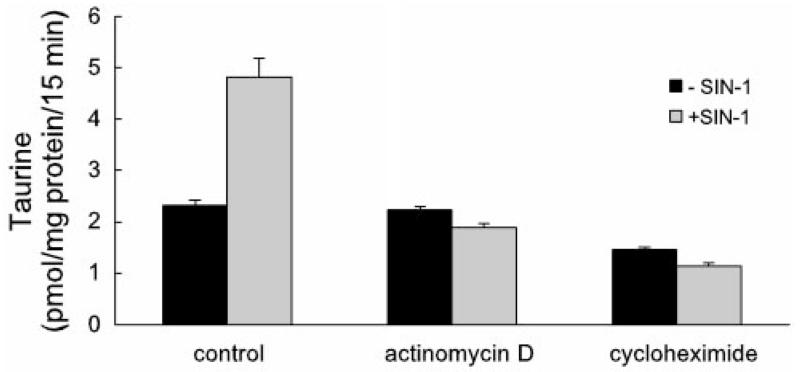
We next examined the influence of cycloheximide (an inhibitor of translation) and actinomycin D (an inhibitor of transcription) on the SIN-1-induced increase in taurine transporter activity. Both compounds significantly decreased the stimulatory effect of SIN-1. As shown in Fig. 8, incubation of cells with SIN-1 in the absence of actinomycin D or cycloheximide (control) resulted in a stimulation of taurine transport as described above. When cells were pretreated with either of the inhibitors for 2 h and then incubated with these compounds in the presence of SIN-1, the stimulatory effect of SIN-1 was eliminated. These data suggest that the stimulatory effect of SIN-1 on taurine transporter activity involves de novo synthesis of RNA and protein.
Fig. 8.
Effects of cycloheximide and actinomycin D on SIN-1-induced increase in taurine transporter activity. Confluent ARPE-19 cells were pretreated with cycloheximide (100 μg/ml) or actinomycin D (10 μg/ml) for 2 h and then incubated with these compounds in the presence or absence of 1 mM SIN-1. Uptake of [3H]taurine (80 nM) was then measured for 15 min. Values are means ± SE for 3 determinations from 3 independent experiments.
Semiquantitative RT-PCR analysis of NO-induced stimulation of the taurine transporter
We then investigated the influence of SIN-1 treatment on the steady-state levels of mRNA transcripts specific for the taurine transporter. mRNA samples isolated from control and SIN-1-treated ARPE-19 cells were used for semiquantitative RT-PCR for the determination of the levels of mRNA transcripts. As an internal control, we determined the steady-state levels of the GAPDH mRNA in the samples in parallel. RT-PCR was done with a wide range of PCR cycles (i.e., 9–30). The resultant products were run on a gel and then subjected to Southern hybridization with 32P-labeled cDNA probes specific for the taurine transporter and GAPDH. The hybridization signals were quantified using the STORM PhosphorImaging System, and the intensities that were in the linear range with the PCR cycle number were used for analysis. The results of these experiments (Fig. 9) showed that in the cells treated with SIN-1, the steady-state levels of taurine transporter mRNA increased markedly compared with control cells. These results demonstrate that the SIN-1-induced increase in taurine transporter activity is likely due to increased expression of the gene coding for the transporter.
Fig. 9.
Analysis of steady-state levels of mRNA for taurine transporter (TAUT) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in control (−SIN-1) and SIN-1 treated (+SIN-1) ARPE-19 cells. Confluent cells were treated with or without 1 mM SIN-1 for 24 h. Poly(A)+ RNA was then isolated from these cells and used for semiquantitative RT-PCR. Primer pairs specific for human TAUT mRNA and human GAPDH mRNA were used. RT-PCR was done with a wide range of PCR cycles (9–30). Resultant products were run on a gel and then subjected to Southern hybridization with 32P-labeled cDNA probes specific for TAUT and GAPDH. Hybridization signals were quantified using the STORM PhosphorImaging System, and intensities that were in the linear range with the PCR cycle number were used for analysis. GAPDH-specific band intensity was used as an internal control. Ratios of TAUT-specific band intensity to GAPDH-specific band intensity were then compared between control and SIN-1 treated cells. Ratio in control cells was taken as 1.
