Abstract
Cancer-associated fibroblasts (CAFs), as the activated fibroblasts in the tumor stroma, are important modifiers of tumour progression. In the present study, we observed that azoxymethane and dextran sodium sulfate treatments induced increasingly severe colorectal mucosal inflammation and the intratumoural accumulation of CAFs. Fibroblast growth factor (FGF)-1 and FGF-3 were detected in infiltrating cells, and FGFR4, the specific receptor for FGF-1 and FGF-3, was detected in colon cancer tissues. The phosphorylation of FGFR4 enhanced the production of metalloproteinase (MMP)-7 and mitogen-activated protein kinase kinase (Mek)/extracellular signal-regulated kinase (Erk), which was accompanied by excessive vessel generation and cell proliferation. Moreover, we separated CAFs, pericarcinoma fibroblasts (PFs), and normal fibroblasts (NFs) from human colon tissue specimens to characterize the function of CAFs. We observed that CAFs secrete more FGF-1/-3 than NFs and PFs and promote cancer cell growth and angiogenesis through the activation of FGFR4, which is followed by the activation of Mek/Erk and the modulation of MMP-7 expression. The administration of FGF-1/-3-neutralizing antibodies or the treatment of cells with FGFR4 siRNA or the FGFR4 inhibitor PD173074 markedly suppressed colon cancer cell proliferation and neovascularization. These observations suggest a crucial role for CAFs and FGF signaling in the initiation and progression of colorectal cancer. The inhibition of the FGF signaling pathway may be a useful strategy for the treatment of colon cancer.
Keywords: Cancer-associated fibroblasts, colon cancer, extracellular signal-regulated kinase, fibroblast growth factor, metalloproteinase-7
Ulcerative colitis (UC) is well documented to increase the risk of colitis-associated cancer (CAC), a subtype of colorectal cancer (CRC).1 CRC and CAC are common malignant neoplasms and leading causes of death.2 Thus, a thorough understanding of the pathogenesis of CRC and CAC at the molecular and cellular levels is urgently required for the prevention of cancer development.
Accumulating clinical and experimental data have revealed that tumor development is intimately associated with the complex interactions of carcinomas with several distinct stromal cell types that together create the microenvironment of cancer cells.3,4 It is becoming increasingly clear that cancer-associated fibroblasts (CAFs) are one of the most crucial components of the tumor microenvironment.5 CAFs are a major constituent of the tumor stroma and can be identified by an increased rate of proliferation and an activated myofibroblastic phenotype, which includes prominent contractile ability and the expression of α-smooth muscle actin (α-SMA).3,5 Other markers expressed by CAFs include platelet-derived growth factor receptor-β (PDGFRβ), fibroblast-specific protein-1 (FSP-1), vimentin, and fibroblast activation protein (FAP).6,7 CAFs promote tumor growth through the direct stimulation of tumor cell proliferation, enhanced angiogenesis, and ECM remodelling.4,8–10 Moreover, CAFs mediate tumor-promoting inflammation and modulate the components of the inflammatory microenvironment that facilitate tumour initiation, progression, and metastasis.11–14 However, the mechanisms underlying the regulation of cancer cells by CAFs remain largely unknown.
In the present study, we used the well-established murine model of colitis-associated cancer based on the use of azoxymethane (AOM) and dextran sodium sulfate (DSS). We observed that combined treatment with AOM and DSS led to the development of multiple tumors in the mouse colon,15 the induction of increasingly severe colorectal mucosal inflammation and the increased infiltration of CAFs, based on the increased expression of FGF-1 and FGF-3 and enhanced phosphorylation of FGFR4. Moreover, CAFs isolated from human colon tumor tissue secreted more FGF-1 and FGF-3, leading to the autophosphorylation of FGFR4. The activation of FGFR4 subsequently activated the downstream signaling proteins mitogen-activated protein kinase kinase (Mek) and extracellular signal-regulated kinase 1/2 (Erk1/2), modulated the expression of metalloproteinase (MMP-7) and promoted the growth of colorectal cancer cells. Thus, these observations suggest that CAFs promote colorectal carcinogenesis and that FGF signaling may play a pivotal role in the regulatory circuit between colon cancer cells and stromal fibroblasts.
Materials and Methods
Cells, recombinant proteins and antibodies
All cells were cultured in RPMI, McCoy’s 5A or DMEM with 10% FBS and antibiotics. The following cell lines were used: SW480, HT29, HCT116 (colorectal cancer) and human umbilical vein endothelial cells (HUVECs). Human recombinant FGF-3 (R&D, Minneapolis, MN, USA) and FGF-1 (PeproTech, New Jersey, NY, USA) were used. PD173074 was from Selleck, Houston, TX, USA. The antibodies used in this study are described in the Data S1.
Immunohistochemical analyses of mouse colon tissues
The CAC mouse model was developed using AOM/DSS treatment as described previously.15 Immunohistochemical analysis was performed. Detailed information is provided in the Data S1.
Real-time quantitative reverse transcription PCR
Total RNA was extracted from colon tissue using TRIzol (Invitrogen, Carlsbad, CA, USA). mRNA was reverse transcribed using SYBR Premix Ex Taq™ II (Takara, Dalian, China). Real-time PCR was performed using an Applied Biosystems HT7900 PCR system with 2× QuantiFast SYBR Green PCR Master Mix (QIAGEN, Hilden, Germany), primers (1 μM; Table S1) and <100 ng cDNA in a 25 μl reaction mixture. The relative expression of target genes was analysed using the ∆∆Ct method.
