Abstract
Cancer stem cells (CSCs) drive tumor initiation and metastasis in several types of human cancer. However, the contribution of ovarian CSCs to peritoneal metastasis remains unresolved. The cell adhesion molecule CD44 has been identified as a major marker for CSCs in solid tumors, including epithelial ovarian cancer. CD44 exists as a standard form (CD44s) and also as numerous variant isoforms (CD44v) generated by alternative mRNA splicing. Here we show that disseminated ovarian tumors in the pelvic peritoneum contain highly enriched CD44v6-positive cancer cells, which drive tumor metastasis and are responsible for tumor resistance to chemotherapy. Clinically, an increased number of CD44v6-positive cancer cells in primary tumors was associated with a shortened overall survival in stage III–IV ovarian cancer patients. Furthermore, a subpopulation of CD44v6-positive cancer cells manifested the ability to initiate tumor metastasis in the pelvic peritoneum in an in vivo mouse model, suggesting that CD44v6-positive cells show the potential to serve as metastasis-initiating cells. Thus, the peritoneal disseminated metastasis of epithelial ovarian cancer is initiated by the CD44v6-positive subpopulation, and CD44v6 expression is a biomarker for the clinical outcome of advanced ovarian cancer patients. Given that a distinct subpopulation of CD44v6-positive cancer cells plays a critical role in peritoneal metastasis, definitive treatment should target this subpopulation of CD44v6-positive cells in epithelial ovarian cancer.
Keywords: Cancer stem cell, CD44, ovarian cancer, peritoneal metastasis, prognosis
Epithelial ovarian cancer is the leading cause of death from gynecological malignancies.1 Because most patients with ovarian malignancies are generally asymptomatic until the cancer has progressed and metastasized, more than two-thirds of tumors are diagnosed at an advanced stage with multiple disseminated tumors in the pelvic peritoneum.2 The clinical outcomes for women diagnosed with advanced epithelial ovarian cancer are poor even after treatment with extirpative surgery and proper chemotherapy. Although the cancer may respond to primary therapy, chemoresistant residual cancer cells can persist in a dormant state for many months in the pelvic peritoneum, leading to relapse.3,4 Therefore, elucidating the molecular events that control peritoneal metastasis may provide potential molecular targets for the treatment of advanced epithelial ovarian cancer with multiple peritoneal disseminated tumors.
Cell adhesion molecule CD44 is a polymorphic integral membrane glycoprotein that binds hyaluronic acid and contributes to tumor growth, invasion, and metastasis.5–7 CD44 exists as a standard form (CD44s) and also as numerous variant isoforms (CD44v) generated by alternative mRNA splicing of up to 10 variant exons that encode parts of the extracellular domain.6–10 Among CD44v isoforms, CD44v6 was initially found to promote the metastatic potential of a rat pancreatic adenocarcinoma cell line.11 Furthermore, several previous studies supported the premise that CD44v6 plays a key role in cancer proliferation, migration, and invasion in a variety of human cancers, such as colorectal, breast, lung, and ovarian cancer.12–15 In epithelial ovarian cancer, it is known that CD44v6 promotes tumor metastasis by binding hyaluronic acid on peritoneal mesothelial cells.16
In recent history, the cancer stem cell (CSC) theory has proposed that the bulk of tumor cells are generated by a rare population of tumor-initiating cells, conceptually termed CSCs.17–19 CSCs possess the ability to self-renew and differentiate into a heterogeneous lineage of cancer cells and inherently drive the metastatic process.18,20 CD44 has been identified as one of the major cell surface markers associated with CSCs in several types of epithelial tumors, including ovarian cancer.6,21–23 Intriguingly, recent studies indicated that a subpopulation of CD44v6-positive cells shows a characteristic phenotype of CSCs in colorectal cancer, bladder cancer, and brain tumor.24–26 These findings led us to hypothesize that CD44v6-positive ovarian cancer cells may possess CSC traits and play a key role in tumor initiation and disseminated metastasis.
Uncovering the molecular mechanisms underlying peritoneal metastasis is the final frontier in ovarian cancer biology. Even though ovarian CSCs have not been fully elucidated, these cells are thought to play a crucial role in disseminated metastasis and relapse at peritoneal metastatic sites.27 The present study was designated to evaluate the role of CD44v6 in peritoneal disseminated metastasis and the potential relevance of CD44v6 to the clinical outcome of patients with advanced epithelial ovarian cancer with long-term follow-up.
Materials and Methods
Patients and tissue preparation
From January 2002 to December 2012, the clinical records of stage III–IV epithelial ovarian cancer patients were reviewed retrospectively, and 59 patients with peritoneal disseminated tumors who underwent primary standard surgery followed by proper chemotherapy at Kumamoto University Hospital were included in this study. Patients were excluded when they had borderline tumors, multiple primary cancers, or non-epithelial tumors. The eligible patients were followed-up until December 2014. Written informed consent was obtained from all patients before treatment, in accordance with the institutional guidelines of our hospital.
