Abstract
We carried out a phase I/II trial of chemoradiotherapy concurrent with S-1 and cisplatin to determine the maximum tolerated dose and recommended dose and to evaluate the efficacy and safety of this treatment in patients with esophageal carcinoma. Thoracic esophageal cancer patients with clinical stage II/III disease, excluding T4, were eligible. Chemotherapy consisted of S-1 at a dose of 60–80 mg/m2/day on days 1–14, and cisplatin at 75 mg/m2 on day 1, repeated twice every 4 weeks. Single daily radiation of 50.4 Gy was given in 28 fractions concurrently starting on day 1. Patients achieving an objective response after chemoradiotherapy underwent two additional cycles of chemotherapy. Patient accrual was terminated early due to slow enrolment after 44 patients were accrued. In the phase I part, two of six patients experienced dose-limiting toxicities at each level of S-1 (S-1 60 or 80 mg/m2/day). Considering treatment compliance, the recommended dose was determined to be S-1 60 mg/m2/day. The complete response rate, the primary endpoint of phase II, was 59.5% (22/37; 90% confidence interval, 44.6–73.1%; weighted threshold, 57.2%; P = 0.46 by the exact binomial test) on central review. In the phase II part, 3-year progression-free survival was 48.4%, with a 3-year overall survival of 61.9%. Grade 3 or 4 toxicity in phase II included leukopenia (57.9%), neutropenia (50%), hyponatremia (28.9%), anorexia (21.1%), anemia (18.4%), thrombocytopenia (18.4%), and febrile neutropenia (2.6%). No treatment-related deaths were observed. Although this combination showed acceptable toxicity and favorable 3-year survival, the study did not meet its primary endpoint. This trial was registered at the UMIN Clinical Trials Registry as UMIN000000710.
Keywords: Chemoradiotherapy, cisplatin, esophageal carcinoma, S-1, stage II/III
For patients with resectable stage II/III esophageal carcinoma, preoperative chemotherapy with 5-FU plus CDDP is regarded as one of the standards of care in Japan.1 Chemoradiotherapy with concurrent 5-FU plus CDDP is the standard of care for patients with clinical stage II/III esophageal carcinoma who refuse radical surgery.2 Half of these cases will recur and clinical outcomes remain limited, indicating an unmet need for further therapeutic intervention.
S-1 is a novel oral fluoropyrimidine derivative that consists of tegafur, gimeracil, and potassium oxonate.3 S-1 has been approved in Japan for many malignancies, including gastric, lung, breast, colorectal, pancreatic, and head and neck cancers. At the time this study commenced, however, S-1 had not been approved for esophageal carcinoma.
A previous study showed that S-1 had a greater effect on radiosensitivity in human non-small-cell lung cancer xenografts in mice than uracil–ftorafur, which is also an oral fluoropyrimidine derivative but which does not contain CDHP.4,5 5-Chloro-2,4-dihydropyrimidine enhanced radiosensitivity in human lung cancer cells in a dose escalation-dependent manner, suggesting that S-1 might be a more powerful enhancer of radiosensitivity in cancer than 5-FU or uracil–ftorafur. In our previous phase I study for unresectable locally advanced squamous cell carcinoma of the head and neck, CRT given concurrently with S-1 plus CDDP showed highly promising activity with a CR rate of 86%.6
In Japan, approximately 90% of esophageal cancer is histologically squamous cell carcinoma, indicating that this combination could demonstrate promising efficacy.
Here, we carried out a phase I/II trial of CRT with concurrent S-1 and CDDP to determine the MTD and RD for clinical stage II/III esophageal carcinoma. We then evaluated the efficacy and safety of this treatment in patients with esophageal carcinoma. This study was carried out as an investigator-initiated registration trial for label extension of S-1 in the treatment of esophageal carcinoma in Japan.
Patients and Methods
Patients
For inclusion in the study, patients had to fulfill all of the following criteria: histologically proven squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma; primary lesion located at the thoracic esophagus; clinical stage II/III, excluding T4 disease; age between 20 and 75 years; Eastern Cooperative Oncology Group performance status of 0 or 1; no prior treatment for esophageal cancer; no prior chemotherapy or radiotherapy for other cancers; sufficient fluid intake; no bilateral recurrent nerve paralysis; adequate organ function; refusal of esophagectomy; and written informed consent.
Patients were excluded if they had any of the following conditions: simultaneous or metachronous double cancers within 5 years, with the exception of intramucosal tumor curable with local therapy; requirement for treatment with phenytoin or warfarin potassium; pregnant or lactating women or women with potentiality of being pregnant; poorly controlled diabetes mellitus (HbA1c ≥ 6.5%); and any serious complications.
Treatment
The treatment protocol consisted of concurrent CRT and adjuvant chemotherapy. First, patients received concurrent CRT with S-1 plus CDDP. Chemotherapy consisted of oral S-1 twice daily at doses of 40–80 mg/m2/day on days 1–14 and i.v. CDDP at a dose of 75 mg/m2 on day 1, repeated every 4 weeks for two cycles.