Isolation and culture of primary human colon fibroblasts
Human colon tissue specimens of at least five individual patients undergoing colon cancer surgery were obtained from the tissue bank of Fudan University Shanghai Cancer Center. All patients signed informed consent for donating their samples to the tissue bank of Fudan University Shanghai Cancer Center, and approval was given by the Human Subjects Research Ethical Committee of Fudan University Shanghai Cancer Center. We separated the tumor, adjacent, and normal tissues, the latter of which were at least 2 cm away from the edge of tumor foci and were histologically confirmed as free from adenocarcinoma cells. We immediately isolated the fibroblasts from samples.
For human colon fibroblast isolation, the colon tissues were divided into small pieces before digestion in HBSS containing 1 mg/mL collagenase, 2.5 mg/mL trypsin, and 30 μg/mL DNase I at 37°C in a shaking water bath. Three digestion cycles were conducted for 30 min each. The pooled cells were cultured in 10% FBS-DMEM medium. By the third passage, the cells formed homogeneous monolayers morphologically consistent with fibroblast-like cells and were confirmed to contain >90% vimentin-positive cells.
Colon fibroblasts were cultured in 10% FBS-DMEM for 24 h. Conditioned medium was collected and passed through 0.45-μm syringe filters. The human colon cancer cell lines SW480, HT29, and HT116 were incubated with the fibroblast-conditioned media for 48 h to obtain cell lysates for immunoblotting analysis. In another experiment, HUVECs were incubated with the fibroblast-conditioned media for the indicated times to examine cell proliferation and endothelial tube formation.
Immunofluorescence analysis
The immunofluorescence analysis was performed using anti-CD8+ (Abcam, Cambridge, UK) primary antibodies directed against mouse T cells and visualized with a fluorescence microscope (IX51; Olympus, Tokyo, Japan). For two-colour immunofluorescence analysis, the isolated fibroblasts were fi fl and incubated with anti-vimentin, anti-α-SMA, or anti-FAP (Abcam) and then with goat anti-mouse IgG fluorescein-conjugated antibodies for detecting vimentin or with goat anti-rabbit IgG fluorescein conjugate antibodies (Calbiochem, Merck Serono, China) for detecting FAP and α-SMA. Immunofluorescence was visualized using a fluorescence microscope.
Analysis of cell proliferation
Cell proliferation was determined using the CellCounting Kit-8 (Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer’s instructions. Detailed information is provided in the Data S1.
Endothelial tube formation assays
Briefly, 0.1 mL of growth-factor-reduced Matrigel (Becton Dickinson, NJ, USA) was added per well, and HUVECs (4 × 104 cells) were added and incubated in media obtained from colon CAFs. All assays were performed with n = 6/group. Tube formation was quantified 18 to 20 h later. The supernatants were maintained throughout experiments. Digital images of the endothelial tubes were obtained using brightfield light microscopy.
Determination of extracellular FGF-1 and FGF-3 levels
The conditioned media of fibroblasts and colon cancer cells were collected and centrifuged to obtain supernatants. FGF-1 levels were determined using a specific ELISA kit against FGF-1 (Elabscience, Wuhan, China) according to the manufacturer’s instructions. The data are expressed as the target molecule (pg) per total media (mL) for each sample. To detect FGF-3, the cell lysates were analyzed by immunoblotting using an anti-FGF-3 primary antibody. The signals were detected using a Las-4000 mini CCD camera (GE Healthcare, Marlborough, USA).
Knockdown of FGFR4 expression
The selected short interfering RNA target sequence in FGFR4 (5′-CCACCACAUUGACUACUAUdTdT-3′) and nonspecific control short interfering RNA duplexes (5′-UUCUCCGAACGUGUCACGUdTdT-3′) were designed and synthesised by Biotend (Shanghai). SW480, HT29, and HT116 cells in 6-cm dishes were transfected for 48 h with the siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The transfected cells were then incubated with the fibroblast-conditioned media for an additional 48 h to obtain cell lysates, which were subjected to immunoblotting analysis.
Protein isolation, co-immunoprecipitation and immunoblotting analysis
Colon tissues were homogenised and sonicated in RIPA lysis buffer (Beyotime Biotechnology, Suzhou, China) supplemented with protease and phosphatase inhibitors (Roche, Rotkreuz, Switzerland) to detect phospho-tyrosine, phospho-Erk, total Erk, phospho-Mek, total Mek, MMP7, and FGFR4. In some experiments, the supernatants of cell lysates were incubated with 2 μg of rabbit anti-FGFR4 antibody and then precipitated with 20 μL of protein G Sepharose 4 Fast Flow (GE Healthcare) to detect the level of tyrosine phosphorylation with an anti-phospho-tyrosine antibody. To detect activated MMP-7, the proteins precipitated from conditioned medium using 10% trichloroacetic acid were analyzed using an anti-MMP7 primary antibody. The signals were detected using a Las-4000 mini CCD camera (GE Healthcare).
Statistical analysis
The data were analyzed using one-way ANOVA followed by the Fisher protected least significant difference test or the Mann–Whitney U-test. P < 0.05 was considered statistically significant.
Results
Combined AOM and DSS treatment increased the proportion of CAFs, inflammatory cells and proinflammatory cytokines in the CAC mouse model
In a previous study, we showed that combined AOM and DSS treatment increased CXCL2 expression and CXCR2-expressing neutrophil infiltration into the lamina propria and submucosal regions of the colon.15 In the present study, immunohistochemical analysis revealed increased numbers of α-SMA-positive cells and FAP-positive CAFs in the mucosa, with a peak on day 56 (Fig.1). Moreover, we observed increasing numbers of infiltrated macrophages and CD8-positive T cells, consistent with previous reports (Suppl. Fig. S1). Furthermore, we evaluated the mRNA expression of the proinflammatory gene signature, and the mRNA expression levels of IL-1α, IL-1β, IL-6 and tumour necrosis factor (TNF)-α were significantly increased (Suppl. Fig. S2a–d). Consistent with a previous study,15 we detected increased expression of CXCL-2 and PDGF-α, which mediates the proliferation of fibroblasts and T cells (Suppl. Fig. S2e,f).