Tumor tissues obtained surgically were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 4-μm thickness for histological diagnosis. Sections were stained with H&E, and histologic typing was carried out according to the WHO classification of surface epithelial–stromal ovarian tumors.28 All tumors were staged according to the International Federation of Gynecology and Obstetrics criteria.29
Evaluation of immunohistochemical staining
Immunohistochemical analysis was carried out as described previously.30 Briefly, the sections were washed with PBS, subjected to antigen retrieval by heating in a microwave in 0.01 M sodium citrate buffer (pH 6.0) for 15 min, and exposed to 3% H2O2 in methanol before staining with the primary antibody. Immune complexes were detected with use of the avidin–biotin–peroxidase complex (ABC kit; Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine substrate (Vector Laboratories), and the sections were counterstained with hematoxylin. CD44v6 was detected with the mouse mAb CD44v6 (2F10; R&D Systems, Minneapolis, MN, USA). The expression level of CD44v6 was quantified as a percentage of the total number of stained cells. The primary ovarian tumors that contained at least 10% CD44v6-positive cancer cells were categorized as the “CD44v6-high” group, whereas the tumors that contained less than 10% CD44v6-positive cells were categorized as the “CD44v6-low” group. The percentage of CD44v6-positive cancer cells in primary tumors was evaluated by counting cells in at least three microscopic fields per slide.
Mice
BALB/c nude mice were obtained from CLEA (Tokyo, Japan) and maintained according to institutional guidelines. All animal experiments were carried out in accordance with protocols approved by the animal ethics committee of Kumamoto University.
Cell line
A human ovarian cancer cell line, ES-2, was obtained from ATCC (Manassas, VA, USA). ES-2 cells were maintained in RPMI-1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FBS at 37°C in a 5% CO2-containing atmosphere.
Flow cytometry and transplantation assay
Cell sorting and flow cytometric analysis were carried out with the use of a FACS Aria II (BD Biosciences, San Jose, CA, USA). Cells were incubated with allophycocyanin-conjugated mouse mAb CD44v6 (2F10; R&D Systems) and phycoerythrin-conjugated rat mAb CD44 (IM7; BioLegend, San Diego, CA, USA) for 30 min. The FACS-sorted CD44v6-positive or -negative cancer cells were suspended in RPMI-1640 medium and injected i.p. into 7-week-old female BALB/c nude mice. Tumor-initiating frequencies were assessed with the use of ELDA software for limiting dilution analysis.31
Immunoblot analysis
Immunoblot analysis was carried out as previously described.27 In brief, equal amounts of cell lysate protein were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to anti-CD44v6 antibody (VFF-18; Abcam, Cambridge, UK), anti-E-cadherin (36/E-cadherin; BD Biosciences), anti-N-cadherin (32/N-cadherin; BD Biosciences), anti-fibronectin (10/fibronectin; BD Biosciences), anti-vimentin (V9; DakoCytomation, Glostrup, Denmark), and anti-β-actin (13E5; Cell Signaling Technology, Beverly, MA, USA). Immune complexes were visualized by chemiluminescence detection (Pierce Biotechnology, Rockford, IL, USA).
Proliferation and chemosensitivity assay
Cell viability was assessed with MTS assay according to the manufacturer’s protocol (CellTiter 96 Aqueous One Solution Cell Proliferation assay; Promega, Madison, WI, USA). Briefly, cells (3 × 103/100 μL per well) were plated in 96-well flat bottom plates and serum starved overnight. Ovarian cancer cells were treated with paclitaxel or cisplatin at the indicated concentrations. At 12 h post-drug treatment, 20 μL MTS assay solution was added to each well for 2 h. Absorbance was recorded at 490 nm on an SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Experiments were carried out in triplicate and repeated three times and the percentage of cell survival was defined as the relative absorbance of untreated versus treated cells.
Statistical analysis
The prognosis of patients was determined according to the cumulative survival rate after treatment. Survival rates were calculated using the Kaplan–Meier method, and differences between curves were assessed with the log–rank test. Correlations between variables were evaluated with the χ2-test, Fisher’s exact test, Mann–Whitney U-test, or Wilcoxon test. Data are presented as mean ± SD and were analyzed with the Student’s t-test. Univariate and multivariate Cox proportional hazard model analyses were carried out to calculate hazard ratios (HRs) using SPSS version 21.0 (IBM, Armonk, NY, USA). In all analyses, a P-value of <0.05 was considered statistically significant.