Nuclear run-on assay
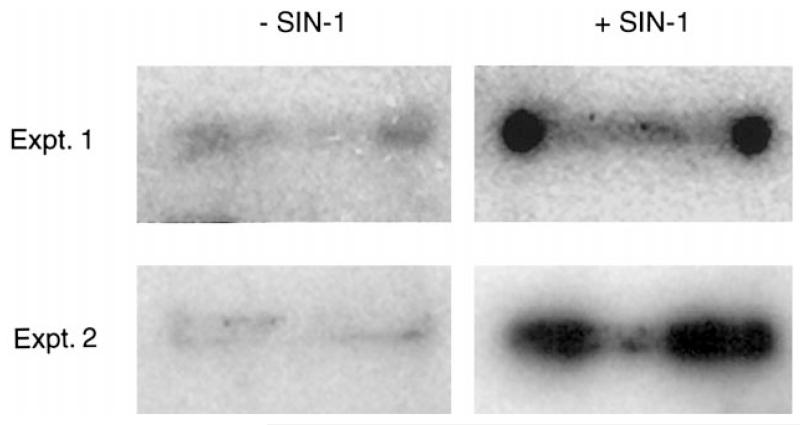
To confirm the results of the semiquantitative RT-PCR data, we used the nuclear run-on transcription assay, which is a sensitive method for direct measurement and comparison of specific gene transcription in cells (17). We isolated nuclei from ARPE-19 cells that had been incubated for 24 h in the presence or absence of SIN-1 and prepared 32P-labeled nascent RNA as described by Greenberg and Bender (17). The RNA was subsequently used in slot-blot analysis on membranes to which the human taurine transporter cDNA had been applied to quantitate specifically the taurine transporter mRNA in the nascent RNA samples. As shown in Fig. 10, the intensity of the signal for the taurine transporter mRNA from ARPE-19 cells incubated with SIN-1 was much greater than that in cells not incubated in SIN-1. Densitometric scans of the bands revealed a 12-fold greater intensity in the SIN-1-treated than in the control cells. This experiment was carried out twice, and the results were the same in both cases. The data provide strong support that transcription of the taurine transporter gene is stimulated in the presence of the NO donor SIN-1.
Fig. 10.
Nuclear run-on transcription assay. ARPE-19 cells were incubated for 24 h with or without 1 mM SIN-1. Nuclei were isolated, and nascent RNA was labeled with [α-32P]UTP. This radiolabeled RNA was then hybridized to membranes with cross-linked human taurine transporter cDNA. Membranes were hybridized overnight at 68°C and exposed to X-ray film.
DISCUSSION
The present study was designed to assess the effects of NO on the transport of taurine by cultured human RPE cells. NO is a mediator of many physiological processes, such as vasodilation and neurotransmission; it is recognized also for its toxic effects (14). NO has been implicated in the pathogenesis of diabetic retinopathy (2, 14, 29, 42, 48, 57, 58). We have shown recently that, in cultured RPE cells, NO stimulates the transport of cystine, the disulfide form of cysteine, which is required for synthesis of the antioxidant glutathione (4). In the present study, we asked whether the transport of taurine would be affected similarly. Taurine is thought to have antioxidant properties (28, 37). Moreover, there is convincing evidence that it functions in osmoregulation (5, 39, 50).
To determine whether NO altered the activity of the taurine transporter, we used the well-differentiated ARPE-19 cell line. These cells are a rapidly growing RPE cell line established in the laboratory of Dr. Larry Hjelmeland (University of California, Davis). They form a uniform population of polarized epithelial monolayers on porous filter supports. They retain features characteristic of RPE cells, including defined cell borders, a cobblestone appearance, noticeable pigmentation (9, 10), and the capacity to phagocytose outer segment disks (11). The usefulness of these cells in studying the transport of folate was established recently (6). In the present study, we used immunohistochemical methods to determine whether the taurine transporter is present in ARPE-19 cells. With the use of an affinity-purified antibody against the transporter, our confocal microscopic analysis clearly showed that the transporter is present in ARPE-19 cells. The horizontal scans suggested that the protein was available on the apical membrane and was present also throughout the cells. A number of functional studies from our laboratory and others had suggested the presence of the transporter on the apical surface of cultured RPE cells (30, 32, 33); however, this report represents the first immunohistochemical evidence to that effect. To confirm the localization of the taurine transporter to RPE in vivo, we used cryosections of normal mouse eye. The antibody detected a strong positive signal in several layers of the retina, including the retinal ganglion cells, outer plexiform layer, photoreceptor cell inner segments, and the RPE. Although recent in situ hybridization studies have localized the mRNA encoding mouse taurine transporter to these same retinal layers (54), our data represent the first immunohistochemical evidence of the location of the transporter in any mammalian retina. The detection of the taurine transporter in intact RPE is important confirmation of the presence of the protein in RPE in vivo and validates the use of RPE cells to study its function in vitro.
