Abstract
Angiogenesis plays an important role in tumor growth and metastasis and has been reported to be inversely correlated with overall survival of osteosarcoma patients. It has been shown that apurinic/apyrimidinic endonuclease 1 (APE1), a dually functional protein possessing both base excision repair and redox activities, is involved in tumor angiogenesis, although these mechanisms are not fully understood. Our previous study showed that the expression of transforming growth factor β (TGFβ) was significantly reduced in APE1-deficient osteosarcoma cells. Transforming growth factor β promotes cancer metastasis through various mechanisms including immunosuppression, angiogenesis, and invasion. In the current study, we initially revealed that APE1, TGFβ, and microvessel density (MVD) have pairwise correlation in osteosarcoma tissue samples, whereas TGFβ, tumor size, and MVD were inversely related to the prognosis of the cohort. We found that knocking down APE1 in osteosarcoma cells resulted in TGFβ downregulation. In addition, APE1-siRNA led to suppression of angiogenesis in vitro based on HUVECs in Transwell and Matrigel tube formation assays. Reduced secretory protein level of TGFβ of culture medium also resulted in decreased phosphorylation of Smad3 of HUVECs. In a mouse xenograft model, siRNA-mediated silencing of APE1 downregulated TGFβ expression, tumor size, and MVD. Collectively, the current evidence indicates that APE1 regulates angiogenesis in osteosarcoma by controlling the TGFβ pathway, suggesting a novel target for anti-angiogenesis therapy in human osteosarcoma.
Keywords: Angiogenesis, apurinic/apyrimidinic endonuclease 1 (APE1), osteosarcoma, redox, transforming growth factor β (TGFβ)
Osteosarcoma is a bone malignancy frequently diagnosed among children and adolescents.1 Therapeutic approaches for osteosarcoma patients combine neoadjuvant chemotherapy and limb-sparing operations.2 Although adjuvant and neoadjuvant chemotherapy have significantly improved the long-term survival rate for patients, approximately 30–40% have recurrent disease and approximately 80% develop metastasis following surgery, so that recurrence and metastatic relapse remain problematic.3–5 Furthermore, the 5-year survival rate for patients with metastasis or relapsed osteosarcoma has remained at approximately 20% for decades.6 Therefore, to facilitate the therapeutic effect and inhibit metastasis, the exploration of novel prognostic factors is of great importance in the development of new treatment strategies for osteosarcoma patients.
Osteosarcoma is a highly vascularized tumor characterized by an early metastatic dissemination potential, which is the main reason for treatment failure and death, and minimal vascularization at the time of diagnosis predicts good response to neoadjuvant chemotherapy, along with a prolonged overall and relapse-free survival.7–9 Thus, targeting angiogenesis is a promising therapeutic approach to osteosarcoma treatment. It is widely accepted that tumor growth and metastasis need vascular support and tumor-associated angiogenesis is a multistep process orchestrated by positive and negative regulatory factors; therefore, the imbalance of angiogenic regulators, such as vascular endothelial growth factor (VEGF) and angiopoietins, may be the cause of the abnormal structure and function of tumor vessels.10,11
Human apurinic/apyrimidinic endonuclease 1 (APE1) is a multifunctional protein involved in redox regulation of transcription factors and the DNA base excision pathway, in which the oxidative base damage is caused by both endogenous and exogenous agents.12,13 In particular, its redox activity is now considered to participate in multiple cancer survival mechanisms such as tumor growth, proliferation, and metastasis, and it plays a key role in pathological angiogenesis by regulating various transcription factors.14,15 Our previous study reported that APE1 expression correlates with poor prognosis of osteosarcoma.16 In subsequent studies, when knocking down APE1, a synergistic growth attenuation together with angiogenic inhibition was observed with recombinant human endostatin in osteosarcoma cell xenografts in null mice, suggesting APE1 involvement in osteosarcoma angiogenesis.17 To explain this phenomenon, we found both VEGF and fibroblast growth factor 2 (FGF2) are regulated by APE1 redox activity through transcription factors, hypoxia-inducible factor-1α (HIF-1α), and signal transducer and activator of transcription 3, separately.17,18 Interestingly, we observed an additional inhibition of angiogenesis by knocking down APE1, even when supplemented with exogenous VEGF and FGF2, implying there might be other APE1-regulated mechanisms involved in tumor angiogenesis.
Transforming growth factor β (TGFβ) constitutes a family of multifunctional peptides that take part in diverse processes of cellular biology, as well as metastasis.19,20 Additionally, TGFβ participates in tumor angiogenesis in cooperation with other angiogenic activators and inhibitors. Transforming growth factor β stimulates angiogenesis through its effects on tumor cells and angiogenic cytokine networks that induce a pro-angiogenic environment, so that increased TGFβ expression results in robust angiogenic responses that further promote tumor metastasis.21 Franchi et al. reported a higher mRNA level of TGFβ1 in high-grade osteosarcoma than in lower-grade, suggesting that TGFβ1 may predict aggressive behavior of osteosarcoma.22 It was also reported that APE1 was correlated with poor prognosis of osteosarcoma. Based on our previous observations,23 we thereby hypothesized that TGFβ could contribute to the regulatory role of APE1 in osteosarcoma angiogenesis.