Figure 1.
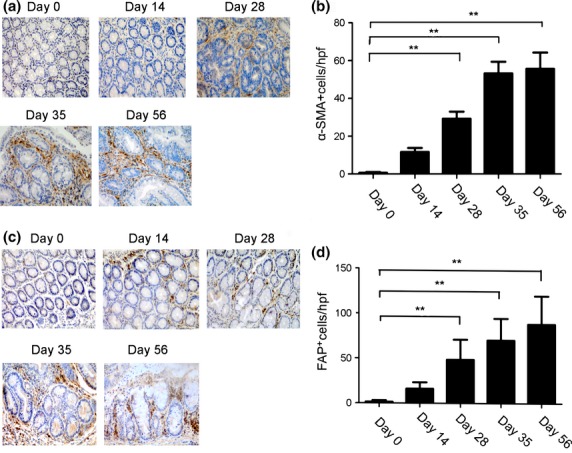
The infiltration of cancer-associated fibroblasts into the colon tissues of a colitis-associated cancer (CAC) mouse model. Colon tissues removed from mice treated with azoxymethane (AOM) and dextran sodium sulfate (DSS) at the indicated times were immunostained with anti-α-smooth muscle actin (α-SMA) (a, b) or anti-fibroblast activation protein (FAP) (c, d). The selected visual fields are at 400× magnification. Each value represents the mean ± SD (n = 7 mice/group). **P < 0.01 versus day 0 mice.
Enhanced cell proliferation and increased neovascularization through FGF signaling in the CAC mouse model
Cancer-associated fibroblasts secrete fibroblast growth factors (FGFs) such as FGF1 and FGF3, which can promote tumourigenesis.16 In the present study, we observed that FGF-1 and FGF-3 expression increased from day 35 to 56 in the colon tissues of CAC mice (Fig.2a,b). The expression of PCNA, a marker of cancer cell proliferation, was markedly increased in the nuclei of tumor cells after AOM and DSS treatment, although PCNA-positive epithelial cells were minimally detected on day 0 (Fig.2c). Consistent with a previous study, intracolonic CD31-positive vascular densities were markedly increased, peaking on day 56 in AOM/DSS-treated mice (Fig.2d).
Figure 2.
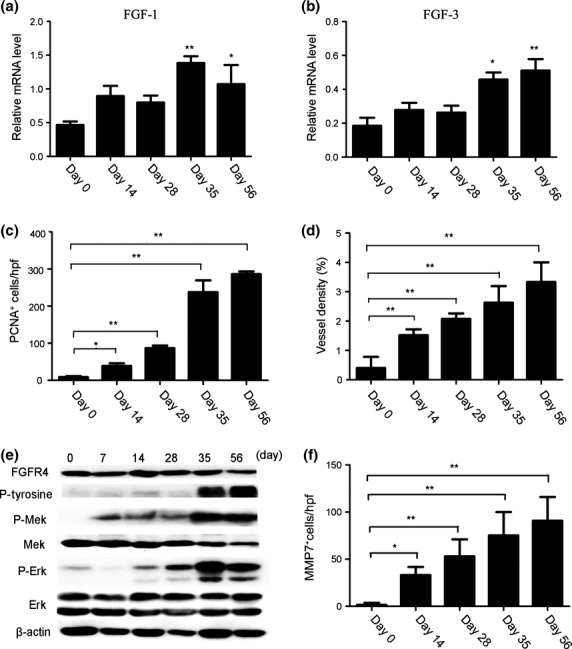
Enhanced cell proliferation and increased neovascularization through fibrtoblast growth factor (FGF) in the colitis-associated cancer (CAC) mouse model. (a, b) The relative mRNA levels of FGF-1 and FGF-3 in mouse colon tissues. *P < 0.05, **P < 0.01 versus day 0 mice. (c) PCNA+ cells were detected in the colon tissues. The numbers of cells per field were counted in five randomly selected visual fields at 400× magnification. Values shown represent the means ± SD (n = 7 mice/group). *P < 0.05, **P < 0.01 versus day 0 mice. (d) Colon tissues were immunostained with anti-CD31 antibody. Each value represents the mean ± SD (n = 7 mice/group). **P < 0.01 versus day 0 mice. (e) Immunoblotting analysis with the indicated antibodies was performed using cell colon tissue lysates from mice treated with azoxymethane (AOM) and dextran sodium sulfate (DSS) and killed at the indicated times. (f) Immunohisto-chemical detection of MMP-7+ cells in the mouse colon tissues. The cell numbers per field were counted in five randomly selected visual fields at 400× magnification. Values shown represent the means ± SD (n = 7 mice/group). *P < 0.05, **P < 0.01 versus day 0 mice.
FGFR4 is the receptor for FGF-1 and FGF-3, and the phosphorylation and activation of FGFR4 initiate downstream signal transduction pathways,17,18 including Mek/Erk signaling. We observed that the phosphorylation of FGFR4 and Mek/Erk was enhanced by treatment with AOM and DSS compared with day 0, but the total levels of FGFR4 and Mek/Erk were unaffected (Fig.2e). Several lines of evidence suggest that CAFs are potent initiators of angiogenesis because these cells promote the secretion of MMPs (angiogenic factors) from cancer cells.19,20 We observed that the mRNA expression of MMP-7 was markedly enhanced (Suppl. Fig. S3a), and the number of MMP-7-positive cells also increased from day 28 to 56 in mice treated with AOM and DSS (Fig.2f, Suppl. Fig. S3b). These results suggest that FGF signaling is essential for the formation and maintenance of colon cancer.