Having established the presence of the transporter in ARPE-19 cells, we sought to address the effects of NO on taurine transport. We used the NO donors SIN-1 and SNP. Incubation of ARPE-19 cells with SIN-1 or SNP caused a marked stimulation of uptake of taurine by the transporter. Inasmuch as SIN-1 was more potent than SNP in triggering this effect, the remaining experiments were conducted with SIN-1. The effects on taurine transporter activity by SIN-1 were not immediate. Rather, the increased activity was observed after 18 h of incubation with the NO donor. Incubation of cells for 24 h with SIN-1 led to a 3.5-fold stimulation of taurine transporter activity. Incubation with SIN-1 did not affect the Na+ or Cl− dependency of the taurine transporter, the Na+- and Cl−-activation kinetics, or the capacity of β-alanine to compete with taurine for the uptake process. Taurine uptake in ARPE-19 cells was not only stimulated by NO donors directly, but also by exposure of the cells to agents such as LPS that are known to induce NOS (45). Additional experiments suggested that the SIN-1 effects on taurine transporter activity were mediated via NO. Treatment of ARPE-19 cells with NO scavengers inhibited SIN-1 stimulation of taurine transporter activity. Moreover, immunohistochemical analysis assessing nitrotyrosine production in SIN-1-treated cells showed positive reactivity, indicating that NO was produced.
Kinetic analysis showed that the increased activity of the taurine transporter in the presence of SIN-1 was not due to an increase in substrate affinity but, rather, to an increase in the maximal velocity of taurine uptake. Such data suggest that synthesis of the taurine transporter may be increased in the presence of NO donors. To test this further, we cultured ARPE-19 cells in the presence of SIN-1 plus agents that inhibit transcription and translation, actinomycin D and cycloheximide, respectively. In both cases, the SIN-1-induced increase in taurine transporter activity was completely inhibited. Semiquantitative RT-PCR provided further evidence that NO increases the steady-state levels of taurine transporter mRNA ~3.5-fold. Although NO stimulated the uptake of taurine by ARPE-19 cells, it had little effect on the transport of other amino acids such as alanine or glutamate. The observed increase in steady-state levels of taurine transporter mRNA levels may be due to an increase in the transcription rate of the taurine transporter gene or an increase in the stability of the taurine transporter mRNA. The finding that the increased mRNA levels are completely abolished by the transcription inhibitor actinomycin D argues in favor of the gene expression as the site of regulation of the NO-mediated increase in mRNA levels. Moreover, the data obtained from the nuclear run-on transcription assay provide strong evidence that the increase in taurine transporter mRNA levels is due to increased transcriptional activity.
These data provide the first evidence that taurine transporter activity is regulated by NO. They are important, because NO levels are increased in diabetic retinopathy, the leading cause of blindness among working-aged Americans (56). Other investigators have examined taurine transporter activity under hyperglycemic conditions. Stevens et al. (47) showed that taurine transporter activity decreased within 4 h of exposure of human RPE cells to 30 mM glucose. On the other hand, Vilchis and Salceda (53) studied uptake of taurine in RPE isolated from rats that had been diabetic for 3 and 6 wk. They found a stimulation of taurine uptake in diabetic RPE compared with normal RPE. The differences in the experimental outcomes from these two laboratories may reflect differences in the length of time RPE cells were exposed to hyperglycemic conditions. In the case of cultured cells (47), the earliest effect of high glucose appears to be a down-regulation of the transporter. Thus the acute effect of hyperglycemia on the taurine transporter in RPE cells seems to be a downregulation of the transporter expression. This effect was accompanied by a decrease in intracellular levels of taurine. The long-term effects of hyperglycemia on the taurine transporter in the RPE cells used in that study are not known. Interestingly, in the present study, exposure of ARPE-19 cells to high glucose for 24 h did not affect taurine transporter function. In the case of the studies of diabetic rats (53), taurine transporter activity increased. In this in vivo model, the retinas would have been subjected constantly to a hyperglycemic state for several weeks. It is noteworthy that the increased taurine transporter activity was greater after 6 wk of diabetes than after 3 wk. Because animal studies have shown that chronic hyperglycemia increases taurine transport activity in RPE cells (53), we speculate that long-term diabetes may be associated with elevated NO production, which in turn stimulates the taurine transporter expression. Our experimental findings showing NO-induced stimulation of taurine uptake in cultured RPE cells support this speculation. That is, they may suggest that, in diabetic retinopathy, NO is one of the molecules responsible for the observed upregulation of taurine transporter expression and activity. Our experiments address an important aspect of the altered diabetic state on RPE cells, that of increased levels of NO. Thus our data showing an upregulation of taurine transport in cultured cells may reflect conditions of longer-standing diabetes, rather than immediate effects of the initial exposure of the cells to hyperglycemia.