Results
Correlation between CD44v6 expression pattern and clinicopathological features in patients with stage III–IV epithelial ovarian cancer
The association between CD44v6 expression and the clinicopathological characteristics of the 59 patients is shown in Table1. Thirteen (22.0%) cases belonged in the CD44v6-high group, and 46 (78.0%) cases to the CD44v6-low group. The median age of all patients at diagnosis was 57 years (range, 37–82 years). There were no significant differences in the median age between the CD44v6-high and CD44v6-low groups. In addition, no significant correlation was observed between the immunohistochemical (IHC) expression of CD44v6 and clinicopathological characteristics, such as tumor histological type, tumor marker CA125, and tumor size. Adjuvant systematic chemotherapy was given as clinically indicated in accordance with standard practices, and almost all patients (57/59, 96.6%) received paclitaxel–carboplatin as first-line adjuvant chemotherapy. No significant differences were recorded in the distribution of the number of cycles of chemotherapy between CD44v6-high and CD44v6-low groups (Table1).
Table 1.
Association between CD44 variant 6 (CD44v6) expression pattern and clinicopathological characteristics in patients with stage III–IV ovarian cancer
| All cases, n (%) | CD44v6-high group, n (%) | CD44v6-low group, n (%) | P-value | |
|---|---|---|---|---|
| All cases | 59 | 13 | 46 | |
| Median age, years (range) | 57 (37–82) | 59 (43–82) | 56 (37–77) | 0.84 |
| Age, years | ||||
| <50 | 18 (30.5) | 3 (23.1) | 15 (32.6) | 0.51 |
| ≥50 | 41 (69.5) | 10 (76.9) | 31 (67.4) | |
| Histological type | ||||
| Serous | 42 (71.2) | 7 (53.8) | 35 (76.1) | 0.12 |
| Clear | 3 (5.1) | 2 (15.4) | 1 (2.2) | |
| Endometrioid | 5 (8.5) | 1 (7.7) | 4 (8.7) | |
| Mucinous | 1 (1.7) | 1 (7.7) | 0 (0.0) | |
| Mixed | 7 (11.8) | 1 (7.7) | 6 (13.0) | |
| Undifferentiated | 1 (1.7) | 1 (7.7) | 0 (0.0) | |
| CA125, U/mL | ||||
| <500 | 18 (30.5) | 6 (46.2) | 12 (26.1) | 0.16 |
| ≥500 | 41 (69.5) | 7 (53.8) | 34 (73.9) | |
| Tumor size, cm | ||||
| <10 | 40 (67.8) | 7 (53.8) | 33 (71.7) | 0.22 |
| ≥10 | 19 (32.2) | 6 (46.2) | 13 (28.3) | |
| First-line chemotherapy regmen | ||||
| Paclitaxel/carboplatin | 57 (96.6) | 12 (92.3) | 45 (97.8) | 0.33 |
| Other | 2 (3.4) | 1 (7.7) | 1 (2.2) | |
| No. of cycles of chemotherapy | ||||
| <2 | 41 (69.5) | 8 (61.5) | 33 (71.7) | 0.48 |
| ≥3 | 18 (30.5) | 5 (38.5) | 13 (28.3) | |
Highly enriched CD44v6-positive ovarian cancer cells in peritoneal disseminated tumors
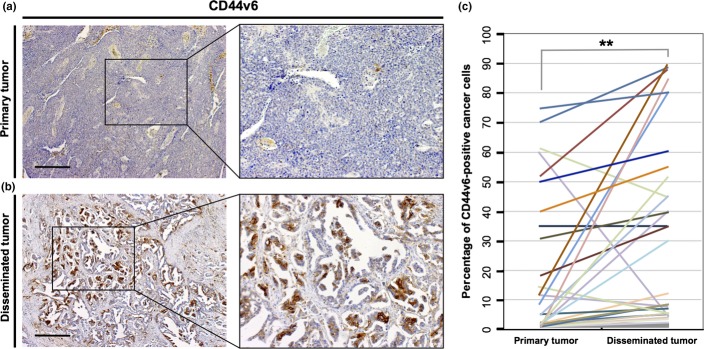
To investigate whether CD44v6-positive cancer cells are associated with peritoneal metastasis, we compared the average number of CD44v6-positive cells among the 59 samples of primary ovarian tumors to that in samples of peritoneal disseminated tumors taken from the same patients. Representative IHC staining patterns for CD44v6 in primary and disseminated tumors are shown in Figure 1(a,b). Immunohistochemical analysis revealed a significantly higher percentage of CD44v6-positive cells detected in peritoneal disseminated tumors than in corresponding primary ovarian tumors (P < 0.01; Fig. 1c). These findings indicated that CD44v6-positive cells are correlated with peritoneal dissemination, and the pelvic peritoneum may have the potential to form a part of the niche microenvironment involved in tumor initiation and metastasis.
Figure 1.
Disseminated ovarian tumors in the pelvic peritoneum contain highly enriched CD44 variant 6 (CD44v6)-positive cancer cells. (a) Immunohistochemical analysis with an anti-CD44v6 antibody in primary epithelial ovarian tumors. Scale bar = 500 μm. (b) Immunohistochemical staining with an anti-CD44v6 antibody in peritoneal disseminated tumors. Scale bar = 500 μm. (c) The percentage of CD44v6-positive cancer cells in primary and disseminated tumors. Peritoneal disseminated tumors contained significantly higher percentages of CD44v6-positive cells than primary tumors (Mann–Whitney U-test, **P < 0.01).