Contribution of CAFs to colorectal cancer cell and HUVEC proliferation and tube formation in vitro
To better understand the involvement of cancer-associated fibroblasts in the regulation of CAC and CRC, we isolated fibroblasts from the colon tissue specimens of patients who underwent colon cancer surgery. We separated the tumor, adjacent, and normal tissues, the latter of which were at least 2 cm away from the edge of the tumor foci, and we designated the resulting cells as CAFs, pericarcinoma fibroblasts (PFs), and normal fibroblasts (NFs), respectively. In the present study, the CAFs, PFs, and NFs were successfully isolated from a colon cancer patient, and each fibroblast population exhibited hallmark fibroblast characteristics: a long, spindle-shaped morphology and the strong expression of fibroblastic markers such as vimentin (Fig.3a). Moreover, CAFs showed elevated expression of α-SMA and FAP compared with patient-matched NFs and PFs (Fig.3a). Flow cytometry revealed that the cell population comprised >90% fibroblasts (Fig.3b), and subsequent experiments were performed using CAFs, PFs, and NFs.
Figure 3.
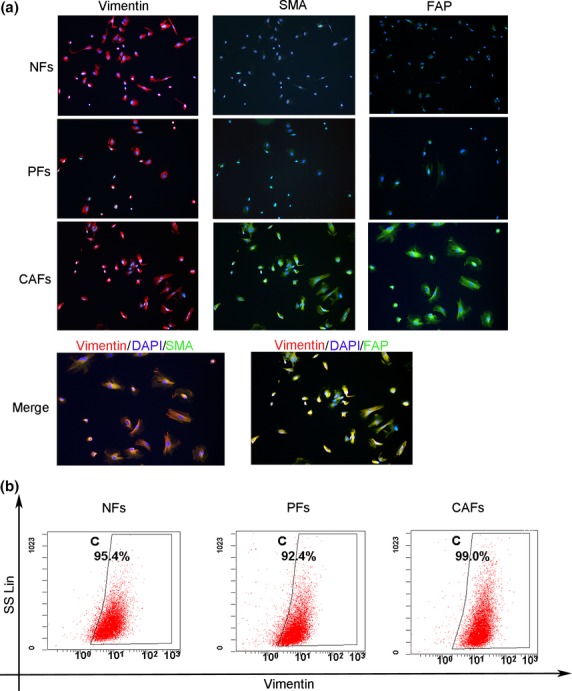
Isolation of fibroblasts from human colon tissues. (a) Primary human fibroblasts were isolated from tumor, adjacent, and normal tissues, the latter of which were at least 2 cm away from the edge of the tumor foci of colon tissue. All experiments were repeated three times, and representative results are shown here. Original magnification, 200×. (b) The purity of isolated normal fibroblasts (NFs), pericarcinoma fibroblasts (PFs) and cancer-associated fibroblasts (CAFs). All experiments were repeated three times, and representative results are shown here.
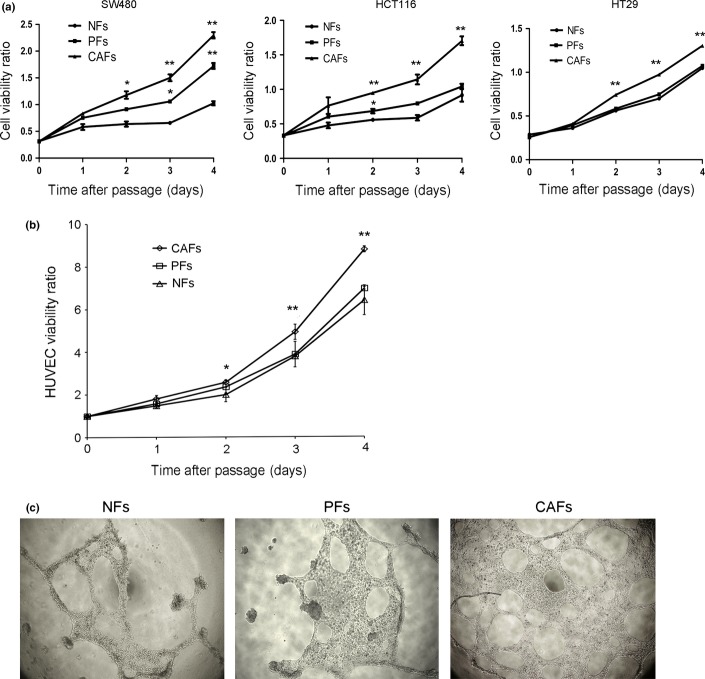
To investigate the role of CAFs in cancer cell proliferation and angiogenesis in vitro, we cultured three types of colon cancer cells (SW480, HCT116, HT29) in CAF-, PF- or NF-conditioned media and then examined the proliferation of these cells. Consistently, the cell numbers were markedly increased for cells incubated with supernatants from CAF-conditioned media but not for cells incubated with PF- or NF-conditioned media (Fig.4a). Similarly, HUVEC proliferation was enhanced in CAF-conditioned media but not in PF- or NF-conditioned media (Fig.4b). Next, we investigated HUVEC tube and loop formation on Matrigel as an angiogenic potential assay in vitro. As shown in Figure4(c), the differentiation of HUVECs into primitive capillary-like structures was observed in the presence of CAF-conditioned media, but this effect was smaller in the presence of NF- or PF-conditioned media (Fig.4c). Together, these results indicate that CAFs isolated from human colon cancer tissue significantly contribute to cell proliferation and angiogenic behavior.
