Abstract
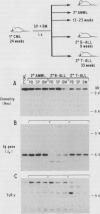
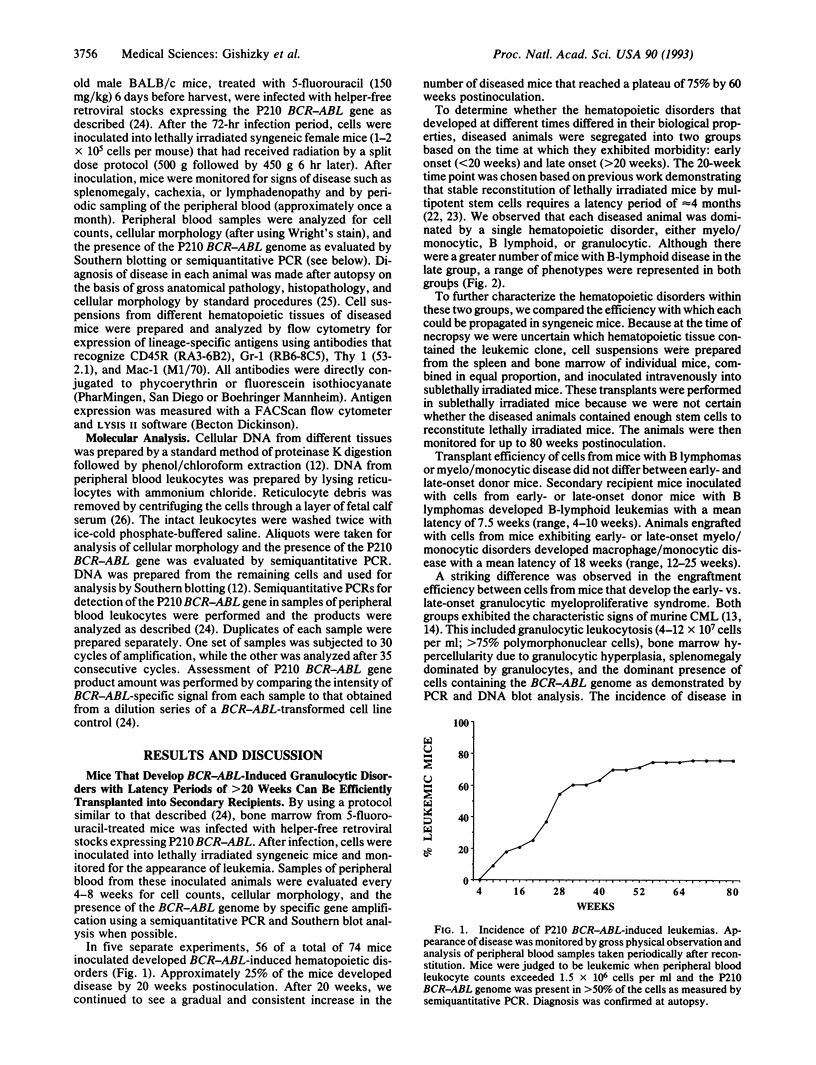
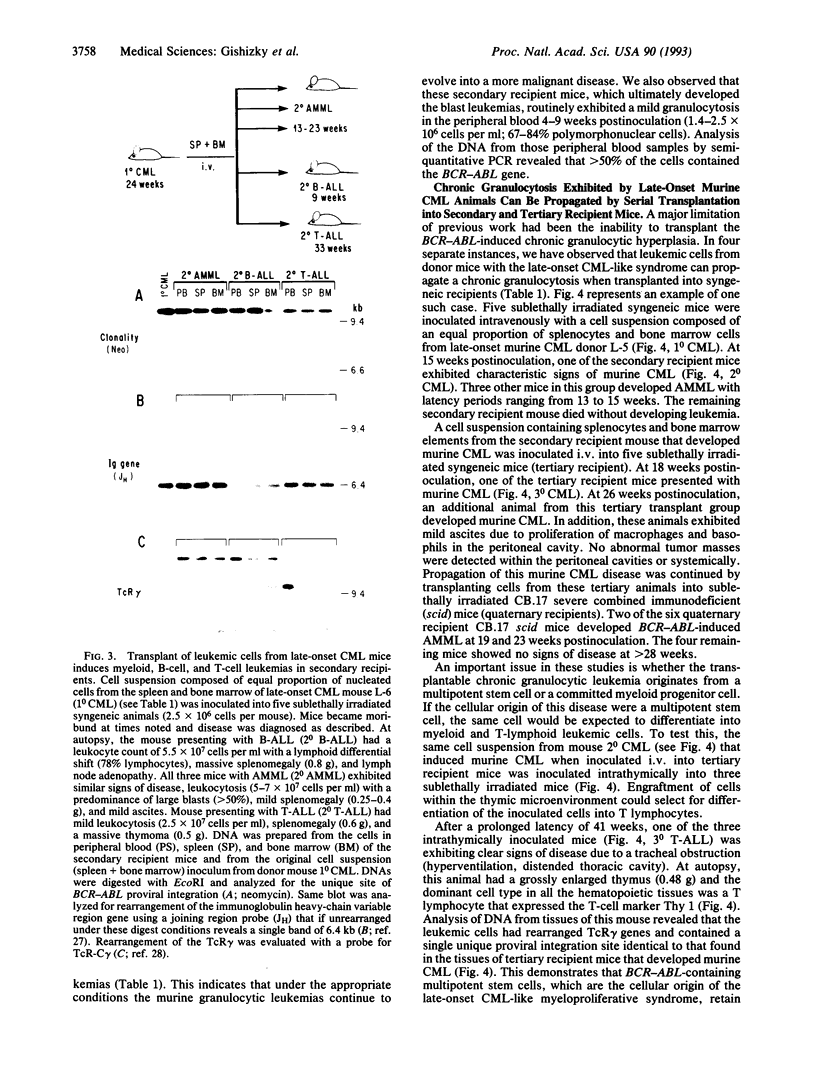
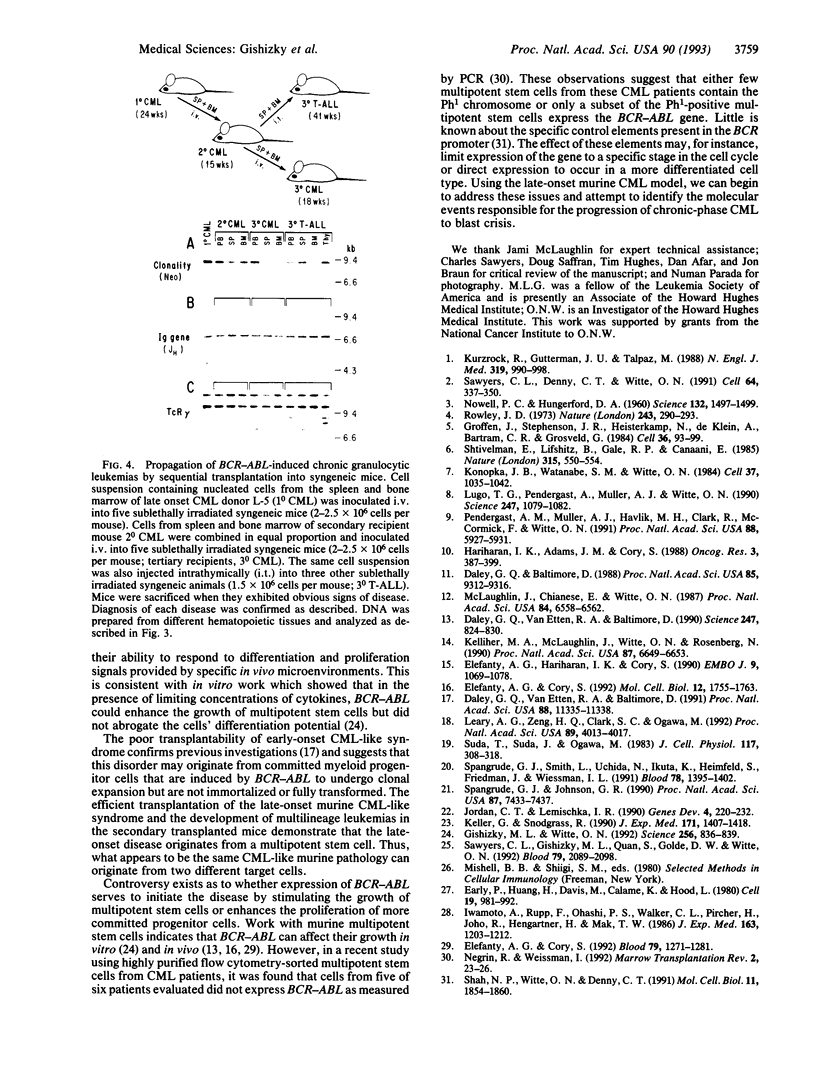
Lethally irradiated mice reconstituted with bone marrow expressing P210 BCR-ABL can develop myeloproliferative syndromes that resemble the initial phase of human chronic myelogenous leukemia (CML). Mice that develop the CML-like syndrome can be segregated into two groups based on the latency with which the granulocytic disease appears--early onset (< 20 weeks) and late onset (> 20 weeks). Only cells from mice exhibiting the late-onset CML-like syndrome can efficiently propagate the disease when transplanted into sublethally irradiated syngeneic recipients. Mice engrafted with late-onset murine CML cells develop a range of hematopoietic disorders that originate from multipotent stem cells. The chronic granulocytic hyperplasia can be propagated by serial transplantation into secondary and tertiary recipient mice. The majority of transplanted mice succumb to acute myeloid and B- and T-lymphoid leukemias. These data support the idea that late-onset murine CML originates from a multipotent progenitor cell with a high replicating capacity. The inability to transplant the disease from mice developing the early-onset CML-like syndrome suggests that this disorder may originate from more differentiated progenitor cells with limited replication capacity that have undergone clonal expansion but are