Radiation therapy consisted of 50.4 Gy in 28 fractions over 6 weeks, delivered with megavoltage equipment (≥6 MV) using the multiple-field technique. Computed tomography-based 3-D treatment planning was required for all enrolled patients. The clinical target volume included the primary tumor with a 2-cm margin for subclinical craniocaudal extension, metastatic lymph nodes, and regional lymph nodes. The initial PTV was defined as the clinical target volume plus 1–2 cm craniocaudally and 0.5–1 cm circumferentially, with compensation for internal organ motion and daily set-up variations. Among regional lymph nodes, the supraclavicular, upper mediastinal, and subcarinal lymph nodes were irradiated for upper thoracic esophageal cancer. The mediastinal and perigastric lymph nodes were included for tumors of the middle or lower esophagus, to which were added the celiac lymph nodes for primary tumors of the lower esophagus. The boost PTV included the primary tumor and metastatic lymph nodes only, with adequate margins. The initial PTV was irradiated with up to 41.4 Gy in 23 fractions, before a booster dose of 9.0 Gy in 5 fractions was delivered to the boost PTV. Irradiation using three or four portals was strongly recommended for carcinoma of the middle or lower esophagus to avoid excessive dosing to the heart. Correction for lung inhomogeneity was not used in this study. Dose constraints for normal tissues were defined as: spinal cord, <48 Gy; mean heart dose, <40 Gy; and lung, V10 < 50%, V15 < 40%, V20 < 25%.
Copies of pretreatment diagnostic chest radiographs and computed tomography scans, simulation and portal films, worksheets for monitor unit calculation of the prescribed dose, and RT charts were collected for the QA review of RT. Information on the total RT course, including both the initial and boost plans, was to be sent to the radiotherapy support center (Tokyo, Japan) within 7 days of completion of RT. The QA reviews were done at the support center regularly, with feedback sent to each institution by the RT study coordinator (K.N.).
For patients achieving an objective response after CRT, two additional cycles of chemotherapy with S-1 plus CDDP at the same dose level as during CRT were repeated with a 4-week interval, starting 4 weeks after the completion of CRT. When a patient achieved CR after completion of adjuvant chemotherapy, additional treatment was not permitted unless recurrence was observed. When a patient had persistent disease or recurrence after completion of adjuvant chemotherapy, salvage surgery was considered as a post-protocol treatment.
Treatment evaluation and dose modification
Baseline evaluation consisted of history, physical examination, upper gastrointestinal endoscopy, radiographic imaging, routine laboratory studies, and electrocardiogram. Safety assessments were repeated weekly during the protocol treatment. Toxicities were evaluated according to the Common Toxicity Criteria for Adverse Events version 3.0.
Any of the following adverse events observed within 28 days of completion of CRT was deemed a DLT: (i) grade 4 neutropenia persisting 4 days or more; (ii) grade 3 febrile neutropenia persisting 4 days or more; (iii) grade 4 thrombocytopenia; (iv) grade 3 or 4 diarrhea persisting 4 days or more despite adequate supportive care; (v) grade 3 or 4 non-hematological toxicities, except grade 3 anorexia, nausea, vomiting, stomatitis, esophagitis, and abnormal laboratory values; (vi) suspension of chemotherapy for 8 days or more in total; (vii) delay of the second cycle for more than 7 days due to adverse events; (viii) suspension of radiotherapy for more than 13 days due to adverse events; and (ix) discontinuation of protocol treatment during CRT, except due to patient refusal.
The MTD was defined as the dose at which more than two of six patients developed a DLT. If MTD was not reached at dose level 2 (S-1 80 mg/m2/day), RD was basically determined to be dose level 2. However, the final decision on RD was made by the coordinating investigator committee of the study, with endorsement from the Data and Safety Monitoring Committee of the JCOG.
Doses of chemotherapy were modified in cases of severe hematological or non-hematological toxicities. As patients received two chemotherapeutic agents, dose adjustment was carried out for each agent individually according to the type of toxicity observed. If an observed toxicity was assumed to be related with both agents, the doses of both agents were reduced.
Grade 4 hematological toxicities or grade 3 infection required a dose reduction of both drugs. Grade 3 diarrhea, mucositis, esophagitis, or skin reaction required a reduction in S-1 dose. Increased aspartate aminotransferase, alanine aminotransferase of 200 IU/L or more, or increased total bilirubin of 3.0 mg/dL or more also required a reduction in S-1 dose. Grade 2 neurotoxicity required a reduction in CDDP dose. Grade 3 neurotoxicity required the discontinuation of CDDP. Creatinine clearance was calculated at the beginning of each cycle according to the Cockcroft–Gault formula. Reduction in dose levels depended on creatinine clearance values: ≥60 mL/min, no dose modification; <60, ≥50 mL/min, reduction in both S-1 and CDDP by one dose level; <50, ≥40 mL/min, reduction of both S-1 and CDDP by two dose levels; and <40 mL/min, cessation of both S-1 and CDDP. Protocol treatment was terminated if more than two dose reductions were required or if there was a treatment delay of >14 days due to toxicity.
