Abstract
Magnolol, the major active compound found in Magnolia officinalis has a wide range of clinical applications due to its anti-inflammation and anti-oxidation effects. This study investigated the effects of magnolol on the growth of human gallbladder carcinoma (GBC) cell lines. The results indicated that magnolol could significantly inhibit the growth of GBC cell lines in a dose- and time-dependent manner. Magnolol also blocked cell cycle progression at G0/G1 phase and induced mitochondrial-related apoptosis by upregulating p53 and p21 protein levels and by downregulating cyclin D1, CDC25A, and Cdk2 protein levels. When cells were pretreated with a p53 inhibitor (pifithrin-a), followed by magnolol treatment, pifithrin-a blocked magnolol-induced apoptosis and G0/G1 arrest. In vivo, magnolol suppressed tumor growth and activated the same mechanisms as were activated in vitro. In conclusion, our study is the first to report that magnolol has an inhibitory effect on the growth of GBC cells and that this compound may have potential as a novel therapeutic agent for the treatment of GBC.
Keywords: Apoptosis, cell cycle arrest, gallbladder carcinoma, magnolol, p53 pathways
Gallbladder carcinoma (GBC) is the most common cancer of the biliary tract, with an incidence rate of 2.5 per 100 000 individuals.1,2 Patients with GBC often do not show early symptoms and may receive late diagnoses; thus, only a minority of patients are candidates for curative resection, and no reported benefit of neoadjuvant therapy for advanced GBC exists.3 The median survival time for GBC patients is less than 1 year.4,5 Therefore, identifying new therapeutic agents against GBC is critical to improving patient health and survival.
The tumor suppressor p53 is a key transcription factor and regulates the expression of genes involved in the apoptosis, angiogenesis, arrest, and DNA repair pathway.6 It exerts its function at the beginning of the intrinsic apoptotic pathway and causes cell cycle arrest at G1 phase in response to DNA damage. When the DNA damage is irreparable, the p53 protein will activate the appropriate cellular signaling cascades to execute apoptosis. Thus, p53 is critical for maintaining genome integrity (ploidy and structure) and for regulating both cell growth and proliferation.7–9 Moreover, previous studies have indicated that p53 status is a poor predictor of patient prognosis in various types of cancer.10 Consistent with these critical roles, p53 may be a promising target for cancer treatment.
Traditional herbal medicines containing various biologically active natural compounds are claimed to have therapeutic efficacy with minimal adverse effects.11,12 Magnolol, a primary active ingredient of the Chinese medicinal herb Magnolia officinalis, displays a variety of pharmacological activities including anti-oxidant and anti-inflammatory activities and central nervous system depressant effects.13,14 Recent studies have indicated that magnolol induces antiproliferative effects by blocking DNA synthesis and by inducing apoptosis and cell cycle arrest in a wide variety of tumor cells including colon, liver, and ovarian cancer cells.15–17 However, the effects and mechanism of magnolol on human GBC cells have never been elucidated. In the present study, we show that magnolol effectively inhibited the proliferation of GBC cells by arresting the cell cycle at G0/G1 phase and blocked the induction of apoptosis by upregulating p53. This inhibition leads to the reduction of localized tumor growth in mice, indicating that magnolol is a promising therapeutic agent for the treatment of GBC.