This study aims to investigate the interaction between APE1 and TGFβ in the angiogenesis of osteosarcoma. First, our data showed that high expression of APE1, TGFβ, and microvessel density (MVD) correlate with poor prognosis of osteosarcoma patients, with a pairwise correlation between each. Second, TGFβ, tumor size, and MVD were important indicators and inversely related to prognosis. According to the above analysis, we speculated that APE1 may indirectly regulate angiogenesis through a TGFβ-dependent pathway and carried out in vivo and in vitro assays to confirm this. This is the first study of the role of APE1 regulating TGFβ in tumor angiogenesis in osteosarcoma.
Materials and Methods
Clinical cases
Eighty patients with long bone intramedullary osteosarcoma in the extremities were treated in Daping Hospital (Chongqing, China) between 1968 and 1993. There were 52 male and 28 female patients, who provided signed, informed consent. Among these patients, 75 had surgery and 5 had biopsy; 54 (67.50%) were in their twenties, and 74 (92.50%) had a tumor size of 5–25 cm in diameter with soft tissue invasion. The bone tumor types and histological grades were determined based on the Enneking staging system24 and Ross FG classification.25 Thirty-five patients had the affected limb amputated with subsequent chemotherapy, 24 had amputation only, 17 had abscission of the tumoral segment followed by end-to-end connection of the amputated ends or inactivation and replantation, and 4 patients did not receive any treatment.
Immunohistochemical analysis
Tumor tissues were fixed in 4% paraformaldehyde at room temperature overnight, then dehydrated and embedded in paraffin, followed by sectioning (RM2235; Leica, Solms, Germany) of the tumor tissue at 4.5 μm per slide. After applied with the primary antibodies, 3,3′-diaminobenzidine was used as a chromogenic substrate and hematoxylin for counterstaining. The intensity of immunohistochemical (IHC) staining was graded from 0 to 3 as reported in Khoury et al.26 The tissue showed less than 25% positive cells was defined as low staining intensity, 25–50% positive cells was moderate staining intensity, and more than 50% positive cells was high staining intensity. All CD34-positive endothelial cells separated from adjacent microvessels were defined as MVD. The eyepiece graticule was rotated to view the maximum number of stained vessels under 200× magnification for the quantification of MVD. Three fields were captured for each section and the expression of the results was the mean ± SD. Primary antibody was the substitution for PBS as a negative control.
Cell culture, APE1-siRNA transfection, Western blot analysis, and RT-PCR in vivo
Human osteosarcoma 9901 cells (contributed by Prof. Qingyu Fan from the Fourth Military Medical University, Xian, China) were grown in RPMI-1640 (HyCloneLaboratories Inc., Uath, USA) supplemented with 10% FBS and HUVECs (ATCC, Manassas, VA, USA) were cultured in DMEM (HyClone) with 10% FBS. Osteosarcoma 9901 cells were transfected with 0.2 nM APE1-siRNA; the sequences of APE1-siRNA1 were 5′-UACUCCAGUCGUACCAGACCU-3′, 5′-GUCUGGUACGACUGGAGUACC-3′, and that of scramble-siRNA1 were 5′-GACCAUGCUGACCUCAUGGAA-3′, 5′-CCAUGAGGUCAGCAUGGUCUG-3′. The sequences of APE1-siRNA2 were 5′-CUCAAUGUGGCACAUGAAGdTdT-3′, 5′-CUUCAUGUGCCACAUUGAGdTdT-3′, and that of scramble-siRNA2 were 5′-UUCUCCGAACGUGUCACGUTT-3′, 5′-ACGUGACACGUUCGGAGAATT-3′. These siRNAs were carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) in accordance with the manufacturer’s instructions. After 48 h, the cells were harvested for Western blot analysis. Commercial antibodies anti-APE1 (1:5000) and anti-TGFβ (1:50) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
RNA was extracted and reverse-transcripted to cDNA using the RevertAid First-Strand cDNA Synthesis Kit (Thermo Scientific, Massachusetts, USA) for RT-PCR following the manufacturer’s instructions. Reverse transcription–PCR for TGFβ was carried out as previously described,27 and the following cycle profile was used for both APE1 and β-actin: 94°C for 2 min, 40 cycles of denaturation at 94°C for 45 s, annealing at 58°C for 30 s, and extension at 72°C for 20 s.
Enzyme-linked immunosorbent assay, Transwell migration, Matrigel tube formation assay, and Western blot analysis
The supernatant from 9901 cells were measured using a TGF-β1 ELISA kit (Invitrogen). The absorbance of each well was read at 450 nm. The Transwell migration assay was carried out in a 24-well plate with an 8.0-μm pore Transwell chamber (BD Biosciences, San Diego, CA, USA). The serum-free DMEM medium containing 18 000 HUVECs was inoculated into the upper chamber. The lower compartments were filled with the supernatant from culturing 9901 cells with Opti-MEM I medium, 0.2 nM APE1-siRNA, and 0.2 nM APE-1-siRNA supplemented with purified TGFβ protein at the concentration of 2 or 5 ng/mL. After incubation for 20 h, the migrated cells were fixed with formaldehyde and stained with crystal violet, followed by counting from four random zones by light microscopy at 200× magnification. For the Matrigel tube formation assay, supernatant as indicated above was mixed with 20 μL DMEM containing 2 × 104 HUVECs then transferred to the 24-well plate on the Matrigel matrix separately, followed by incubation at 37°C, 5% CO2, for 12 h.