Overall responses were evaluated according to Response Evaluation Criteria in Solid Tumors version 1.0 with endoscopy and computed tomography. Complete response at the primary lesion was declared by endoscopic examination using the modified criteria of the Japanese Society for Esophageal Diseases (9th edition)7 when all of the following criteria were met under observation of the entire esophagus: disappearance of the tumor lesion, disappearance of ulceration or erosion, and absence of cancer cells in biopsy specimens.8 Existence of a granular protruded lesion and Lugol-voiding lesion do not prevent a CR evaluation. The first evaluation was carried out 28 days after the completion of CRT, and the second after the completion of four cycles of chemotherapy. Endoscopic assessments were repeated every 4 weeks until primary CR or progressive disease was confirmed.
All enrolled patients were followed for at least 5 years. Efficacy and safety were evaluated at least every 3 months for 3 years and then every 6 months thereafter.
Study design and statistical analysis
This trial was designed as a multicenter, prospective, single-arm phase I/II study to determine the MTD and RD and to evaluate the efficacy and safety of CRT concurrent with S-1 plus CDDP in patients with esophageal carcinoma. The study protocol was approved by the JCOG Protocol Review Committee and the institutional review board of each participating institution, and carried out in accordance with the Declaration of Helsinki and Good Clinical Practice. This trial was registered at the UMIN Clinical Trials Registry as UMIN000000710 (http://www.umin.ac.jp/ctr/index.htm).
As a previous study showed a remarkable difference in CR rate between patients with T1–2 and T3 disease,9 the planned sample size was determined to be 75 patients based on the weighting of threshold values of CR rate according to the expected registered proportion of T1–2 and T3. This sample size was calculated using Southwest Oncology Group’s two-stage attained design10 based on an expected %CR of 85% and threshold of 70% in patients with T1–2, and an expected %CR of 65% and threshold of 45% in patients with T3, with a one-sided alpha of 0.05 and a power of 0.9.
The goal of phase I was to estimate the MTD and DLT of CRT concurrent with S-1 plus CDDP and to determine RD in patients with clinical stage II/III esophageal carcinoma: the primary endpoint was the incidence of DLT in each dose level, and the secondary endpoint was adverse events. The objective of the phase II part was to evaluate the safety and efficacy of CRT concurrent with S-1 plus CDDP in patients who received the RD in the phase I part: the primary endpoint was CR rate based on central review, and the secondary endpoint was OS, PFS, and adverse events. An interim analysis was not planned. Progression-free survival was defined as the time from enrolment to any disease progression or death from any cause. Overall survival was defined as the number of days from enrolment to death from any cause. Confidence intervals of %CR were estimated by the Clopper–Pearson method. Survival curves were estimated by the Kaplan–Meier method. Analyses were carried out using sas 9.2 (SAS Institute, Cary, NC, USA).
Results
Patients and disease characteristics
Patient accrual was terminated due to slow enrolment after 44 patients were accrued from May 2007 to September 2011. Patient characteristics are listed in Table1. Of the 44 patients, 40 were male and the median age was 62 years (range, 48–72). Disease characteristics were: performance status 0/1 (39/5), squamous cell carcinoma/adenocarcinoma (43/1), T1a/T1b/T2/T3 (3/11/8/22), N0/N1 (8/36), and clinical stage IIA/IIB/III/IVA (8/18/17/1). One patient was diagnosed as stage IVA by the detection of supraclavicular lymph node metastasis (M1) on central review, and was considered to be ineligible for the current study.
Table 1.
Characteristics of patients with clinical stage II/III esophageal carcinoma who participated in phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin (n = 44)
| Characteristic | n |
|---|---|
| Age, years | |
| Median | 62 |
| Range | 48–72 |
| Sex | |
| Female | 4 |
| Male | 40 |
| PS | |
| 0 | 39 |
| 1 | 5 |
| Histology | |
| Squamous cell carcinoma | 43 |
| Adenocarcinoma | 1 |
| TNM | |
| T1a/T1b/T2/T3 | 3/11/8/22 |
| N0/N1 | 8/36 |
| Clinical stage | |
| IIA/IIB/III/IVA† | 8/18/17/1 |
One patient with stage IVA disease; supraclavicular lymph node metastasis (M1) was detected on central review. PS, performance status.
Safety and treatment compliance
A patient flow diagram is shown in Figure 1. Overall toxicities in phase I are listed in Table2. In phase I, two of six patients experienced DLTs at each level of S-1 (S-1 60 or 80 mg/m2/day). That is, MTD was not reached at the dose of S-1 80 mg/m2/day. Dose-limiting toxicities required either the suspension of chemotherapy for a total of 8 or more days or a delay of the second cycle for more than 7 days due to adverse events. Specifically, at an S-1 dose level of 60 mg/m2/day (level 1), one patient experienced a DLT, namely a delay of the second cycle of longer than 7 days behind schedule due to adverse events including leukopenia and thrombocytopenia in the first cycle. A second patient experienced a DLT that required the suspension of chemotherapy for a total of 8 or more days due to adverse events, including leukopenia and thrombocytopenia, in the second cycle. At an S-1 dose level of 80 mg/m2/day (level 2), one patient experienced a DLT requiring the suspension of chemotherapy for more than 8 days due to adverse events including leukopenia and thrombocytopenia in the second cycle. A second patient experienced a DLT that required a delay in the second cycle for more than 7 days behind schedule due to thrombocytopenia in the first cycle.