Because NO is known to function as a vasodilator (14) and because diabetic retinopathy has an ischemic component in which NO levels may be decreased, it may seem paradoxical to implicate NO in the pathogenesis of diabetic retinopathy. This paradox is resolved when it is recognized that the increased level of NO observed in the vitreous of diabetic patients is not an early event (58). During the early stages of diabetic retinopathy, typically called the nonproliferative stage, platelets clump together to form small stable aggregates that can lead to capillary closure (21). During this period of ischemia, there may be a reduction of vascular NO. The nonproliferative stage is followed, however, by a proliferative stage in which new blood vessels are formed. It is this proliferative stage that is associated with increased levels of NO (57). The proliferative stage is associated also with an increase in vascular endothelial growth factor, which has been shown to upregulate NO (22, 52). In addition, proliferative diabetic retinopathy is associated with elevated intravitreal levels of cytokine tumor necrosis factor, interferon, and interleukin-1 (1, 12). These compounds have been shown also to induce production of NO by the expression of iNOS, which is found in Müller and RPE cells (16, 45, 57).
The present study did not address the specific mechanism by which NO regulates the expression of the taurine transporter gene. It is known that NO activates guanylate cyclase. Our data showing that 8-BrcGMP, a cell-permeable cGMP analog, stimulated taurine uptake suggest that the effects of NO donors may be at least partially mediated by cGMP. It is well known also that, in addition to this signaling role, NO interacts with cellular redox systems, especially thiol groups. NO exposure or synthesis may impose a nitrosative stress similar to that imposed by oxygen-derived species. The attenuation of the stimulatory effect of NO on taurine transporter expression by the antioxidants ascorbic acid and glutathione suggests that nitrosative stress may contribute also to the NO-induced action observed in the present study. In summary, these data show that NO regulates the expression and activity of the taurine transporter in RPE cells in vivo. It is becoming apparent that NO regulates other transporters in RPE as well. For example, NO stimulates the transport by RPE of cystine, the disulfide form of cysteine, which is required for synthesis of the antioxidant glutathione (4). On the other hand, NO inhibits the activity of the reduced-folate transporter in cultured RPE cells (44). Studies of the regulation of transporter activity by NO may provide important insights for altered cellular function that occurs in conditions such as diabetes, when NO is elevated.
Acknowledgments
The authors thank Susan Johnson for assistance in preparation of the manuscript and Penny Roon for preparation of the mouse tissue sections.
This research was supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology, Medical College of Georgia, by an award from the Medical College of Georgia Research Institute, and National Eye Institute Grants EY-13089 and EY-12830.