not immortalized. Although both early- and late-onset CML-like syndromes exhibit granulocytic hyperplasia, these disorders represent distinct diseases that appear to originate from different hematopoietic cell types. The late-onset CML-like disease and transfer to secondary recipients provides a useful murine model with features of the chronic and acute phases of human CML.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Blast crisis in a murine model of chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11335–11338. doi: 10.1073/pnas.88.24.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Elefanty A. G., Cory S. Hematologic disease induced in BALB/c mice by a bcr-abl retrovirus is influenced by the infection conditions. Mol Cell Biol. 1992 Apr;12(4):1755–1763. doi: 10.1128/mcb.12.4.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefanty A. G., Cory S. bcr-abl-Induced cell lines can switch from mast cell to erythroid or myeloid differentiation in vitro. Blood. 1992 Mar 1;79(5):1271–1281. [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gishizky M. L., Witte O. N. Initiation of deregulated growth of multipotent progenitor cells by bcr-abl in vitro. Science. 1992 May 8;256(5058):836–839. doi: 10.1126/science.1375394. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990 May 1;171(5):1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Gutterman J. U., Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988 Oct 13;319(15):990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Zeng H. Q., Clark S. C., Ogawa M. Growth factor requirements for survival in G0 and entry into the cell cycle of primitive human hemopoietic progenitors. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4013–4017. doi: 10.1073/pnas.89.9.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Pendergast A. M., Muller A. J., Witte O. N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990 Mar 2;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast A. M., Muller A. J., Havlik M. H., Clark R., McCormick F., Witte O. N. Evidence for regulation of the human ABL tyrosine kinase by a cellular inhibitor. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5927–5931. doi: 10.1073/pnas.88.13.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Gishizky M. L., Quan S., Golde D. W., Witte O. N. Propagation of human blastic myeloid leukemias in the SCID mouse. Blood. 1992 Apr 15;79(8):2089–2098. [PubMed] [Google Scholar]
- Shah N. P., Witte O. N., Denny C. T. Characterization of the BCR promoter in Philadelphia chromosome-positive and -negative cell lines. Mol Cell Biol. 1991 Apr;11(4):1854–1860. doi: 10.1128/mcb.11.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Johnson G. R. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Smith L., Uchida N., Ikuta K., Heimfeld S., Friedman J., Weissman I. L. Mouse hematopoietic stem cells. Blood. 1991 Sep 15;78(6):1395–1402. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]