Prognostic impact of CD44v6 expression in advanced epithelial ovarian cancer patients
Given that a subpopulation of CD44-positive cancer cells in hierarchically organized ovarian cancer manifests CSC properties,21 we hypothesized that CD44v6 expression would correlate with aspects of ovarian cancer survival. To address this issue, we used Kaplan–Meier analyses of overall survival (OS) and progression-free survival (PFS) between the CD44v6-high and CD44-low groups. Representative IHC staining patterns for CD44v6 in CD44-high and CD44-low groups are shown in Figure 2(a,b). In the evaluation of the sites of primary lesions, the 5-year OS rates were 18.0% (95% confidence interval [CI], 0.0–40.2) in the CD44-high group and 59.6% (95% CI, 44.3–74.8) in the CD44-low group. Significant differences were observed in OS between the CD44v6-high and CD44v6-low groups for patients with stage III–IV ovarian cancer (P = 0.0059; Fig. 2c). In contrast, no significant differences were observed in PFS between the CD44v6-high and CD44v6-low groups (P = 0.4290; Fig. 2d). These findings suggested that CD44v6-positive cancer cells in primary tumors play an important role in the survival of advanced ovarian cancer patients.
Figure 2.
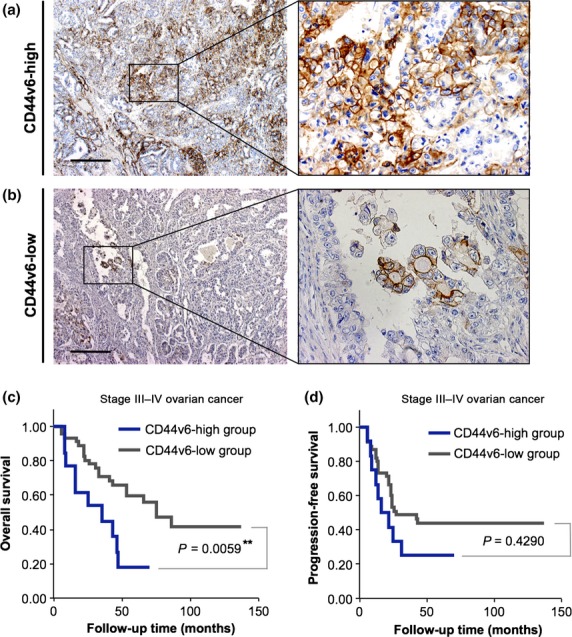
CD44 variant 6 (CD44v6) expression predicts epithelial ovarian cancer survival. (a) Immunohistochemical analysis with an anti-CD44v6 antibody in primary epithelial ovarian tumors. The tumors that contained at least 10% CD44v6-positive cancer cells were categorized as the CD44v6-high group. Scale bar = 500 μm. (b) Immunohistochemical staining with an anti-CD44v6 antibody in primary tumors. The tumors that contained less than 10% CD44v6-positive cancer cells were categorized as the CD44v6-low group. Scale bar = 500 μm. (c) Kaplan–Meier analysis of overall survival in patients with stage III–IV ovarian cancer according to the expression of CD44v6. There were significant differences in overall survival between the CD44v6-high and CD44v6-low groups (**P = 0.0059). (d) Kaplan–Meier analysis of progression-free survival in patients with stage III–IV ovarian cancer according to the expression of CD44v6. Progression-free survival was not significantly different between the CD44v6-high and CD44v6-low groups (P = 0.4290).
Univariate and multivariate analysis of various clinicopathological factors in relation to OS are shown in Table2. Immunohistochemical expression of CD44v6 proved to be a highly predictive factor based on the univariate Cox proportional hazards model (P = 0.007; HR, 2.930; 95% CI, 1.334–6.436) and the multivariate Cox proportional hazards model (P = 0.022; HR, 2.568; 95% CI, 1.149–5.738). In addition, surgical debulking status also significantly correlated with OS based on the univariate Cox proportional hazards model (P = 0.011; HR, 2.568; 95% CI, 1.247–5.288) and the multivariate Cox proportional hazards model (P = 0.028; HR, 2.283; 95% CI, 1.091–4.775).
Table 2.