Figure 4.
Cancer-associated fibroblasts (CAFs) promote the proliferation of human colon cancer cells and HUVECs and endothelial tube formation. (a) The effect of CAFs on cancer cell growth was detected using the CCK8 assay. All experiments were repeated three times, and representative results are shown here. *P < 0.05, **P < 0.01. (b) The effect of CAFs on endothelial cell growth was detected using the CCK8 assay. *P < 0.05, **P < 0.01. All experiments were repeated three times, and representative results are shown here. (c) Endothelial tube formation after HUVECs were incubated in NF-, PF-, or CAF-conditioned media for 24 h. All experiments were repeated three times, and representative results are shown here.
CAFs promote tumor growth through the FGF1/3/FGFR4 signalling pathway
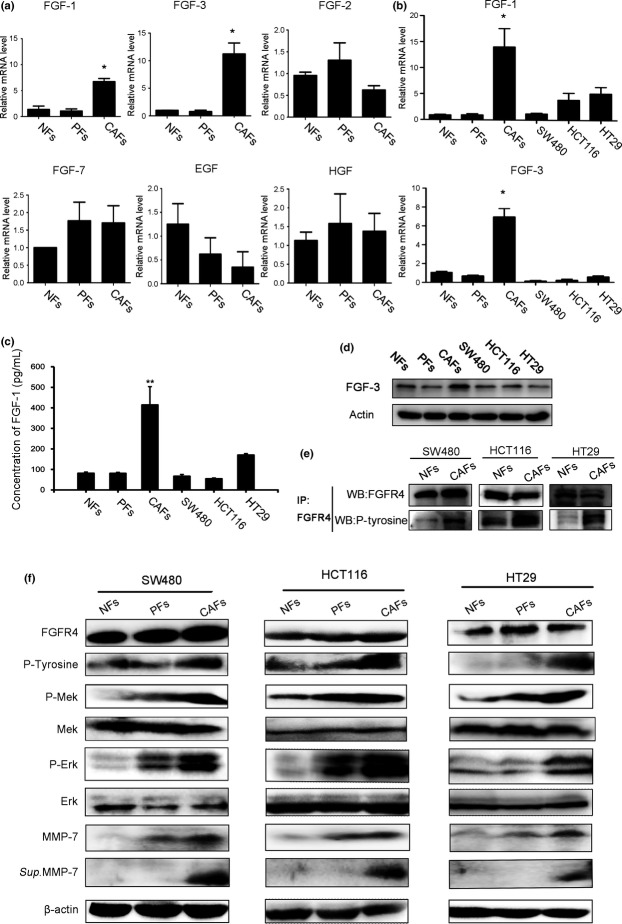
Consistent with the in vivo observations, we found that the mRNA expression of FGF-1 and FGF-3 was remarkably increased in CAFs (Fig.5a). In addition to FGFs, CAFs also secreted other growth factors and chemokines, including SDF-1/CXCL12, VEGF, HGF, and CXCL14, into the tumor microenvironment for the promotion of tumourigenesis.6,10,21,22 However, the growth factors HGF, EGF, FGF-2, and FGF-7 showed no change in expression compared with isolated NFs and PFs (Fig.5a). Moreover, the expression of FGF-1 and FGF-3 in CAFs was abundant not only at the mRNA level but also at the protein level, in contrast to what was observed in colon cancer cells (Fig.5b–d), suggesting that FGF-1 and FGF-3 primarily act as autocrine mediators in CAFs, thereby contributing to the progression of colorectal cancer. Next, we cultured SW480, HCT116, and HT29 human colon cancer cells with CAF-, PF- and NF-conditioned media to examine the expression of FGFR4. Co-immunoprecipitation and immunoblotting analysis showed that FGFR4 phosphorylation (tyrosine kinase phosphorylation) was increased in cancer cells maintained in CAF-conditioned media compared with cells incubated in NF- or PF- conditioned media. However, total FGFR4 expression did not change (Fig.5e,f). The phosphorylation level of Mek/Erk was enhanced in cancer cells maintained in CAF-conditioned media compared with cells incubated in NF- or PF-conditioned media, although no apparent differences were observed in total Mek/Erk expression (Fig.5f). In addition, the protein expression and activation of MMP-7 were upregulated in the lysates and supernatants of SW480, HCT116, and HT29 cells after culture with CAF-conditioned media compared to culture with NF- or PF-conditioned media (Fig.5f).
Figure 5.
Cancer-associated fibroblasts (CAFs) predominantly secrete FGF1 and FGF3 and can upregulate the Mek/Erk pathway and MMP-7 through the activation of FGFR4. (a) mRNA levels of FGF-1, -2, -3, -7, EGF and HGF in NFs, PFs and CAFs were quantified through qRT-PCR. *P < 0.05 versus NFs. All experiments were repeated three times, and representative results are shown here. (b) The relative FGF1 and FGF3 mRNA levels in NFs, PFs, CAFs, and HCT116, SW480, and HT29 colon cancer cells. *P < 0.05 versus NFs. All experiments were repeated three times, and representative results are shown here. (c, d) FGF-1 content in conditioned media was determined (c), and FGF-3 protein (d) was measured in cell lysates from NFs, PFs, CAFs, and HCT116, SW480, and HT29 colon cancer cells. Each value represents the mean ± SEM (n = 6). *P < 0.05 versus NFs. All experiments were repeated three times, and representative results are shown here. (e) Colon cancer cells were incubated with NF- or CAF-conditioned media for 48 h. The resulting cell lysates were subjected to co-immunoprecipitation and immunoblotting analyses with the indicated antibodies. All experiments were repeated three times, and representative results are shown here. (f) Colon cancer cells were incubated with NF-, PF-, or CAF-conditioned media for 48 h. The resulting lysates were subjected to immunoblotting with the indicated antibodies. The soluble MMP-7 was isolated from human colon cancer cell culture media. All experiments were repeated three times, and representative results are shown here.