Three groups of HUVECs were tested for protein levels of Smad3 and phosphorylated Smad3. The culture medium of these groups were supernatants of untreated 9901 cells, 9901 cells treated with 0.2 nM APE1-siRNA, or 9901 cells treated with 0.2 nM APE1-siRNA plus purified recombinant TGFβ protein (2 ng/mL; PeproTech, Rocky Hill, NJ, USA). After 2 h of conditioning culture, three groups of HUVECs were tested for protein levels of pan and phosphorylated Smad3 by Western blot. The anti-Smad3 and anti-Smad3 (phospho) antibodies were purchased from Abcam (Cambridge, MA, USA).
Tumor angiogenesis in osteosarcoma xenograft mouse model and IHC analysis
The xenograft model and treatment were carried out as previously described by Wang et al.(17) Balb/c nude mice were acclimated to autoclaved cages for 1 week and housed with access to food and water ad libitum in HEPA-filtered racks (Dwyer Instruments, Michigan, IN, USA) with close supervision. All experimental protocols were consented to by the Ethics Committee of the Third Military Medical University (Chongqing, China). The 4–5-week-old mice were allocated randomly to each group (n = 5 per group) followed by inoculation with a suspension of 9901 cells in the axilla of the right anterior limbs. Injection of 20 μg APE1-siRNA into these mice was carried out once every 3 days for 14 consecutive days and mice in the control group received the same volume of PBS when the size of the tumor was approximately 50 mm3. Mice were killed on the 14th day, and the tumors were excised and fixed in formalin solution for IHC analysis. The gross tumor volume was calculated according to the formula Dmax (maximum diameters) × Dmin (minimum diameters)2 × 0.52.28 The results were the sum of the integral optical density and quantified by Image-Pro Plus analysis software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The data were calculated from triplicate experiments and quantitative data were represented as the mean ± SD. Student’s t-test or the Kruskal–Wallis test was used for measurement data; the χ2-test or Fisher’s exact probability were used for count data. The Kaplan–Meier survival test was used to compare the overall survival rate between TGFβ-positive and TGFβ-negative groups. Spearman’s rank correlation coefficient was used to evaluate the association between MVD, APE1, and TGFβ expression in osteosarcoma patients. The measurement data are presented as the mean ± SD. A P-value < 0.05 was regarded as having statistical significance.
Results
Expression of APE1, TGFβ, and CD34 in human osteosarcoma
To investigate the potential correlation between APE1, TGFβ1, and CD34 in osteosarcoma patients, 80 osteosarcoma tissues were immunohistochemically stained. Representative IHC staining with high and low expression of APE1, TGFβ1, and CD34 in osteosarcoma patients are shown in Figure 1. APE1 staining was generally localized in both the nucleus and cytoplasm, and 55 cases showed high APE1 expression levels (68.75%). The TGFβ1 staining was mainly localized in the cytoplasm with high expression observed in 31 cases (38.75%). CD34 staining was mostly localized in the membrane and cytoplasm of vascular endothelial cells in the tumor stroma, and was positive on endothelial cells representing the microvessel. Thirty-four cases had MVD surpassing 37.62 (42.50%). Due to the high expression of APE1 and TGFβ, which was postulated to be correlated with high MVD, the investigation was carried out in osteosarcoma tissues. The expression of these three proteins was positively correlated to each other and the results are presented in Table1. The Cox hazard probability regression model was used to evaluate the potential prognostic factors. As shown in Figure 2, the 2- and 5-year survival rates of osteosarcoma patients were 33.8% and 18.3%, respectively. The baseline patient characteristics (Table2) were similar to a previous report from our group. The data revealed that TGFβ, MVD, and tumor size were important prognostic factors for osteosarcoma. APE1 was excluded in the final model, suggesting its prognostic effect may be dependent on other factors. According to the hazard ratio, the rank of effectiveness of these prognostic factors was TGFβ > tumor size > MVD (Table3).
Figure 1.

Immunohistochemical expression of apurinic/apyrimidinic endonuclease 1 (APE1), transforming growth factor β (TGFβ), and CD34 in human osteosarcoma. Magnification, ×200 magnification. Left images, high expression of APE1, TGFβ1, and microvessel density staining in an osteoblastic osteosarcoma patient. Right images, representative low expression of APE1, TGFβ1, and microvessel density staining in a fibroblastic osteosarcoma patient.
Table 1.
Correlation among apurinic/apyrimidinic endonuclease1 (APE1), transforming growth factor β (TGFβ), and microvessel density (MVD) in osteosarcoma patients
| APE1 | TGFβ | MVD | ||
|---|---|---|---|---|
| APE1 | R squared | 1.000 | 0.259† | 0.291 |
| P-value | 0.000 | 0.020 | 0.001 | |
| TGFβ | R squared | 0.259 | 1.000 | 0.324 |
| P-value | 0.020 | 0.000 | 0.001 | |
| MVD | R squared | 0.259 | 0.324† | 1.000 |
| P-value | 0.001 | 0.001 | 0.000 |
Positive correlation. Data evaluated using Spearman’s rank correlation analysis.
Figure 2.