Hazard ratios (HRs) using univariate and multivariate Cox proportional hazard model
| Prognostic factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age, years | ||||||
| <50 | ||||||
| ≥50 | 1.286 | 0.574–2.879 | 0.542 | |||
| CA125, U/mL | ||||||
| <500 | ||||||
| ≥500 | 1.060 | 0.487–2.306 | 0.884 | |||
| Tumor size, cm | ||||||
| <10 | ||||||
| ≥10 | 1.063 | 0.498–2.267 | 0.874 | |||
| First-line chemotherapy | ||||||
| Paclitaxel/carboplatin | ||||||
| Other | 0.905 | 0.122–6.727 | 0.923 | |||
| Surgical debulking status | ||||||
| Optimal surgery (Residual tumor size <1 cm) | ||||||
| Suboptimal surgery (Residual tumor size ≥1 cm) | 2.568 | 1.247–5.288 | 0.011 | 2.283 | 1.091–4.775 | 0.028 |
| CD44v6 expression | ||||||
| Low | ||||||
| High | 2.930 | 1.334–6.436 | 0.007 | 2.568 | 1.149–5.738 | 0.022 |
CD44v6, CD44 variant 6; CI, confidence interval; High,; Low,.
High metastatic ability in a subpopulation of CD44v6-positive ovarian cancer cells
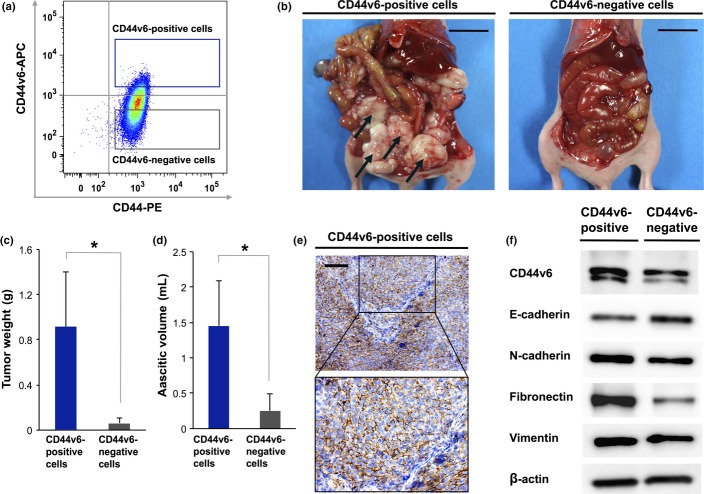
Given that CD44v6-positive cancer cells showed high metastatic potential in patients with advanced ovarian cancer, we next examined the relevance of peritoneal metastasis in a subpopulation of CD44v6-positive cells in an in vivo mouse model. To compare the peritoneal metastatic abilities of CD44v6-positive and CD44v6-negative cancer cells, we sorted CD44v6-positive and CD44v6-negative cells from the ES-2 ovarian cancer cell line (Fig. 3a) and serially transplanted them intraperitoneally into nude mice. Limiting dilution assay revealed that CD44v6-positive cells had a greater tumor initiating ability than CD44v6-negative cells, suggesting that a subpopulation of CD44v6-positive cells is highly efficient at metastatic dissemination (Table3). The CD44v6-positive cells generated extensive disseminated tumors, resulting in massive abdominal distension by hemorrhagic ascites, within 5 weeks of inoculation, whereas CD44v6-negative cells showed little ability to form disseminated tumors in the peritoneal cavity (Fig. 3b). The total weight of peritoneal disseminated tumors formed by CD44v6-positive cells was significantly greater than that of those formed by CD44v6-negative cells (P < 0.05; Fig. 3c). In addition, transplantation of CD44v6-positive cells caused a significant increase in the ascitic volume in comparison with that resulting from transplantation of CD44v6-negative cells (P < 0.05; Fig. 3d). A representative IHC staining pattern for CD44v6 in peritoneal disseminated tumors generated by CD44v6-positive cancer cells is shown in Figure 3(e). These results suggested that CD44v6-positive cells play a crucial role in the formation of disseminated tumors in the pelvic peritoneum and have the potential to contain specialized metastasis-initiating cells.
Figure 3.
Subpopulation of CD44 variant 6 (CD44v6)-positive ovarian cancer cells possesses a high peritoneal metastatic ability. (a) Flow cytometric analysis of CD44v6 expression in ES-2 ovarian cancer cells. (b) Macroscopic appearance of disseminated tumors at 35 days after cell transplantation. CD44v6-positive cells generated more extensive disseminated tumors in the peritoneal cavity than CD44v6-negative cells. Scale bar = 2 cm. (c) Total weight of peritoneal disseminated tumors determined at 35 days after cell injection. Quantitative data are presented as mean ± SD for five mice. *P < 0.05. (d) Ascitic volume determined at 35 days after transplantation. Quantitative data are presented as mean ± SD for five mice. *P < 0.05. (e) Immunohistochemical analysis with antibody to CD44v6 in peritoneal disseminated tumors in a mouse model. Paraffin-embedded sections of disseminated tumors generated by CD44v6-positive cancer cells were subjected to immunohistochemical staining with an anti-CD44v6 antibody. Scale bar = 200 μm. (f) Western blot analysis of CD44v6 and epithelial–mesenchymal transition regulatory proteins, including E-cadherin, N-cadherin, fibronectin, and vimentin in FACS-sorted CD44v6-poisitive cells versusFACS-sorted CD44v6-negative cells.