Figure 1.
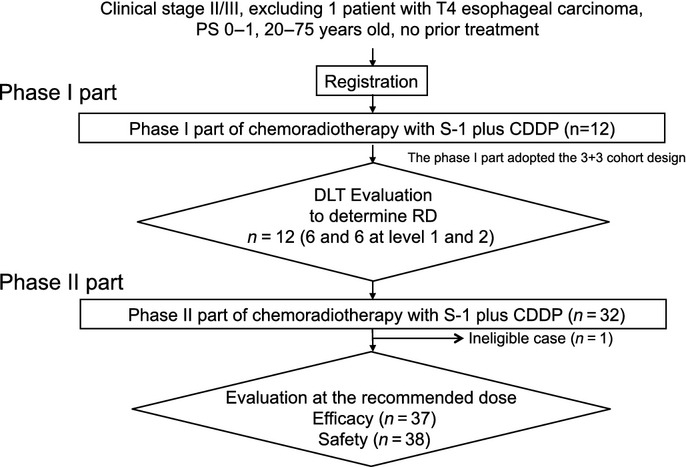
Patient flow diagram of phase I/II trial of chemoradiotherapy with concurrent S-1 and cisplatin (CDDP) for clinical stage II/III esophageal carcinoma. DLT, dose-limiting toxicity; PS, performance status; RD, recommended dose.
Table 2.
Overall toxicities in the phase I part of a phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin in patients with clinical stage II/III esophageal carcinoma (n = 12)
| Hematological toxicities | Level 1 (n = 6) | Level 2 (n = 6) | ||||
|---|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |||
| Adverse event | Grade 3 | Grade 4 | Gr 3 + 4 | Grade 3 | Grade 4 | Gr 3 + 4 |
| Leukopenia | 4 | 0 | 66.7 | 4 | 0 | 66.7 |
| Neutropenia | 4 | 0 | 66.7 | 3 | 0 | 50.0 |
| Anemia | 2 | 0 | 33.3 | 1 | 0 | 16.7 |
| Thrombocytopenia | 2 | 0 | 33.3 | 1 | 0 | 16.7 |
| Febrile neutropenia | 1 | 0 | 16.7 | 0 | 0 | 0.0 |
| Non-hematological toxicities | No. of patients | % | No. of patients | % | ||
| Adverse event | Grade 2 | Grade 3 | Gr 2 + 3 | Grade 2 | Grade 3 | Gr 2 + 3 |
| Esophagitis | 4 | 0 | 66.7 | 5 | 1 | 100 |
| Mucositis | 0 | 0 | 0.0 | 1 | 0 | 16.7 |
| Skin rash | 0 | 0 | 0.0 | 1 | 0 | 16.7 |
| Anorexia | 1 | 4 | 83.3 | 0 | 1 | 16.7 |
| Vomiting | 2 | 0 | 33.3 | 0 | 0 | 0.0 |
| Neuropathy | 1 | 0 | 16.7 | 0 | 0 | 0.0 |
| Upper respiratory infection | 1 | 0 | 16.7 | 1 | 0 | 16.7 |
Adverse events were graded according to the Common Toxicity Criteria for Adverse Events version 3.0. Gr, grade; Level 1, S-1 dose level of 60 mg/m2/day; Level 2, S-1 dose level of 80 mg/m2/day.
Relative dose intensity in phase I is listed in Table3. Intensity was 91.1% at level 1 and 68.5% at level 2. The incidence of esophagitis at level 2 was higher than in level 1 (100% vs 66.7%), and bone marrow recovery was subsequently delayed. In consideration of treatment compliance, RD was determined to be S-1 60 mg/m2/day.
Table 3.