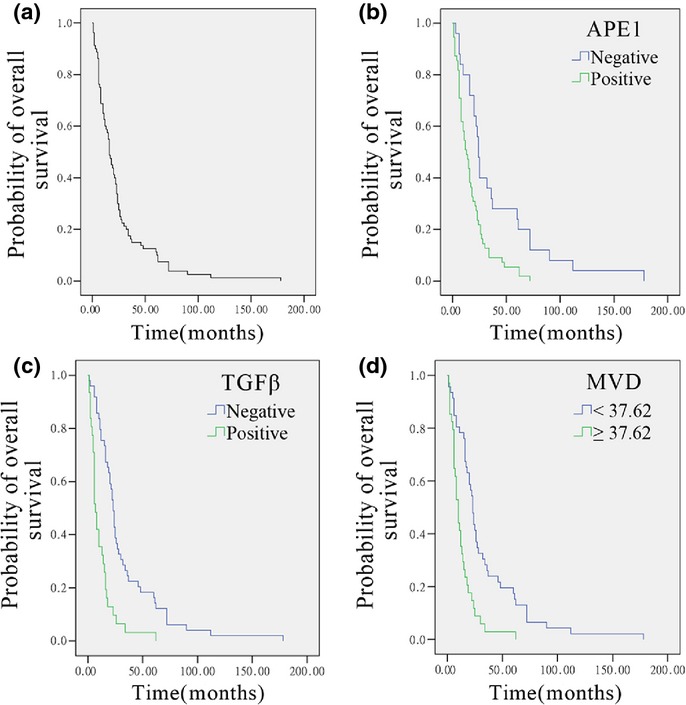
Survival analysis of 80 osteosarcoma patients. (a) Overall survival analysis shows the 2-year survival rate was 33.8% and the 5-year survival rate was 18.3%. (b–d) Univariate analysis reveals that apurinic/apyrimidinic endonuclease 1 (APE1) (b), transforming growth factor β (TGFβ) (c), and microvessel density (MVD) (d) (n = 80; P < 0.05 each) had a significant relation to prognosis.
Table 2.
Univariate analysis for prognosis of osteosarcoma patients
| n (n = 80) | Median OS, months | P-value | |
|---|---|---|---|
| Age, years | |||
| High, ≥19 | 53 | 19.0 | 0.056 |
| Low, <19 | 27 | 12.0 | |
| Gender | |||
| Male | 49 | 16.0 | 0.383 |
| Female | 31 | 20.0 | |
| Histopathology | |||
| Chondroblastic | 16 | 19.0 | 0.102 |
| Fibroblastic | 24 | 15.0 | |
| Mixed | 5 | 14.0 | |
| Small cell | 3 | 7.0 | |
| Osteoblastic | 32 | 16.0 | |
| Enneking staging | |||
| I | 9 | 20.0 | 0.102 |
| II | 36 | 21.0 | |
| III | 35 | 13.0 | |
| Size, mm | |||
| <10.36 | 50 | 21.0 | 0.002* |
| ≥10.36 | 30 | 8.0 | |
| High expression of TGFβ | |||
| − | 49 | 23.0 | 0.000* |
| + | 31 | 7.0 | |
| MVD | |||
| <37.62 | 46 | 23.0 | 0.000* |
| ≥37.62 | 34 | 10.0 | |
| High expression of APE1 | |||
| − | 25 | 22.0 | 0.001* |
| + | 55 | 13.0 | |
P < 0.01. APE1, apurinic/apyrimidinic endonuclease; TGFβ, transforming growth factor; MVD, microvessel density; OS, overall survival.
Table 3.
Hazard ratios for overall survival of osteosarcoma patients
| Variable | Degrees of freedom | Parameter estimate | Standard error | Wald χ2-test | P-value | Hazard risk | 95% CI |
|---|---|---|---|---|---|---|---|
| APE1 | 1 | 0.293 | 0.296 | 0.979 | 0.322 | 1.340 | 0.750–2.393 |
| Size | 1 | 0.097 | 0.043 | 5.152 | 0.023 | 1.102 | 1.013–1.199 |
| Grade | 1 | 0.161 | 0.186 | 0.749 | 0.387 | 1.175 | 0.816–1.692 |
| MVD | 1 | 0.011 | 0.006 | 4.117 | 0.042 | 1.012 | 1.000–1.023 |
| TGFβ | 1 | 0.861 | 0.279 | 9.535 | 0.002 | 2.366 | 1.370–4.086 |
APE1, apurinic/apyrimidinic endonuclease 1; CI, confidence interval; MVD, microvessel density; TGFβ, transforming growth factor β.
Apurinic/apyrimidinic endonuclease 1-siRNA-mediated downregulation in osteosarcoma cells inhibits expression of TGFβ1
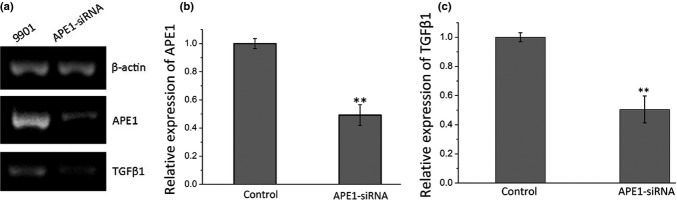
We then tested whether TGFβ1 expression is affected by APE1 exogenous manipulation in 9901 cells by Western blot. As shown in Figure 3, the expression of APE1 was decreased in APE1-siRNA transfected cells when compared with the scramble-siRNA transfected cells and blank control. When APE1 was knocked down, TGFβ1 expression was downregulated. The percentage inhibition of APE1 and TGFβ1 protein was 88.75% and 63.1% compared to the blank control, respectively (P < 0.01), and there were no differences between the scramble and blank control. To evaluate the secretory level of TGFβ1 affected by APE1 deficiency, an ELISA was carried out. The results indicated that knocking down APE1 in 9901 cells results in a significant decrease in TGFβ1 levels in the supernatant when compared with control groups (435.23 ± 22.80 pg/mL for the APE1-siRNA group, 731.610 ± 48.949 pg/mL for the blank control group, and 755.75 ± 51.36 pg/mL for the scramble group with an inhibition level of 40.51% (Fig. 3d). To investigate the mRNA level of TGFβ1 expression after knocking down APE1-siRNA, we carried out an RT-PCR assay (P < 0.01; Fig. 4a). In APE1-siRNA transfected 9901 cells, the expression of APE1 was decreased by 50.81% (P < 0.01; Fig. 4b). As a result of APE1 knockdown, TGFβ1 expression was downregulated by 49.54% (P < 0.01; Fig. 4c). The above experiments confirmed that exogenous manipulation of APE1 results in altered expression of TGFβ1, suggesting a regulatory role of APE1 in TGFβ1 signaling.