We further examined the effects of PD173074, an FGFR-selective inhibitor, and FGFR4-specific siRNA on FGF signalling.17 PD173074 inhibits the FGF-induced phosphorylation of FGFR4, subsequently reducing the levels of phosphorylated FGFR4, Mek and Erk, but it does not change the levels of total FGFR4, Mek, or Erk (Fig.6a). FGFR4-specific siRNA decreased total FGFR4 expression, subsequently reducing the FGF-induced phosphorylation of FGFR4, Mek, and Erk but not total Mek or Erk expression (Fig.6b). Moreover, PD173074 or FGFR4-siRNA treatment reduced the protein expression and activation of MMP-7 in cell lysates and supernatants (Fig.6a,b). Furthermore, human recombinant FGF-1 and FGF-3 activated the phosphorylation of FGFR4, Mek and Erk and upregulated both the protein expression and the activation of MMP-7 (Fig.7a,b). In addition, the proliferation of colon cancer cells and HUVECs was enhanced in CAF-conditioned media, and this enhanced proliferation could be significantly reduced by anti-FGF-1 or anti-FGF-3 treatments (Fig.8). Taken together, these data further indicate that CAFs promote tumor growth through the FGF1/3/FGFR4 signaling pathway.
Figure 6.
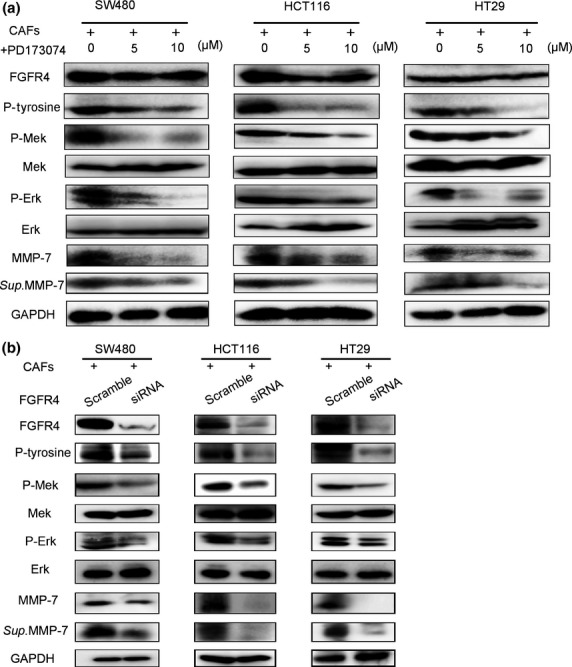
Inhibiting FGFR4 phosphorylation reduces Mek/Erk phosphorylation and MMP-7 expression. (a) Lysates from colon cancer cells incubated in supernatants from CAFs incubated with or without FGFR4 inhibitor (PD173074) for 24 h were immunoblotted with the indicated antibodies. All experiments were repeated three times, and representative results are shown here. (b) The cells were transfected with FGFR4 siRNA for 48 h; then, the transfected cells were incubated with the fibroblast-conditioned media for an additional 48 h to obtain cell lysates and perform immunoblotting analyses. All experiments were repeated three times, and representative results are shown here.
Figure 7.
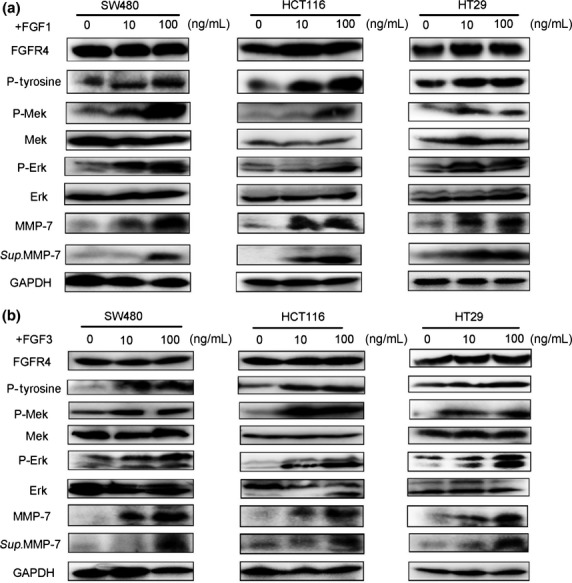
Recombinant FGF1 and FGF3 proteins upregulate Mek/Erk signaling and MMP-7 expression. Colon cancer cells were treated with or without recombinant FGF1 (a) or FGF3 (b) for 24 h, and lysates were immunoblotted with the indicated antibodies. All experiments were repeated three times, and representative results are shown here.
Figure 8.