REFERENCES
- 1.Abu el Asrar AM, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992;114:731–736. doi: 10.1016/s0002-9394(14)74052-8. [DOI] [PubMed] [Google Scholar]
- 2.Bank N, Aynedjian HS. Role of EDRF (nitric oxide) in diabetic renal hyperfiltration. Kidney Int. 1993;43:1306–1312. doi: 10.1038/ki.1993.183. [DOI] [PubMed] [Google Scholar]
- 3.Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Regulation of taurine transport in human colon carcinoma cell lines (HT-29 and Caco-2) by protein kinase C. Am J Physiol Gastrointest Liver Physiol. 1993;264:G939–G946. doi: 10.1152/ajpgi.1993.264.5.G939. [DOI] [PubMed] [Google Scholar]
- 4.Bridges CC, Kekuda R, Wang H, Prasad P, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:47–54. [PubMed] [Google Scholar]
- 5.Burg MB, Kador PF. Sorbitol, osmoregulation, and the complications of diabetes. J Clin Invest. 1988;81:635–640. doi: 10.1172/JCI113366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel J-M, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor α in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AN, Kaczmarek LN. Taurine a possible neurotransmitter? Nature. 1971;234:107–108. doi: 10.1038/234107a0. [DOI] [PubMed] [Google Scholar]
- 8.Do Carmo A, Lopes C, Santos M, Proenca R, Cunha-Vaz J, Carvalho AP. Nitric oxide synthase activity and l-arginine metabolism in the retinas from streptozotocin-induced diabetic rats. Gen Pharmacol. 1998;3:319–324. doi: 10.1016/s0306-3623(97)00363-7. [DOI] [PubMed] [Google Scholar]
- 9.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KC, Marmorstein AD, Bonilha VL, Rodriguez-Boulan E, Giordano F, Hjelmeland LM. Use of the ARPE-19 cell line as a model of RPE polarity: basolateral secretion of FGF5. Invest Ophthalmol Vis Sci. 1998;39:2744–2749. [PubMed] [Google Scholar]
- 11.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks WA, Limb GA, Stanford MR, Ogilvie J, Wolstencroft RA, Chignell AH, Dumonde DC. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11(Suppl):187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- 13.Ganapathy V, Ramamoorthy JD, Del Monte MA, Leibach FH, Ramamoorthy S. Cyclic AMP-dependent up-regulation of the taurine transporter in a human retinal pigment epithelial cell line. Curr Eye Res. 1995;14:843–850. doi: 10.3109/02713689508995807. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein IM, Ostwald P, Roth S. Nitric oxide: a review of its role in retinal function and disease. Vision Res. 1996;36:2979–2994. doi: 10.1016/0042-6989(96)00017-x. [DOI] [PubMed] [Google Scholar]
- 15.Goto H, Wu GS, Chen F, Kristeva M, Sevanian A, Rao NA. Lipid peroxidation in experimental uveitis: sequential studies. Curr Eye Res. 1992;11:489–499. doi: 10.3109/02713689209001805. [DOI] [PubMed] [Google Scholar]
- 16.Goureau O, Hicks D, Courtois Y, de Kozak Y. Induction and regulation of nitric oxide synthase in retinal Müller glial cells. J Neurochem. 1994;63:310–317. doi: 10.1046/j.1471-4159.1994.63010310.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg ME, Bender TP. Current Protocols in Molecular Biology. Wiley; New York: 1997. Identification of newly transcribed RNA. sect. 4.10.1-4.10.11. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg ME, Ziff EB. Stimulation of 3T3 cells induces transcription of the c-fos protooncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 19.Han X, Budreau AM, Chesney RW. Adaptive regulation of MDCK cell taurine transporter (pNCT)mRNA: transcription of pNCT gene is regulated by external taurine concentration. Biochim Biophys Acta. 1997;1351:296–304. doi: 10.1016/s0167-4781(96)00217-5. [DOI] [PubMed] [Google Scholar]
- 20.Hayes KC, Carey RE, Schmidt SY. Retinal degeneration associated with taurine deficiency in the cat. Science. 1975;188:949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- 21.Heath H, Brigden WD, Canever JV, Pollock J, Hunter PR, Kelsey J, Bloom A. Platelet adhesiveness and aggregation in relation to diabetic retinopathy. Diabetologia. 1971;7:308–315. doi: 10.1007/BF01219463. [DOI] [PubMed] [Google Scholar]