Figure 3.
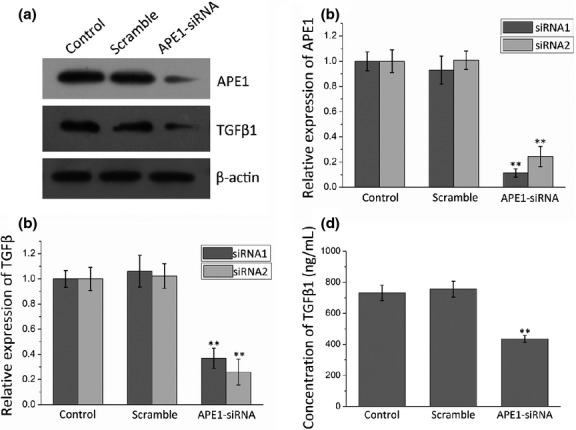
Osteosarcoma 9901 cells transfected with two apurinic/apyrimidinic endonuclease 1 (APE1)-siRNAs, negative control (scramble), or Optimum I as blank (control). (a) Western blot images. (b,c) Western blot analysis shows that APE1-siRNA suppresses expression of both APE1 and transforming growth factor β1 (TGFβ1). (d) ELISA assays show the concentration of TGFβ1 in the supernatant of the control, scramble, and treatment groups. **P < 0.01.
Figure 4.
Osteosarcoma 9901 cells transfected with apurinic/apyrimidinic endonuclease 1 (APE1)-siRNA, with the negative control as scramble control. (a) PCR images. (b, c) PCR reveals that APE1-siRNA suppresses expression of APE1 and transforming growth factor β1 (TGFβ1). **P < 0.01.
Apurinic/apyrimidinic endonuclease 1-siRNA inhibits the capability to enhance HUVEC migration and tube formation of tumor cells through the TGFβ/Smad3 signaling pathway
To test the hypothesis that APE1 regulates tumor angiogenesis through a TGFβ-dependent pathway, supplementation with purified TGFβ protein in APE1 deficient cells was used to measure the in vitro angiogenic capacity in HUVECs using a Transwell migration and tube formation assay. As shown in Figure 5(a,b), fewer HUVECs migrated in the APE1-siRNA transfected group than in the negative control, with a reduction level of 62.6% (P < 0.01). When supplemented with TGFβ, the HUVEC migration was restored in a dose-dependent manner. The differences between the two groups were significant (P < 0.01, respectively). Additionally, the Matrigel tube formation assay revealed that fewer capillary-like structures were formed in the APE1-siRNA transfected group than the negative control (P < 0.01), and that TGFβ supplementation significantly restored the architecture of tube formation (Fig. 5c,d). These results suggested that APE1 regulates TGFβ during tumor angiogenesis in a dose-dependent manner.
Figure 5.

Apurinic/apyrimidinic endonuclease 1 (APE1)-siRNA inhibited the capability of osteosarcoma cells to promote HUVEC migration and tube formation. (a) Typical images of migration. Culture medium of APE1-siRNA treated 9901 cells results in fewer migrations compared to culture medium of the control group. Addition of transforming growth factor β (TGFβ) restored the migration capability of culture medium containing 9901 cells transfected with APE1-siRNA in a dose-dependent way. (b) Quantitative analysis of HUVEC migration. (c) Typical images of tube formation assay. Culture medium of APE1-siRNA treated 9901 cells produced fewer capillary-like structures than culture medium of the control group. The angiogenesis capacity of culture medium containing APE1-siRNA treated 9901 cells was increased by the addition of TGFβ in a dose-dependent manner. (d) Quantitative analysis of tube formation. **P < 0.01.
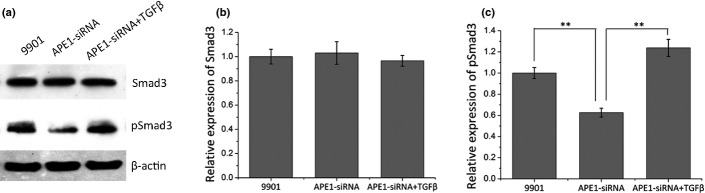
Furthermore, we tested whether the downstream TGFβ signaling pathway was affected by APE1 deficiency by measuring the activation of Smad3. As Figure 6 revealed, the pSmad3 level was remarkably decreased to 62.61% in the group treated with supernatant from culturing 9901 cells treated with 0.2 nM APE1-siRNA compared with those without APE1-siRNA (P < 0.01) (Fig. 6c), while Smad3 levels remained unchanged (Fig. 6b). Moreover, addition of purified TGFβ protein restored the phosphorylated Smad3 level caused by APE1-siRNA (P < 0.01). Taken together, the results indicated that APE1 regulates angiogenesis through the TGFβ/Smad3 signaling pathway.