Table 3.
In vivo tumorigenicity of CD44 variant 6 (CD44v6)-positive and CD44v6-negative cells
| No. of transplanted cells | Frequency of metastasis-initiating cells (95% CI) | |||
|---|---|---|---|---|
| 10, 000 | 1000 | 100 | ||
| CD44v6-positive cells | 6/6 | 6/6 | 4/5 | 62.6**(21.4–185.0) |
| CD44v6-negative cells | 3/12 | 1/12 | 0/12 | 29, 211.2(10, 813.7–78, 910.0) |
CD44v6-positive and -negative cancer cells were separated by FACS, and the indicated numbers of cells were transplanted intraperitoneally into nude mice. The incidence of tumor formation within 8 weeks was scored. Data represent the number of tumors per number of injections. Tumorigenic cell frequencies were estimated with the use of ELDA software for limiting dilution analysis.
P < 0.01.
Epithelial–mesenchymal transition (EMT) is an important step in invasion and metastasis of cancer.32 When the ovarian cancer cells detach and start their metastatic journey, it is believed that they frequently undergo EMT.3 We therefore hypothesized that CD44v6 has an important role in the EMT phenomenon of ovarian cancer. To investigate the relationship between CD44v6 expression and EMT, we evaluated the expression of EMT regulatory proteins, such as E-cadherin, N-cadherin, fibronectin, and vimentin in FACS-sorted CD44v6-poisitive cells versus FACS-sorted CD44v6-negative cells by Western blot analysis. In consequence, E-cadherin expression was downregulated in FACS-sorted CD44v6-positive cells in comparison with FACS-sorted CD44v6-negative cells and concomitant upregulation of N-cadherin, fibronectin, and vimentin was observed in CD44v6 - positive cells (Fig. 3f). These findings suggested that a subpopulation of CD44v6 regulates the metastatic ability of ovarian cancer cells, which is relevant to the process of EMT.
Chemoresistance in a subpopulation of CD44v6-positive ovarian cancer cells
CSCs are inherently responsible for tumor resistance to conventional chemotherapy.17,18 Given that the primary ovarian tumors containing at least 10% CD44v6-positive cancer cells showed significantly poorer prognosis, we next evaluated the relevance of chemoresistance in CD44v6-positive cells as a potential cause of the poor prognosis. To investigate whether the subpopulation of CD44v6-positive cells correlates with resistance to chemotherapy, ES-2 ovarian cancer cells were exposed to paclitaxel or cisplatin in vitro. Flow cytometric analysis showed that treatment with paclitaxel or cisplatin results in enhanced expression of CD44v6 in residual cancer cells as compared to untreated cells (Fig. 4a,b). Furthermore, FACS-sorted CD44v6-positive ovarian cancer cells showed significantly higher viability compared to FACS-sorted CD44v6-negative cells in MTS assay (Fig. 4c,d), indicating that a subpopulation of CD44v6-positive cells is associated with tumor resistance to chemotherapy.
Figure 4.
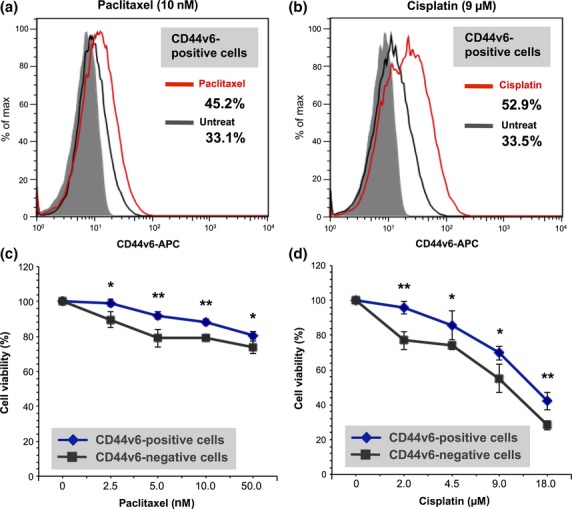
CD44 variant 6 (CD44v6)-positive ovarian cancer cells are associated with chemoresistance. (a) Flow cytometric analysis of CD44v6 expression in ES-2 ovarian cancer cells treated with paclitaxel and untreated ES-2 cells. (b) Flow cytometric analysis of CD44v6 expression in ES-2 ovarian cancer cells treated with cisplatin and untreated ES-2 cells. (c) Chemosensitivity assay in FACS-sorted CD44v6-positive and FACS-sorted CD44v6-negative cells. Cells were subjected to MTS assay to evaluate viability in the presence of paclitaxel. *P < 0.05, **P < 0.01. (d) Chemosensitivity assay. FACS-sorted CD44v6-positive and FACS-sorted CD44v6-negative cells were subjected to MTS assay to assess the viability in the presence of cisplatin. *P < 0.05, **P < 0.01.