- 22.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol Heart Circ Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 23.Huxtable RJ. From heart to hypothesis: a mechanism for the calcium modulatory actions of taurine. In: Huxtable RJ, Franconi F, Giotti A, editors. The Biology of Taurine: Methods and Mechanisms. Plenum; New York: 1987. pp. 371–388. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Nakano M, Yamamoto Y, Hiramitsu T, Mizuno Y. Hemoglobin-induced lipid peroxidation in the retina: a possible mechanism for macular degeneration. Arch Biochem Biophys. 1995;316:864–872. doi: 10.1006/abbi.1995.1116. [DOI] [PubMed] [Google Scholar]
- 25.Jhaing SM, Fithian L, Smanik P, McGill J, Tong Q, Mazzaferri EL. Cloning of the human taurine transporter and characterization of taurine uptake in thyroid cells. FEBS Lett. 1993;318:139–144. doi: 10.1016/0014-5793(93)80008-i. [DOI] [PubMed] [Google Scholar]
- 26.Jones DP, Miller LA, Dowling C, Chesney RW. Regulation of taurine transporter activity in LLC-PK1 cells: role of protein synthesis and protein kinase C activation. J Am Soc Nephrol. 1991;2:1021–1029. doi: 10.1681/ASN.V251021. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy AJ, Voaden MJ. Free amino acids in the photoreceptor cells of the frog retina. J Neurochem. 1974;23:1093–1095. doi: 10.1111/j.1471-4159.1974.tb10766.x. [DOI] [PubMed] [Google Scholar]
- 28.Keys SA, Zimmerman WF. Antioxidant activity of retinol, glutathione, and taurine in bovine photoreceptor cell membranes. Exp Eye Res. 1999;68:693–702. doi: 10.1006/exer.1999.0657. [DOI] [PubMed] [Google Scholar]
- 29.Komers R, Allen TJ, Cooper ME. Role of endothelium-derived nitric oxide in the pathogenesis of renal hemodynamic changes of experimental diabetes. Diabetes. 1994;43:1190–1197. doi: 10.2337/diab.43.10.1190. [DOI] [PubMed] [Google Scholar]
- 30.Leibach JW, Cool DR, Del Monte MA, Ganapathy V, Leibach FH, Miyamoto Y. Properties of taurine transport in a human retinal pigment epithelial cell line. Curr Eye Res. 1993;12:29–36. doi: 10.3109/02713689308999493. [DOI] [PubMed] [Google Scholar]
- 31.Li Y-P, Lombardini JB. Inhibition by taurine of the phosphorylation of specific synaptosomal proteins in the rat cortex: effects of taurine on the stimulation of calcium uptake in the mitochondria and inhibition of phosphoinositide turnover. Brain Res. 1991;553:89–96. doi: 10.1016/0006-8993(91)90234-m. [DOI] [PubMed] [Google Scholar]
- 32.Miller SS, Steinberg RH. Potassium modulation of taurine transport across the frog retinal pigment epithelium. J Gen Physiol. 1979;74:237–259. doi: 10.1085/jgp.74.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto Y, Kulanthaivel P, Leibach FH, Ganapathy V. Taurine uptake in apical membrane vesicles from the bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1991;32:2542–2551. [PubMed] [Google Scholar]
- 34.Neuringer M, Sturman J. Visual acuity loss in rhesus monkey infants fed a taurine-free human infant formula. J Neurosci Res. 1987;18:597–601. doi: 10.1002/jnr.490180413. [DOI] [PubMed] [Google Scholar]
- 35.Orr HT, Cohen AI, Lowry OH. The distribution of taurine in the vertebrate retina. J Neurochem. 1976;26:609–611. doi: 10.1111/j.1471-4159.1976.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 36.Pasantes-Morales H. Current concepts on the role of taurine in the retina. Prog Retinal Res. 1986;5:207–229. [Google Scholar]
- 37.Pasantes-Morales H, Cruz C. Protective effect of taurine and zinc on peroxidation-induced damage in photoreceptor outer segments. J Neurosci Res. 1984;11:303–311. doi: 10.1002/jnr.490110310. [DOI] [PubMed] [Google Scholar]
- 38.Pasantes-Morales H, Klethi J, Ledig M, Mandel P. Free amino acids of chicken and rat retina. Brain Res. 1972;41:494–497. doi: 10.1016/0006-8993(72)90523-9. [DOI] [PubMed] [Google Scholar]
- 39.Pasantes-Morales H, Ochoa de la Paz LD, Sepulveda J, Quesada O. Amino acids as osmolytes in the retina. Neurochem Res. 1999;24:1339–1346. doi: 10.1023/a:1022568203717. [DOI] [PubMed] [Google Scholar]