Figure 6.
Three groups of HUVECs treated with supernatants of untreated osteosarcoma 9901 cells (9901), 9901 cells treated with apurinic/apyrimidinic endonuclease 1 (APE1)-siRNA, or 9901 cells treated with APE1-siRNA plus purified recombinant transforming growth factor β (TGFβ) protein (APE1-siRNA + TGFβ). (a) Western blot images. (b) Western blot analyses show no difference in protein level of Smad3 between the two groups. (c) Western blot analysis shows that HUVECs treated with supernatant of APE1-siRNA treated 9901 cells remarkably decreases the phosphorylation level of Smad3, and addition of TGFβ restores the decrease. **P < 0.01.
Tumor angiogenesis and growth in xenografts suppressed by APE1-siRNA
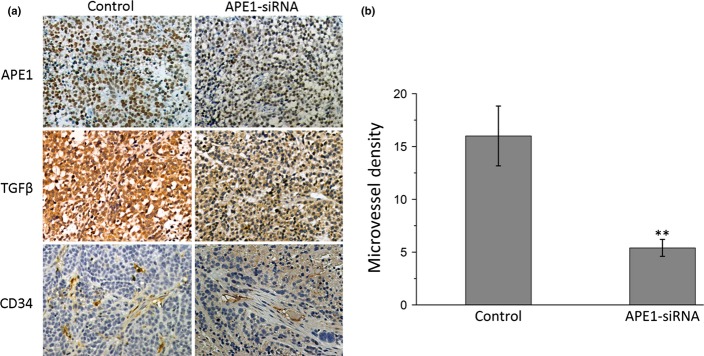
The 9901 cell xenograft model was used to confirm that APE1-siRNA suppresses tumor angiogenesis and growth in vivo. The xenograft model and treatment was the same as in Ren et al.,18 in which the APE1-siRNA treated group resulted in a smaller volume of neoplasm than the PBS treated group (P < 0.01). Tumor sections were analyzed after IHC staining for APE1, TGFβ, and CD34. Figure 7 shows representative images of the treatment and control groups. As expected, expression of APE1, TGFβ, and MVD was significantly lower in the treatment group compared with the control group (P = 0.033 for APE1; P = 0.041 for TGFβ; P = 0.041 for MVD). The data further confirmed the role of APE1 in the angiogenesis of osteosarcoma and suggested it is possibly mediated by TGFβ signaling.
Figure 7.
Immunohistochemical expression of apurinic/apyrimidinic endonuclease 1 (APE1), transforming growth factor β1 (TGFβ1), and CD34 in a xenograft mouse model (magnification, ×200). (a) Left, high expression of APE1, TGFβ, and CD34 in mice from the control group. Right, low expression of APE1, TGFβ, and CD34 of mice in the APE1-siRNA group. (b) Microvessel density of control and APE1-siRNA groups. **P < 0.01.
Discussion
In this study, we have shown that APE1 and TGFβ expression is significantly correlated with poor prognosis in osteosarcoma patients, based on the analysis of clinical data. Subsequently, we confirm that knocking down APE1 in osteosarcoma cells resulted in downregulation of TGFβ at both protein and mRNA levels. Angiogenesis is suppressed by APE1-siRNA through the TGFβ/Smad3 signaling pathway in osteosarcoma; addition of TGFβ protein restored the reduced pSmad3 and the angiogenic capability of HUVECs induced by APE1 deficiency. Taken together, our results show that APE1 regulates angiogenesis in a TGFβ-dependent way in osteosarcoma.
Apurinic/apyrimidinic endonuclease 1 is a multifunctional protein playing crucial roles in DNA base excision repair and redox regulation of gene expression, activities that are functionally and structurally independent of each other. The APE1 redox activity stimulates numerous transcriptional factors, including activator protein-1 (AP-1), nuclear factor-κB (NF-κB), and HIF-1.14,29 These factors are involved in mediating VEGF gene expression; HIF-1 and NF-κB, in particular, increased VEGF expression in response to hypoxia.30 It has been reported that APE1 is an upstream regulator of VEGF in angiogenesis.14 Our previous study showed that APE1 induced angiogenesis in human osteosarcoma cells by upregulating FGF2 and its receptor 3.18 However, a previous study by us revealed that exogenous addition of VEGF and FGF2 failed to restore angiogenesis after inhibition by APE1-siRNA. Thus, we infer that APE1 regulated other factors involved in tumor angiogenesis in addition to VEGF and FGF2. Our previous investigation indicated that knockdown of APE1 resulted in the downregulation of factors involved in angiogenesis, such as epidermal growth factor receptor, FGF2, TGFβ, TGFβ receptor1, and thrombospondin-1 in osteosarcoma cells.23 Transforming growth factor β has a conserved signaling pathway and its dysregulation in a tumor microenvironment, invasive properties, and immune cell functions could cause cancer metastasis. It can stimulate the generation of myofibroblasts, which produce certain factors and regulate key angiogenic mediators to promote angiogenesis.20,21 In particular, TGFβ is closely related with the pathogenesis of osteosarcoma and it has been reported by Cao et al.31 and Zhang et al.32 that higher serum levels of TGFβ were observed in patients with osteosarcoma than in healthy people, although there was no statistical significance between the two groups. However, a significantly higher expression of TGFβ was measured in the serum of osteosarcoma patients with metastasis compared to those without metastasis.23 In this study, we revealed that APE1-siRNA suppresses the capacity of Transwell and tube formation of HUVECs through the TGFβ/Smad3 signaling pathway in osteosarcoma.