Relative dose intensity in the phase I part of a phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin (CDDP) in patients with clinical stage II/III esophageal carcinoma (n = 12)
| S-1 dose level | Patient no. | Drug | Relative dose intensity, % | ||||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Total | |||
| 1 (60 mg/m2/day) | 1 | CDDP | 100.0 | 100.0 | 100.0 | 76.8 | 91.1 |
| S-1 | 100.0 | 100.0 | 100.0 | 80.0 | |||
| 2 | CDDP | 100.0 | 100.0 | 100.0 | 0.0 | ||
| S-1 | 100.0 | 100.0 | 100.0 | 0.0 | |||
| 3 | CDDP | 100.0 | 77.2 | 74.0 | 74.8 | ||
| S-1 | 100.0 | 80.0 | 80.0 | 80.0 | |||
| 4 | CDDP | 100.0 | 100.0 | 100.0 | 97.3 | ||
| S-1 | 100.0 | 100.0 | 100.0 | 100.0 | |||
| 5 | CDDP | 100.0 | 100.0 | 100.0 | 100 | ||
| S-1 | 100.0 | 53.6 | 100.0 | 100.0 | |||
| 6 | CDDP | 100.0 | 100.0 | 100.0 | 100.0 | ||
| S-1 | 100.0 | 100.0 | 100.0 | 100.0 | |||
| 2 (80 mg/m2/day) | 7 | CDDP | 100.0 | 80.2 | 80.2 | 80.2 | 68.5 |
| S-1 | 100.0 | 83.3 | 83.3 | 83.3 | |||
| 8 | CDDP | 100.0 | 79.8 | 79.8 | 79.8 | ||
| S-1 | 100.0 | 56.6 | 83.3 | 83.3 | |||
| 9 | CDDP | 100.0 | 79.8 | 79.8 | 79.8 | ||
| S-1 | 100.0 | 80.0 | 80.0 | 80.0 | |||
| 10 | CDDP | 100.0 | 80.0 | 80.0 | 80.0 | ||
| S-1 | 100.0 | 83.3 | 83.3 | 83.3 | |||
| 11 | CDDP | 100.0 | 79.9 | 0.0 | 0.0 | ||
| S-1 | 100.0 | 83.3 | 0.0 | 0.0 | |||
| 12 | CDDP | 100.0 | 0.0 | 0.0 | 0.0 | ||
| S-1 | 100.0 | 0.0 | 0.0 | 0.0 | |||
Overall toxicities at RD are listed in Table4. Grade 3 or 4 toxicity at RD included leukopenia (57.9%), neutropenia (50%), hyponatremia (28.9%), anorexia (21.1%), anemia (18.4%), thrombocytopenia (18.4%), and febrile neutropenia (2.6%). No treatment-related deaths were observed. Most common late toxicities were pneumonitis (n = 20), pericardial effusion (n = 5), and esophagitis (n = 4). Grade 3 late toxicities included pneumonitis (n = 1), myocardial ischemia (n = 1), and esophageal fistula (n = 1). One patient developed grade 3 myocardial ischemia 62 days after starting the second cycle. Additionally, no new or unexpected toxicities were observed.
Table 4.
Overall toxicities at the recommended dose in a phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin in patients with clinical stage II/III esophageal carcinoma (n = 38)
| Adverse event | No. of patients | % | |
|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 + 4 | |
| Leukopenia | 22 | 0 | 57.9 |
| Neutropenia | 19 | 0 | 50.0 |
| Anemia | 7 | 0 | 18.4 |
| Thrombocytopenia | 7 | 0 | 18.4 |
| Febrile neutropenia | 1 | 0 | 2.6 |
| Anorexia | 8 | 0 | 21.1 |
| Esophagitis | 5 | 0 | 13.2 |
| Vomiting | 1 | 0 | 2.6 |
| Constipation | 1 | 0 | 2.6 |
| Neuropathy | 1 | 0 | 2.6 |
| Upper respiratory infection | 1 | 0 | 2.6 |
Adverse events were graded according to the Common Toxicity Criteria for Adverse Events version 3.0.
Radiation therapy QA data were reviewed and found to be fully evaluable in all 44 cases. All cases were assessed as acceptable per protocol, excluding one patient whose treatment for a primary tumor in the middle esophagus had an acceptable variation: the anterior–posterior opposed fields were used in the initial 5 fractions. However, the RT technique was revised and the optimal multiple-portal technique was adopted for the remaining 23 fractions.
Treatment outcomes
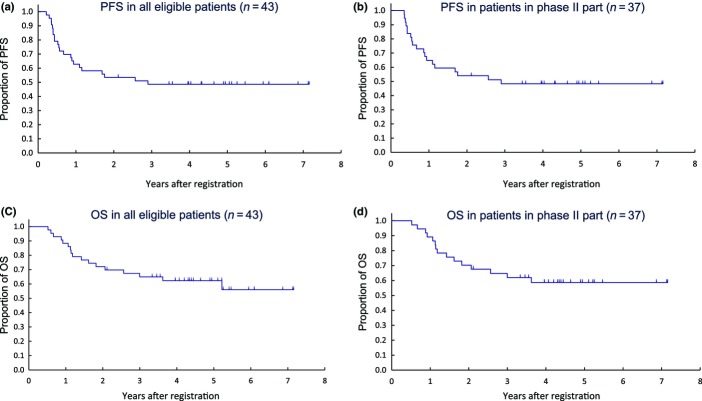
The CR rate, the primary endpoint of phase II, was 59.5% (22/37; 90% CI, 44.6–73.1%; weighted threshold, 57.2%; P = 0.46 by the exact binomial test) on central review versus 65.8% (90% CI, 51.2–78.4; weighted threshold, 58.2%; P = 0.22 by the exact binomial test) on investigator review. In phase II (n = 37), with a median follow-up time for censored patients of 4.4 years, 3-year PFS was 48.4% (95% CI, 31.6–63.2%), and 3-year OS was 61.9% (95% CI, 44.3–75.4%) (Fig. 2). In all eligible patients (n = 43), 3-year PFS was 48.6% (95% CI, 33.1–62.5%) and 3-year OS was 65.0% (95% CI, 48.7–77.2%). All deaths were due to recurrence or disease progression of esophageal cancer.