Discussion
We have identified that disseminated tumors in the pelvic peritoneum are highly enriched in CD44v6-positive cancer cells, which prominently contributes to peritoneal metastasis of advanced epithelial ovarian cancer. Of particular interest in this study was that an increased number of CD44v6-positive cancer cells were associated with a shortened OS in the evaluation of the sites of primary tumors. Furthermore, we showed that a subpopulation of CD44v6-positive ovarian cancer cells possesses a strong ability to initiate tumor metastasis in the pelvic peritoneum in an in vivo mouse model, indicating that CD44v6-positive cells have the potential to serve as metastasis-initiating cells.
Epithelial ovarian cancer is a highly lethal malignancy that represents a great clinical challenge in gynecologic oncology.4 Given that peritoneal dissemination and metastasis is responsible for most cancer-related deaths in patients with advanced ovarian cancer, the elucidation of molecular mechanisms underlying the peritoneal metastasis and the characteristics of ovarian CSCs is essential to combat this fatal disease. Although CD44v6 plays an important role in the tumor growth and metastasis of several types of tumors,24,25 the functions of CD44v6 have not been completely characterized in ovarian cancer metastasis. In the current study, we showed that CD44v6 expression is increased in tumor tissues at the peritoneal metastasis sites compared with those at the corresponding primary tumors, indicating that CD44v6 is clinically associated with the induction of metastasis in the pelvic peritoneum.
Although previous studies have focused on the potential correlation of CD44v with ovarian cancer survival to address the diagnostic and prognostic values of CD44v, there is no unified view on this issue.33 Some authors suggested that the expression of the CD44v6 is not correlated with tumor development and prognosis of epithelial ovarian cancer,12,34 whereas others showed that CD44v6 expression levels are involved in ovarian cancer progression, metastasis, and relapse.35 Taken together, several questions regarding the relationship between CD44v6 expression and prognosis remain to be resolved. In the light of these unanswered questions, we evaluated the association between CD44v6 expression and OS and PFS in the sites of primary lesions. As a result, the tumors containing at least 10% CD44v6-positive cancer cells showed significantly poorer prognosis in terms of OS than those containing less than 10% CD44v6-positive cells in the evaluation of the sites of primary tumors. Furthermore, the multivariate Cox proportional hazards model showed that the expression of CD44v6 is an independent prognostic factor for the OS of patients with advanced ovarian cancer.
In recent years, emerging evidence has provided support for the existence of CSCs in various cancers, including epithelial ovarian cancer.17,20 Even though previous studies indicated that a CD44v6-positive cell population possesses CSC properties in several types of tumors,24,25 the correlation between CD44v6-positive cells and ovarian CSCs remained unclear. To investigate whether a subpopulation of CD44v6-positive cancer cells manifest highly metastatic activity, we compared the tumorigenic and peritoneal metastatic potential of CD44v6-positive and CD44v6-negative cells in an in vivo mouse model. Consistent with our clinical observations, we found that a subpopulation of CD44v6-positive cells is prominently involved in peritoneal metastasis in a mouse model. In a set of experiments, we also showed that CD44v6 expression demarcates a highly tumorigenic ovarian CSC population with peritoneal metastatic potential and CD44v6-positive cells possess the potential to serve as metastasis-initiating cells. Recent evidence indicates the existence of a “CSC niche,” a specialized microenvironment that regulates CSC properties and contributes to tumor initiation, growth, and metastasis.36,37 The present study revealed the close relationship between CD44v6 expression and the pelvic peritoneum and thereby, raises the possibility that the microenvironment of the pelvic peritoneum forms a possible CSC niche for epithelial ovarian cancer.38
Recent evidence suggested that CD44v manifests enhanced protection against species (ROS), rendering them resistant to chemotherapy in several types of solid tumors.22,23 In the current study, we showed that a subpopulation of CD44v6-positive cancer cells is correlated with tumor resistance to chemotherapy. In view of this, the results of our present study raise the possibility that CD44v6 potentiates the ability of ovarian cancer cells to defend themselves against chemotherapy-induced ROS.
In conclusion, the biological and molecular heterogeneity of ovarian CSCs represents a highly promising area of research that may provide new insights that could lead to prognostic and therapeutic breakthroughs for advanced epithelial ovarian cancer. CD44v6-positive cancer cells may be a potential molecular therapeutic target for eliminating ovarian CSCs and metastasis-initiating cells. The finding that a distinct subpopulation of CD44v6-positive CSCs plays a central role in peritoneal metastasis suggests that definitive treatment should target the CD44v6-positive cell population in epithelial ovarian cancer.
Acknowledgments
We thank A. Aoki for technical assistance and Y. Hisako, K. Kawaguchi, S. Miyaji, and R. Tanaka for help in the preparation of the manuscript.