- 40.Ramamoorthy S, Del Monte MA, Leibach FH, Ganapathy V. Molecular identity and calmodulin-mediated regulation of the taurine transporter in a human retinal pigment epithelial cell line. Curr Eye Res. 1994;13:523–529. doi: 10.3109/02713689408999884. [DOI] [PubMed] [Google Scholar]
- 41.Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J. 1994;300:893–900. doi: 10.1042/bj3000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmetterer L, Findl O, Fasching P, Ferber W, Strenn K, Breiteneder H, Adam H, Eichler HG, Wolzt M. Nitric oxide and ocular blood flow in patients with IDDM. Diabetes. 1997;46:653–658. doi: 10.2337/diab.46.4.653. [DOI] [PubMed] [Google Scholar]
- 43.Smith KE, Borden LA, Wang CH, Hartig PR, Branchek TA, Weinshank RL. Cloning and expression of a high-affinity taurine transporter from rat brain. Mol Pharmacol. 1992;42:563–569. [PubMed] [Google Scholar]
- 44.Smith SB, Huang W, Chancy C, Ganapathy V. Regulation of the reduced folate transporter by nitric oxide in cultured human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1999;257:279–283. doi: 10.1006/bbrc.1999.0452. [DOI] [PubMed] [Google Scholar]
- 45.Sparrow JR, Nathan CF, Vodovotz Y. Cytokine regulation of nitric oxide synthase in mouse retinal pigment epithelial cells in culture. Exp Eye Res. 1994;59:129–139. doi: 10.1006/exer.1994.1091. [DOI] [PubMed] [Google Scholar]
- 46.Stevens MJ, Henry DN, Thomas TP, Killen PD, Greene DA. Aldose reductase gene expression and osmotic dysregulation in cultured human retinal pigment epithelial cells. Am J Physiol Endocrinol Metab. 1993;265:E428–E438. doi: 10.1152/ajpendo.1993.265.3.E428. [DOI] [PubMed] [Google Scholar]
- 47.Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Physiol Endocrinol Metab. 1999;277:E760–E771. doi: 10.1152/ajpendo.1999.277.4.E760. [DOI] [PubMed] [Google Scholar]
- 48.Tilton RG, Chang K, Hasan KS, Smith SR, Petrash JM, Misko TP, Moore WM, Currie MG, Corbett JA, McDaniel ML. Prevention of diabetic vascular dysfunction by guanidines. Inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993;42:221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- 49.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 50.Uchida S, Moo Kwon HM, Preston AS, Handler JS. Taurine behaves as an osmolyte in MDCK cells: protection by polarized, regulated transport of taurine. J Clin Invest. 1991;88:656–662. doi: 10.1172/JCI115350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchida S, Moo Kwon H, Yamauchi A, Preston AS, Marumo F, Handler JS. Molecular cloning of the cDNA for an MDCK cell Na+- and Cl−-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci USA. 1992;89:8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 53.Vilchis C, Salceda R. Effect of diabetes on levels and uptake of putative amino acid neurotransmitters in rat retina and retinal pigment epithelium. Neurochem Res. 1996;21:1167–1171. doi: 10.1007/BF02532391. [DOI] [PubMed] [Google Scholar]
- 54.Vinnakota S, Qian X, Egal H, Sarthy V, Sarkar HK. Molecular characterization and in situ localization of a mouse retinal taurine transporter. J Neurochem. 1997;69:2238–2250. doi: 10.1046/j.1471-4159.1997.69062238.x. [DOI] [PubMed] [Google Scholar]
- 55.Voaden MJ, Lake N, Marshall J, Morjaria B. Studies on the distribution of taurine and other neuroactive amino acids in the retina. Exp Eye Res. 1977;24:249–257. doi: 10.1016/0014-4835(77)90091-4. [DOI] [PubMed] [Google Scholar]
- 56.Wu G. Retina: The Fundamentals. Saunders; Philadelphia, PA: 1995. Diabetic retinopathy; p. 31. [Google Scholar]
- 57.Yamamoto R, Bredt DS, Snyder SH, Stone RA. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience. 1993;54:189–200. doi: 10.1016/0306-4522(93)90393-t. [DOI] [PubMed] [Google Scholar]
- 58.Yilmaz G, Esser P, Kociek N, Aydin P, Heimann K. Elevated vitreous nitric oxide levels in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2000;130:87–90. doi: 10.1016/s0002-9394(00)00398-6. [DOI] [PubMed] [Google Scholar]