Figure 2.
Clinical outcomes for patients with clinical stage II/III esophageal carcinoma who participated in phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin. (a) Progression-free survival (PFS) in all eligible patients (n = 43). (b) PFS in patients in phase II of trial (n = 37). (c) Overall survival (OS) in all eligible patients (n = 43). (d) OS in patients in phase II of trial (n = 37).
Recurrence/progression during phase II is listed in Table5. Nineteen (51.4%) of 37 patients developed recurrence or disease progression, while 6 (27.3%) of 22 patients who achieved CR developed recurrence or disease progression. Six of 7 patients who developed recurrence at the primary site were eligible for salvage therapy by EMR (Table6). Distant metastasis was observed in 11 patients (29.7%) during phase II. In the overall population, 24 patients received salvage therapy, including chemotherapy (n = 9), surgery (n = 8), and EMR (n = 7) (Table6). No salvage therapy-related death was observed.
Table 5.
Recurrence/progression at recommended dose (RD) in a phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin in patients with clinical stage II/III esophageal carcinoma (n = 37)
| Patients at RD dose (n = 37) | Patients with CR† (n = 22) | |||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
| Recurrence or progression | ||||
| No | 18 | 48.6 | 16 | 72.7 |
| Yes | 19 | 51.4 | 6 | 27.3 |
| Recurrent site | ||||
| Primary site | 7 (6)‡ | 18.9 | 1 (1)‡ | 4.5 |
| Lymph node | 7 | 18.9 | 3 | 13.6 |
| Distant metastasis | 11 | 29.7 | 4 | 18.2 |
Complete response (CR) evaluated by central review.
Patients who could receive salvage therapy by endoscopic mucosal resection.
Table 6.
Incidence of salvage therapy in all patients with clinical stage II/III esophageal carcinoma who participated in a phase I/II trial of chemoradiotherapy and concurrent S-1 and cisplatin (n = 44)
| Salvage therapy | All (n = 44) | S-1 dose level 1 (n = 38) | S-1 dose level 2 (n = 6) |
|---|---|---|---|
| No | 20 (1)† | 18 (1)† | 2 |
| Yes | 24 | 20 | 4 |
| EMR | 7 | 6 | 1 |
| Surgery | 8 | 6 | 2 |
| Chemotherapy | 9 | 8 | 1 |
| RT | 1 | 1 | 0 |
| Others | 3 | 3 | 0 |
One ineligible patient. S-1 dose level 1, 60 mg/m2/day; S-1 dose level 2, 80 mg/m2/day. EMR, endoscopic mucosal resection; RT, radiation therapy.
Discussion
This study was carried out as an investigator-initiated registration trial for label extension of S-1 in the treatment of esophageal carcinoma in Japan. However, patient accrual was terminated early due to slow enrolment after 44 patients were accrued. The main reason for poor accrual was the change in patients’ treatment preference. During the study period, preoperative chemotherapy with 5-FU plus cisplatin has become a standard treatment for resectable stage II/III esophageal carcinoma in Japan,1 and the number of patients who preferred to receive definitive CRT has decreased.
During phase I, two of six patients experienced DLTs at each level of S-1 (60 or 80 mg/m2/day) and MTD was not reached at the level of 80 mg/m2/day, the maximum dose of S-1. The incidence of esophagitis with delayed bone marrow recovery was higher at 80 mg/m2/day than at 60 mg/m2/day, leading to an increased risk of treatment interruption. Furthermore, relative dose intensity was inferior to that at 60 mg/m2/day, indicating that no marked improvement in clinical activity would be expected. Based on these concerns, RD was determined to be S-1 60 mg/m2/day. During phase II, grade 3 or 4 toxicity included neutropenia (50%), anemia (18.4%), thrombocytopenia (18.4%), and febrile neutropenia (2.6%), and no treatment-related deaths were observed, indicating that the current combination at RD will be manageable with acceptable acute toxicity.
In a previous study, the AUC of 5-FU appeared higher in Caucasian than Japanese patients in a comparable dose range of S-1.11 This difference is primarily attributable to different polymorphisms in the CYP2A6 gene among Asians and Caucasians.12,13 Accordingly, the dose of S-1 in the present study is likely unsuitable for Caucasian patients, and further study to determine the RD of S-1 concurrent with CRT for these patients appears necessary.
One concern of CRT for esophageal cancer is RT-related late toxicity, which leads to high mortality. A previous phase II study reported that grade 3 or 4 late toxicities included pericardial (16%) and pleural (9%) effusion, and pneumonitis (4%), leading to four deaths related to these toxicities.9 Furthermore, in a retrospective analysis of 78 patients who achieved CR after the completion of CRT, two died of myocardial infarction and eight (10.2%) died of pericardial or pleural effusion.14 Both of these studies used a much wider and longer radiation field to cover lymph node dissections. To reduce these late toxicities, the multiple-field technique has been adapted in CRT for esophageal cancer. In the current study, grade 3 or 4 late toxicities included pneumonitis (n = 1) and esophageal fistula (n = 1), and no death related to these late toxicities was observed, indicating that the multiple-field technique might reduce late toxicities.