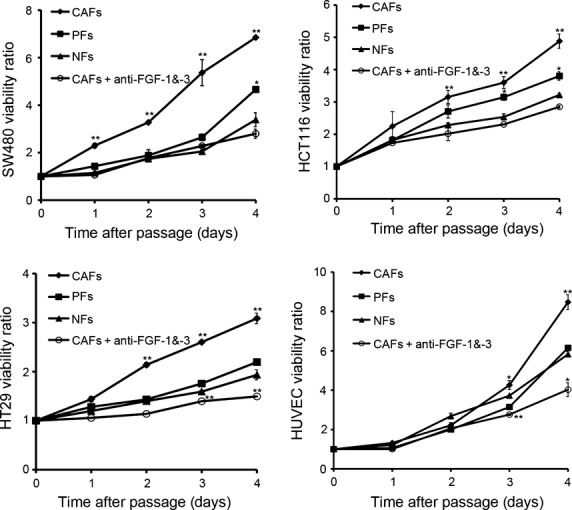
Effects of anti-FGF-1/-3 antibodies on colon cancer cell proliferation. Colon cancer cells were incubated in supernatants from NF-, PF-, or CAF-conditioned media with or without anti-FGF-1/-3 antibodies. The cell viability was detected using the CCK8 assay. All experiments were repeated three times, and representative results are shown here. *P < 0.05, **P < 0.01.
Discussion
In colorectal cancer, the tumor microenvironment promotes the progression from a normal colonic epithelium to an adenomatous polyp and ultimately to an invasive colon carcinoma.13,23 Increasing evidence has shown that fibroblasts are among the most crucial components of the tumor microenvironment and are prominent modulators of cancer progression. Reportedly, α-SMA,24 S100A4,25 and FAP26 can be used as predictors of colorectal cancer recurrence, and cancer-associated fibroblast and M2 macrophage markers together predict colorectal cancer outcomes.24 Moreover, the tumor-promoting characteristics of CAFs have prompted interest in exploiting these cells as drug targets for anticancer therapy. The continued characterization of the interaction between tumor epithelial cells and stromal elements and the molecular identification of key mediators will likely provide insights into tumor biology and suggest novel therapeutic options. Herein, we show that CAFs can promote the proliferation of colorectal cancer cells and tumor angiogenesis both in vitro and in vivo. CAFs promote colonic tumourigenesis through FGF signaling, thereby highlighting a crucial role for CAFs in the initiation and progression of colorectal cancer.
Several families of growth factors implicated as autocrine and paracrine mediators of stromal-epithelial interactions are involved in the initiation and progression of carcinoma. It has been suggested that the EGF signaling pathway regulates the growth and differentiation of intestinal epithelial and stem/progenitor cells.27 Moreover, HGF secreted from CAFs promotes cell invasion and motility by inducing the phosphorylation of Akt and ERK 1/2 in colon cancer cell lines.28 However, no apparent differences were observed among CAFs, PFs and NFs, the three types of fibroblasts. In contrast, we observed that the mRNA and protein levels of FGF-1 and FGF-3, but not other growth factors (FGF-2 and FGF-7), were increased in CAFs isolated from tumor tissue. In addition, FGF-1 and FGF-3 act as primary autocrine mediators of stromal-epithelial interactions in the progression of colorectal carcinoma, and this promotion was inhibited by treatment with antibodies against FGF-1 or FGF-3.
Previous studies have reported that FGFR4, a receptor for FGF-1 and FGF-3 that is highly expressed in cancer cells, promotes colon cancer growth and metastasis.18,29 MAPK/ERK is a classical pathway that promotes the growth of cancers, including colorectal cancer, and the tumor fibroblast-derived protein epiregulin promotes the growth of colitis-associated neoplasms through Erk.30 Therefore, we speculate that the FGF1/3/FGFR4 macromolecular signaling complex activates the MAPK signaling pathway in colon cancer development and that the downstream effect of MAPK/Mek/Erk signaling is cell proliferation. In our mouse model, the mRNA levels of FGF-1 and FGF-3 and the phosphorylation of FGFR4 and Erk were obviously increased with increasing cell proliferation. Consistent with the in vivo observations, FGFR4 and Mek/Erk phosphorylation were upregulated by CAFs but not by NFs or PFs. Furthermore, FGF-1 and FGF-3 upregulated Mek/Erk expression in colorectal cancer cells via FGFR4 phosphorylation, whereas the FGFR4 inhibitor or the silencing of FGFR4 expression inhibited the expression of Mek and Erk in the colon cancer cells. These observations indicate that the FGF/FGFR axis, mediated through CAFs, plays a key role in promoting colon tumor growth.
Other pathways are also activated through FGFRs, including STAT-dependent signalling.31,32 STAT-3 signaling induces MMP-7 expression in PDA cells. Some studies have suggested that MMP-7 and other MMPs are elevated in human CRC and regulate the angiogenic activity of VEGF in colorectal tissue to promote angiogenesis.19,20 Indeed, CAFs induce greater MMP-7 expression than NFs or PFs can in cancer cells; FGF-1/-3 induced MMP-7 expression both in vitro and in vivo, and inhibiting FGFR4 phosphorylation using PD173074 or FGFR4 silencing reduced MMP-7 expression. These observations support the hypothesis that CAFs, mediated by FGF signaling, upregulate MMP-7 or directly secrete MMP-7 to drive tumor-associated angiogenesis in colorectal cancer.
The tumor microenvironment primarily comprises tumor-infiltrating cells, vasculature, extracellular matrix (ECM), and other matrix-associated molecules.6 In the present study, the colon carcinomas were infiltrated by various cell types, including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), CD8 T-cells, and CAFs. Colon carcinomas also showed increasing mRNA expression of IL-1α, IL-1β, IL-6, TNFα, CXCL2, and PDGF-α. Some studies have suggested that the activation of CAFs is dependent on NF-κB pathway activation and the secretion of IL-1β IL-6, IL-8, and SDF-1.33 Among the paracrine factors acting on fibroblasts, members of the TGF-β, PDGF and HGF families have also been recognized as major regulators of CAFs.5,6 It has been reported that fibroblasts, through the CCL3-CCR5 axis, play an important role in CAC.34 Thus, we speculated that the cytokines, chemokines or paracrine factors secreted from immune and tumor cells instruct the NFs adjacent to colorectal cancer to become CAFs or to recruit fibroblasts to inflammatory tissue. Indeed, the molecular mechanisms involved in the activation of CAFs in colon cancer require further exploration.