Angiogenesis plays a key role in tumor growth and progression, which is considered an indicator of a higher incidence of metastasis and poor prognosis.10,33,34 Moreover, angiogenesis is regulated by both angiogenic stimulators, including members of FGF and VEGF families, as well as angiogenic inhibitors like angiostatin and endostatin.34 In tumor metastasis, TGFβ stimulates angiogenesis by inducing a pro-angiogenic environment and also promotes epithelial–mesenchymal transition (EMT) as a major inducer of both Smad-dependent and Smad-independent signaling pathways.21 During the process of EMT, epithelial cells lose their polarity, disassemble the cell junction, increase cell motility, and acquire invasive properties to become mesenchymal cells.21,35 However, in the case of osteosarcoma, which is a mesenchymal-derived malignant bone tumor, EMT is no longer the key issue, which implies that the overexpressed TGFβ might be involved in some pathological process other than EMT. In the aspect of angiogenesis, TGFβ1 signaling machinery exerts a protumorigenic effect in canine osteosarcoma.36 Therefore, we speculate that TGFβ promotes tumor progression and metastasis through angiogenesis in osteosarcoma. Sanchez-Elsner et al. discovered that TGFβ and the hypoxia signaling pathway synergize in the induction of VEGF gene expression, which stimulates angiogenesis.37 In primary breast cancer, multiple angiogenic factors, including VEGF, TGFβ, and FGF are expressed,38 and it has been confirmed that TGFβ-induced angiogenesis depending on Smad3 promotes bone metastasis in breast cancer.39 Moreover, TGFβ promotes tumor progression probably by stimulating angiogenesis in prostate cancer.40 It has been confirmed that Smad3, a critical intracellular signaling molecule, has a pro-angiogenic role in response to TGFβ1.41 However, the specific effect APE1 has in these processes is yet to be determined.
It has been confirmed that AP-1 is the main transcription factor that plays a significant role in inducing the second promoter-derived transcription of the TGFβ1 gene (sequences between nucleotides +1 and +271), and a family member of AP-1 (Fos-related antigen 2/FOSL2) regulates TGFβ by interacting with Smad3 in NSCLC.42,43 There was also a report that when NF-κB interacted with CCAAT/Enhancer Binding Protein β (C/EBPβ), the activation of TGFβ induced by C/EBPβ can be modulated.44 Moreover, there is evidence that NF-κB activity is involved in AP-1 activation and regulated TGFβ expression in angiogenesis.45,46 In accordance with previous studies, we propose that APE1, as a redox effector, regulates TGFβ, possibly by an AP-1 and NF-κB transcriptional signaling pathway in the angiogenesis of osteosarcoma.
As an additive to our previous studies on the role of APE1 in tumor angiogenesis, our current observations further confirmed TGFβ as an important factor controlled by APE1 in contributing to the process of angiogenesis. We believe that our current study, together with previous ones, provides solid evidence that APE1 is a key regulator of tumor angiogenesis through more complicated mechanisms than we initially expected.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81172117).
Disclosure Statement
The authors have no conflict of interest.
References
- Duong LA, Richardson LC. Descriptive epidemiology of malignant primary osteosarcoma using population-based registries, United States, 1999-2008. J Registry Manag. 2013;40:59–64. [PMC free article] [PubMed] [Google Scholar]
- Jentzsch T, Robl B, Husmann M, Bode-Lesniewska B, Fuchs B. Worse prognosis of osteosarcoma patients expressing IGF-1 on a tissue microarray. Anticancer Res. 2014;34:3881–9. [PubMed] [Google Scholar]
- Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–61. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- Zhao J, Xu H, He M. Rho GTPase-activating protein 35 rs1052667 polymorphism and osteosarcoma risk and prognosis. BioMed research international. 2014;2014:396947. doi: 10.1155/2014/396947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–32. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–35. doi: 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med. 2014;17:301–7. [PubMed] [Google Scholar]
- Kunz P, Fellenberg J, Moskovszky L, et al. Improved survival in osteosarcoma patients with atypical low vascularization. Ann Surg Oncol. 2015;22:489–96. doi: 10.1245/s10434-014-4001-2. [DOI] [PubMed] [Google Scholar]
- Zwaga T, Bovee JV, Kroon HM. Osteosarcoma of the femur with skip, lymph node, and lung metastases. Radiographics. 2008;28:277–83. doi: 10.1148/rg.281075015. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Gasparini G. The rationale and future potential of angiogenesis inhibitors in neoplasia. Drugs. 1999;58:17–38. doi: 10.2165/00003495-199958010-00003. [DOI] [PubMed] [Google Scholar]
- Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK. APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med. 2014;46:e106. doi: 10.1038/emm.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, McNeill DR, Abbotts R, et al. Synthetic lethal targeting of DNA double-strand break repair deficient cells by human apurinic/apyrimidinic endonuclease inhibitors. Int J Cancer. 2012;131:2433–44. doi: 10.1002/ijc.27512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MR, Georgiadis MM, Fishel ML. APE1/Ref-1 role in redox signaling: translational applications of targeting the redox function of the DNA repair/redox protein APE1/Ref-1. Curr Mol Pharmacol. 2012;5:36–53. doi: 10.2174/1874467211205010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu X, Zhou T, et al. Suppression of choroidal neovascularization through inhibition of APE1/Ref-1 redox activity. Invest Ophthalmol Vis Sci. 2014;55:4461–9. doi: 10.1167/iovs.14-14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Luo M, Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 2004;3:679–86. [PubMed] [Google Scholar]