Disclosure Statement
The authors have no conflict of interest.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–81. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- Tanabe KK, Nishi T, Saya H. Novel variants of CD44 arising from alternative splicing: changes in the CD44 alternative splicing pattern of MCF-7 breast carcinoma cells treated with hyaluronidase. Mol Carcinog. 1993;7:212–20. doi: 10.1002/mc.2940070403. [DOI] [PubMed] [Google Scholar]
- Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–74. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe KK, Saya H. The CD44 adhesion molecule and metastasis. Crit Rev Oncog. 1994;5:201–12. doi: 10.1615/critrevoncog.v5.i2-3.50. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Tsuchihashi K, Ishimoto T, Yae T, Motohara T, Sugihara E, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–66. doi: 10.1158/0008-5472.CAN-12-3609-T. [DOI] [PubMed] [Google Scholar]
- Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Sliutz G, Tempfer C, Winkler S, Kohlberger P, Reinthaller A, Kainz C. Immunohistochemical and serological evaluation of CD44 splice variants in human ovarian cancer. Br J Cancer. 1995;72:1494–7. doi: 10.1038/bjc.1995.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, et al. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–55. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afify AM, Tate S, Durbin-Johnson B, Rocke DM, Konia T. Expression of CD44s and CD44v6 in lung cancer and their correlation with prognostic factors. Int J Biol Markers. 2011;26:50–7. doi: 10.5301/jbm.2011.6291. [DOI] [PubMed] [Google Scholar]
- Kamura T, Sakai K, Kaku T, Kobayashi H, Mitsumoto M, Tsuneyoshi M, et al. Comparison of p53 and CD44 variant 6 expression between paired primary and recurrent ovarian cancer: an immunohistochemical analysis. Oncol Rep. 1999;6:97–101. doi: 10.3892/or.6.1.97. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Catterall JB, Jones LM, Turner GA. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis. 1996;14:325–34. doi: 10.1007/BF00123391. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–28. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–56. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Jijiwa M, Demir H, Gupta S, Leung C, Joshi K, Orozco N, et al. CD44v6 regulates growth of brain tumor stem cells partially through the AKT-mediated pathway. PLoS ONE. 2011;6:e24217. doi: 10.1371/journal.pone.0024217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Chang JW. Bladder cancer initiating cells (BCICs) are among EMA-CD44v6 + subset: novel methods for isolating undetermined cancer stem (initiating) cells. Cancer Invest. 2008;26:725–33. doi: 10.1080/07357900801941845. [DOI] [PubMed] [Google Scholar]
- Motohara T, Masuko S, Ishimoto T, Yae T, Onishi N, Muraguchi T, et al. Transient depletion of p53 followed by transduction of c-Myc and K-Ras converts ovarian stem-like cells into tumor-initiating cells. Carcinogenesis. 2011;32:1597–606. doi: 10.1093/carcin/bgr183. [DOI] [PubMed] [Google Scholar]
- Tavassoli FA, Devilee P World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. [Google Scholar]
- Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. Int J Gynaecol Obstet. 2003;83:135–66. doi: 10.1016/s0020-7292(03)90118-4. [DOI] [PubMed] [Google Scholar]
- Motohara T, Tashiro H, Miyahara Y, Sakaguchi I, Ohtake H, Katabuchi H. Long-term oncological outcomes of ovarian serous carcinomas with psammoma bodies: a novel insight into the molecular pathogenesis of ovarian epithelial carcinoma. Cancer Sci. 2010;101:1550–6. doi: 10.1111/j.1349-7006.2010.01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–8. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Unternaehrer JJ, Zhao R, Kim K, Cesana M, Powers JT, Ratanasirintrawoot S, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SC, Song JY, Lee JK, Lee NW, Kim SH, Yeom BW, et al. Significance of CD44v6 expression in gynecologic malignancies. J Obstet Gynaecol Res. 2006;32:379–86. doi: 10.1111/j.1447-0756.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Sakai K, Kaku T, Kamura T, Kinukawa N, Amada S, Shigematsu T, et al. Comparison of p53, Ki-67, and CD44v6 expression between primary and matched metastatic lesions in ovarian cancer. Gynecol Oncol. 1999;72:360–6. doi: 10.1006/gyno.1998.5266. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhou Z, Di W, Li N. Correlation of CD44v6 expression with ovarian cancer progression and recurrence. BMC Cancer. 2013;13:182. doi: 10.1186/1471-2407-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Mitsui H, Shibata K, Suzuki S, Umezu T, Mizuno M, Kajiyama H, et al. Functional interaction between peritoneal mesothelial cells and stem cells of ovarian yolk sac tumor (SC-OYST) in peritoneal dissemination. Gynecol Oncol. 2012;124:303–10. doi: 10.1016/j.ygyno.2011.10.006. [DOI] [PubMed] [Google Scholar]