In summary, our study provides the first evidence of the crucial involvement of CAFs, which are dependent on the FGF signaling pathway, in promoting the initiation and progression of colon carcinogenesis. Our data suggest that the inhibition of FGF signaling results in anti-proliferative and/or anti-angiogenic effects in vitro, possibly providing a useful strategy for the treatment of colon cancer. However, the effects of inhibiting the FGF/FGFR axis in vivo require further investigation to confirm the validity of the FGF/FGFR axis as a potential therapeutic target.
Acknowledgments
This work was supported through grants from The National Science Foundation of China (81272727, 82472223).
Disclosure Statement
The authors have no conflicts of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 Inflammatory cells in mouse colon tissues.
Fig. S2 Expression of cytokines and chemokines in mouse colon tissues.
Fig. S3 MMP-7 expression in mouse colon tissues.
Data S1. Antibodies; Immunohistochemical analyses of mouse colon tissues; Analysis of cell proliferation
Table S1 Primer sequences for quantitative RT-PCR
References
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes – bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;8:1461–2. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Calado DP, Zhang B, Srinivasan L, et al. Constitutive canonical NF-kappaB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell. 2010;18:580–9. doi: 10.1016/j.ccr.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Lebret SC, Newgreen DF, Thompson EW, Ackland ML. Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res. 2007;9:R19. doi: 10.1186/bcr1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang K, Bai YP, Wang C, et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One. 2012;7:e51848. doi: 10.1371/journal.pone.0051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Ye YW, Hu S, Shi YQ, et al. Combination of the FGFR4 inhibitor PD173074 and 5-fluorouracil reduces proliferation and promotes apoptosis in gastric cancer. Oncol Rep. 2013;30:2777–84. doi: 10.3892/or.2013.2796. [DOI] [PubMed] [Google Scholar]
- Liu R, Li J, Xie K, et al. FGFR4 promotes stroma-induced epithelial-to-mesenchymal transition in colorectal cancer. Cancer Res. 2013;73:5926–35. doi: 10.1158/0008-5472.CAN-12-4718. [DOI] [PubMed] [Google Scholar]
- Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- Kostova E, Slaninka-Miceska M, Labacevski N, et al. Expression of matrix metalloproteinases 2, 7 and 9 in patients with colorectal cancer. Vojnosanit Pregl. 2014;71:52–9. doi: 10.2298/vsp121221024k. [DOI] [PubMed] [Google Scholar]
- Augsten M, Sjoberg E, Frings O, et al. Cancer-associated fibroblasts expressing CXCL14 rely upon NOS1-derived nitric oxide signaling for their tumor-supporting properties. Cancer Res. 2014;74:2999–3010. doi: 10.1158/0008-5472.CAN-13-2740. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen X, Zhou Q, et al. Hepatocyte growth factor activates tumor stromal fibroblasts to promote tumorigenesis in gastric cancer. Cancer Lett. 2013;335:128–35. doi: 10.1016/j.canlet.2013.02.002. [DOI] [PubMed] [Google Scholar]
- O’Brien P, O’Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta. 2008;1784:1130–45. doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Herrera M, Herrera A, Dominguez G, et al. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013;104:437–44. doi: 10.1111/cas.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Lee HJ, Kim SH, et al. Expression of protein S100A4 is a predictor of recurrence in colorectal cancer. World J Gastroenterol. 2010;16:3897–904. doi: 10.3748/wjg.v16.i31.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LR, Lee HO, Lee JS, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–41. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- Feng Y, Dai X, Li X, et al. EGF signalling pathway regulates colon cancer stem cell proliferation and apoptosis. Cell Prolif. 2012;45:413–9. doi: 10.1111/j.1365-2184.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713–22. doi: 10.3748/wjg.v16.i6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Zhang SX, Liu HJ, et al. Fibroblast growth factor receptor 4 as a potential prognostic and therapeutic marker in colorectal cancer. Biomarkers. 2014;19:81–5. doi: 10.3109/1354750X.2013.876555. [DOI] [PubMed] [Google Scholar]
- Neufert C, Becker C, Tureci O, et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J Clin Invest. 2013;123:1428–43. doi: 10.1172/JCI63748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Prochazkova J, Bryja V, et al. Fibroblast growth factor inhibits interferon gamma-STAT1 and interleukin 6-STAT3 signaling in chondrocytes. Cell Signal. 2009;21:151–60. doi: 10.1016/j.cellsig.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer LR, Chuntova P, Bade LK, et al. Activation of the FGFR-STAT3 pathway in breast cancer cells induces a hyaluronan-rich microenvironment that licenses tumor formation. Cancer Res. 2014;74:374–86. doi: 10.1158/0008-5472.CAN-13-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Baba T, Shinagawa K, Matsushima K, Mukaida N. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int J Cancer. 2014;135:129–306. doi: 10.1002/ijc.28779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Inflammatory cells in mouse colon tissues.
Fig. S2 Expression of cytokines and chemokines in mouse colon tissues.
Fig. S3 MMP-7 expression in mouse colon tissues.
Data S1. Antibodies; Immunohistochemical analyses of mouse colon tissues; Analysis of cell proliferation
Table S1 Primer sequences for quantitative RT-PCR