A previous study showed an obvious difference in CR rate between patients with T1–2 and T3 disease (78.3% vs 54.9%).9 In the present phase II trial, therefore, the planned sample size was calculated based on the weighting of threshold values of CR rate according to the registered proportion of T1–2 and T3, based on an expected %CR of 85% and a threshold of 70% in patients with T1–2 disease, and an expected %CR of 65% and a threshold of 45% in patients with T3. During phase II, 18 (48.6%) patients had T1/2 disease. The CR rate was 59.5% (90% CI, 44.6–73.1%; weighted threshold, 57.2%; P = 0.46) on central review, and thus the lower limit of the confidence interval did not exceed the weighted threshold of CR rate. In other words, this study did not meet its primary endpoint.
One possible reason for this was the small sample size, which was due to the early termination of patient accrual. A second is that CDHP did not enhance radiosensitivity as we had expected. A Japanese phase II study of CRT concurrent with 5-FU plus CDDP for stage II–III esophageal carcinoma15 administered 5-FU at 1000 mg/m2/day on days 1–4 and CDDP at 75 mg/m2 on day 1 every 4 weeks for two cycles. Patients received an additional two cycles of chemotherapy 4 weeks after CRT. Radiation therapy consisted of 50.4 Gy in 28 fractions over 6 weeks, delivered with megavoltage equipment (≥6 MV) using the multiple-field technique. Except for the administration of 5-FU, therefore, treatment was the same as in the current study. A total of 41 patients were enrolled. The CR rate was 70.6% (80% CI, 58.3–84.1%), which was similar to that by investigator review in the current phase II study (65.8%; 90% CI, 48.7–80.4%). Three-year PFS was 56.6% and 3-year OS was 63.8%, indicating no difference in efficacy between this previous and the present studies. Namely, CDHP could not enhance radiosensitivity as we had expected.
In the present study, CR at the primary lesion was evaluated by endoscopic examination based on our previous study,8 which indicated a remarkable difference in 5-year survival rates between patients evaluated as having primary CR versus non-CR (46% and 6%, P < 0.0001). These findings suggest that primary CR is an appropriate surrogate endpoint. Although PFS would have been a more appropriate primary endpoint in the treatment of locally advanced esophageal cancer, CR is a useful means of avoiding unnecessary therapy in treatment decision-making after the completion of CRT. Disease progression was observed in 19 (51.4%) of 37 patients during phase II and recurrence occurred in 6 (27.3%) of 22 patients who achieved CR. Furthermore, recurrence at the primary site was observed in only 1 of 22 patients who achieved CR and in 7 of 37 in phase II, indicating that the current endoscopic CR criteria will likely predict primary control.
A previous study indicated that salvage surgery after local failure following CRT had high in-hospital mortality (11.4%) with 5-year OS of 25–35%.16 Salvage therapy has recently progressed, however, including salvage surgery and EMR, and one study reported no operative mortality or hospital death.15 Long-term results of salvage EMR in patients with local failure after CRT for esophageal cancer showed 5-year OS from the initiation of salvage EMR of 49.1%.17 Our present study showed the favorable 3-year OS of 62.9%, and 3-year PFS was 44.4%. During phase II, 20 patients received salvage therapy, including salvage surgery (n = 6) and EMR (n = 6), which might have contributed to the favorable survival.
In the present study, recurrence at the primary site or a regional lymph node was observed in seven (18.9%) patients each, indicating that this treatment achieved excellent local control. However, activity for distant control was limited, with a distant metastasis rate of 29.7%. Accordingly, next efforts should focus on distant control to improve outcomes. One promising treatment strategy for distant control is induction chemotherapy, which demonstrated decreased distant metastasis in a randomized trial of squamous cell carcinoma of the head and neck.18 A phase I/II study of induction chemotherapy with DCF followed by CRT in patients with unresectable locally advanced esophageal carcinoma demonstrated excellent distant control, with a distant metastasis rate of 9%.19 Furthermore, DCF showed promising activity as neoadjuvant chemotherapy for stage II/III esophageal cancer,20 leading to an ongoing phase III trial.21
In conclusion, although this combination showed acceptable toxicity and favorable 3-year survival, this study did not meet its primary endpoint. Further evaluation of the efficacy of S-1 in the treatment of esophageal cancer is required.