- Wang D, Zhong ZY, Li MX, Xiang DB, Li ZP. Vector-based Ape1 small interfering RNA enhances the sensitivity of human osteosarcoma cells to endostatin in vivo. Cancer Sci. 2007;98:1993–2001. doi: 10.1111/j.1349-7006.2007.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Qing Y, Dai N, et al. Apurinic/apyrimidinic endonuclease 1 induced upregulation of fibroblast growth factor 2 and its receptor 3 induces angiogenesis in human osteosarcoma cells. Cancer Sci. 2014;105:186–94. doi: 10.1111/cas.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Oshima Y, Hikita A. Regulation of TGF-beta family signalling by ubiquitination and deubiquitination. J Biochem. 2013;154:481–9. doi: 10.1093/jb/mvt097. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- Franchi A, Arganini L, Baroni G, et al. Expression of transforming growth factor beta isoforms in osteosarcoma variants: association of TGF beta 1 with high-grade osteosarcomas. J Pathol. 1998;185:284–9. doi: 10.1002/(SICI)1096-9896(199807)185:3<284::AID-PATH94>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dong W, Zhaoyang Z, Zengpeng L, Zhenzhou Y, Kelley MR. The altered gene profile of human osteosarcoma cell after knock-down of double functional gene apurinic/apyrimidinic endonuclease. J Third Mil Med Univ. 2007;01:1–4. [Google Scholar]
- Enneking WF. A system of staging musculoskeletal neoplasms. Clinical orthopaedics and related research. Clinical orthopaedics and related research. 1986;204:9–24. [PubMed] [Google Scholar]
- Ross FG. Osteogenic sarcoma. Br J Radiol. 1964;37:259–76. doi: 10.1259/0007-1285-37-436-259. [DOI] [PubMed] [Google Scholar]
- Khoury JD, Abrahams NA, Levin HS, MacLennan GT. The utility of epithelial membrane antigen and vimentin in the diagnosis of chromophobe renal cell carcinoma. Ann Diagn Pathol. 2002;6:154–8. doi: 10.1053/adpa.2002.33901. [DOI] [PubMed] [Google Scholar]
- Jokhi PP, King A, Sharkey AM, Smith SK, Loke YW. Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J Immunol. 1994;153:4427–35. [PubMed] [Google Scholar]
- Zhou B, Ma R, Si W, et al. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159–69. doi: 10.1016/j.canlet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Jiang A, Gao H, Kelley MR, Qiao X. Inhibition of APE1/Ref-1 redox activity with APX3330 blocks retinal angiogenesis in vitro and in vivo. Vision Res. 2011;51:93–100. doi: 10.1016/j.visres.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–15. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chen QX, Hou ZY, Hu J, Cao YG. Clinical predictive value of serum angiogenic factor in patients with osteosarcoma. Asian Pac J Cancer Prev. 2012;13:4823–6. doi: 10.7314/apjcp.2012.13.9.4823. [DOI] [PubMed] [Google Scholar]
- Xu S, Yang S, Sun G, Huang W, Zhang Y. Transforming growth factor-Beta polymorphisms and serum level in the development of osteosarcoma. DNA Cell Biol. 2014;33:802–6. doi: 10.1089/dna.2014.2527. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Seshadri M, Oven SD, Toth K, Vaughan MM, Rustum YM. Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs. Clin Cancer Res. 2008;14:3926–32. doi: 10.1158/1078-0432.CCR-08-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–24. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- Yang G, Yuan J, Li K. EMT transcription factors: implication in osteosarcoma. Med Oncol. 2013;30:697. doi: 10.1007/s12032-013-0697-2. [DOI] [PubMed] [Google Scholar]
- Portela RF, Fadl-Alla BA, Pondenis HC, et al. Pro-tumorigenic effects of transforming growth factor beta 1 in canine osteosarcoma. J Vet Intern Med. 2014;28:894–904. doi: 10.1111/jvim.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–35. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- Relf M, LeJeune S, Scott PA, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–9. [PubMed] [Google Scholar]
- Petersen M, Pardali E, van der Horst G, et al. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene. 2010;29:1351–61. doi: 10.1038/onc.2009.426. [DOI] [PubMed] [Google Scholar]
- Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Li JH, Garcia G, et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66:605–13. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989;264:19373–8. [PubMed] [Google Scholar]
- Wang J, Sun D, Wang Y, et al. FOSL2 positively regulates TGF-beta1 signalling in non-small cell lung cancer. PLoS ONE. 2014;9:e112150. doi: 10.1371/journal.pone.0112150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S, Sweet T, Khalili K, Sawaya BE, Amini S. Evidence for activation of the TGF-beta1 promoter by C/EBPbeta and its modulation by Smads. J Interferon Cytokine Res. 2009;29:1–7. doi: 10.1089/jir.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley G, Harvey K, Slivova V, Jiang J, Sliva D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem Biophys Res Commun. 2005;330:46–52. doi: 10.1016/j.bbrc.2005.02.116. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Niu J, Schmidt C, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–19. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]