Acknowledgments
The authors would like to thank the patients and families who participated in this study. They also wish to acknowledge the support of the following participating institutions (five institutions, from north to south): National Cancer Center Hospital East, National Cancer Center Hospital Central, Keio University Hospital, Shizuoka Cancer Center, and Aichi Cancer Center Hospital. The authors are grateful to Mr. Hidenobu Yamada from the JCOG Data Center for his support in data management and the following central review members: Dr. Yasuaki Arai, Dr. Hirofumi Kuno, Dr. Yasuo Hamamoto, Dr. Shuichi Hironaka, Dr. Yoshinori Miyata, and Dr. Mototaka Miyake. This study was supported by a clinical trial promotion program of the Japan Medical Association subsidized by the Ministry of Health, Labor and Welfare, a Grant from the Ministry of Health, Labor and Welfare for the Third Term Comprehensive Strategy for Cancer Control, a Grant-in-Aid for Cancer Research (17S-3, 17S-5, 20S-3, and 20S-6), and the National Cancer Center Research and Development Fund (23-A-16, 23-A-19, and 26-A-4).
Glossary
- CDDP
cisplatin
- CDHP
5-chloro-2,4-dihydropyrimidine
- CI
confidence interval
- CR
complete response
- CRT
chemoradiotherapy
- DCF
docetaxel, cisplatin, fluorouracil
- DLT
dose-limiting toxicity
- EC
esophageal carcinoma
- EMR
endoscopic mucosal resection
- 5-FU
5-fluorouracil
- JCOG
Japan Clinical Oncology Group
- MTD
maximum tolerated dose
- OS
overall survival
- PFS
progression-free survival
- PTV
planning target volume
- QA
quality assurance
- RD
recommended dose
- RT
radiation therapy
- Vxx
percentage of the volume receiving xx Gy
Disclosure Statement
Nozomu Fuse received research funding from Taiho Pharmaceutical. Akihiro Sato received research funding from Taiho Pharmaceutical. Kentaro Yamazaki received honoraria from Taiho Pharmaceutical. Kei Muro received honoraria and research funding from Taiho Pharmaceutical. Narikazu Boku received honoraria and research funding from Taiho Pharmaceutical. The other authors have no conflict of interest. JCOG Data Center collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and final responsibility for the decision to submit for publication.
References
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakano K, Takechi T, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–6. [PubMed] [Google Scholar]
- Fukushima M, Ohshita H, Taguchi T. Combined therapy with radiation and S-1, an oral new 5FU prodrug, is markedly effective against non-small cell lung cancer xenografts in mice. Eur J Cancer. 2005;3:343. [Google Scholar]
- Fukushima M, Sakamoto K, Sakata M, Nakagawa F, Saito H, Sakata Y. Gimeracil, a component of S-1, may enhance the antitumor activity of X-ray irradiation in human cancer xenograft models in vivo. Oncol Rep. 2010;24:1307–13. doi: 10.3892/or_00000987. [DOI] [PubMed] [Google Scholar]
- Tahara M, Minami H, Kawashima M, et al. Phase I trial of chemoradiotherapy with the combination of S-1 plus cisplatin for patients with unresectable locally advanced squamous cell carcinoma of the head and neck. Cancer Sci. 2011;102:419–24. doi: 10.1111/j.1349-7006.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- Japanese Society for Esophageal Diseases. Guidelines for clinical and pathologic studies on carcinoma of the esophagus ninth edition: part II. Esophagus. 2004;1:107–25. [Google Scholar]
- Tahara M, Ohtsu A, Hironaka S, et al. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol. 2005;35:316–23. doi: 10.1093/jjco/hyi095. [DOI] [PubMed] [Google Scholar]
- Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–90. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Green SJ, Dahlberg S. Planned versus attained design in phase II clinical trials. Stat Med. 1992;11:853–62. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol. 2005;23:6957–65. doi: 10.1200/JCO.2005.01.917. [DOI] [PubMed] [Google Scholar]
- van der Weide J, Steijns LS. Cytochrome P450 enzyme system: genetic polymorphisms and impact on clinical pharmacology. Ann Clin Biochem. 1999;36(Pt 6):722–9. doi: 10.1177/000456329903600604. [DOI] [PubMed] [Google Scholar]
- Daigo S, Takahashi Y, Fujieda M, et al. A novel mutant allele of the CYP2A6 gene (CYP2A6*11) found in a cancer patient who showed poor metabolic phenotype towards tegafur. Pharmacogenetics. 2002;12:299–306. doi: 10.1097/00008571-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- Kato K, Nakajima TE, Ito Y, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43:608–15. doi: 10.1093/jjco/hyt048. [DOI] [PubMed] [Google Scholar]
- Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg. 2007;94:1059–66. doi: 10.1002/bjs.5865. [DOI] [PubMed] [Google Scholar]
- Yano T, Muto M, Hattori S, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40:717–21. doi: 10.1055/s-2008-1077480. [DOI] [PubMed] [Google Scholar]
- Cohen EW, Karrison K, Kocherginsky M, et al. DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2012;30(Suppl) abstr 5500. [Google Scholar]
- Satake H, Tahara M, Mochizuki S, et al. Phase I/II trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil (DCF) followed by concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. J Clin Oncol. 2013;31(Suppl) abstr 4074. [Google Scholar]
- Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60. doi: 10.1111/cas.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study) Jpn J Clin Oncol. 2013;43:752–5. doi: 10.1093/jjco/hyt061. [DOI] [PubMed] [Google Scholar]