Materials and Methods
Reagents
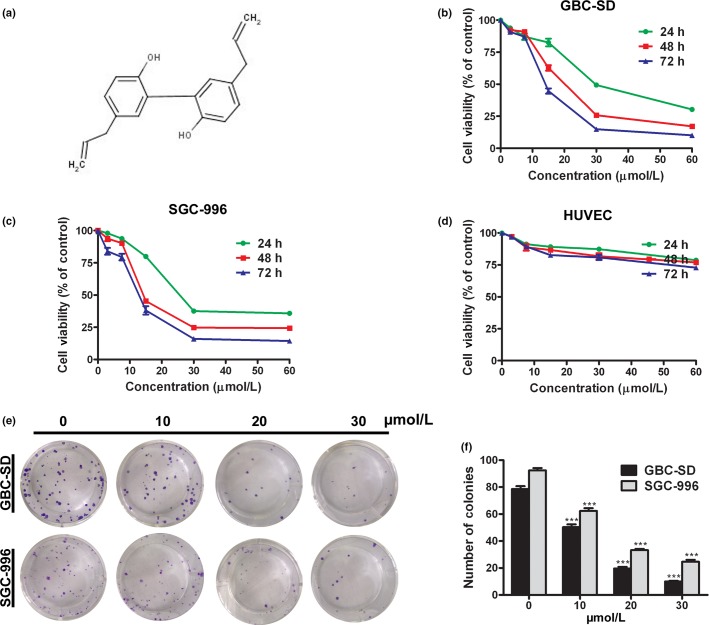
Magnolol was obtained from Sigma-Aldrich (St. Louis, MO, USA) (Fig.1a), dissolved in 100% DMSO, stored at −20°C, and then diluted with cell culture media before use. The final DMSO concentration did not exceed 0.1%. The p53 inhibitor pifithrin-a (PFT-a) was purchased from Beyotime Institute of Biotechnology (Nantong, Jiangsu, China). A Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies (Kumamoto, Japan). All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All cell culture supplies were obtained from Invitrogen Gibco (Carlsbad, CA, USA).
Figure 1.
Magnolol inhibits cell proliferation and colony formation in gallbladder cancer (GBC) cells. (a) Chemical structure of magnolol. (b–d) HUVECs, GBC-SD, and SGC-996 cells were treated with various concentrations of magnolol for 24, 48, and 72 h. Cell viability was assessed by CCK-8 assay. (e,f) Magnolol suppressed the colony formation abilities of GBC-SD and SGC-996 cells. Cells were treated with magnolol and then cultured in fresh medium for 14 days to form colonies. The values represent the mean ± SD of three independent experiments. ***P < 0.001.
Cell culture
The GBC-SD cell line and HUVEC line were purchased from the Shanghai Institutes for Biological Sciences (Shanghai, China). The GBC-SD cell line was established from a 61-year-old male patient with poorly differentiated GBC. The SGC-996 cell line, isolated from the primary mastoid adenocarcinoma of the gallbladder obtained from a 61-year-old female patient, was provided by the Academy of Life Science, Tongji University (Shanghai, China). All cells were maintained in DMEM with 10% (v/v) bovine calf serum and were routinely cultivated in a humidified incubator at 37°C and 5% CO2.
Cell viability assay
Cell viability was determined using CCK-8 according to the manufacturer’s instructions. Briefly, cancer cells were seeded in 96-well plates and were treated for either 48 h with magnolol at serial concentrations (0, 10, 20, and 30 μmol/L) or with magnolol at various times (0, 24, 48, or 72 h). After each treatment, CCK-8 solution (10 μL) was added to each well, and the cells were incubated for 3 h. The absorbance was recorded at an optical density of 450 nm using a microplate reader (Bio-Tek, Norcross, GA, USA) to calculate the percentages of cell survival. The IC50 values were determined by plotting a linear regression curve.
Colony formation assay
The GBC-SD and SGC-996 cell lines treated with different concentrations of magnolol were counted and seeded in 12-well plates (in triplicate) at 100 cells per well. The medium was replaced with fresh culture medium every 3 days. Colonies were counted only if they contained more than 50 cells. The number of colonies was counted from the sixth day after seeding, and then the cells were stained with crystal violet. The rate of colony formation was calculated using the following equation: colony formation rate = (number of colonies/number of seeded cells) × 100%.
Cell cycle and apoptosis analyses
Propidium iodide (PI) staining was used to analyse DNA content and cell cycle distribution. After exposure to different concentrations of magnolol for 24 h, the cells were harvested and fixed in 70% ethanol. Then, the cells were centrifuged (875g, 5 min), incubated with RNase (100 mg/mL) at 37°C for 30 min, and stained with PI (50 mg/mL in PBS). The cellular DNA content and cell cycle distribution were analyzed by flow cytometry (FACSCalibur; BD, Bedford, MA, USA).
Apoptosis analyses were carried out using an annexin V–FITC Apoptosis Detection Kit (BioVision, Milpitas, CA, USA). Cells (5 × 105) were exposed to different concentrations of magnolol for 24 h. Then, the cells were collected by centrifugation and resuspended in 500 μL of 1 × binding buffer. Next, annexin V–FITC (5 μL) and PI (5 μL) were added to the cells. After the cells were incubated at room temperature for 5 min in the dark, they were analyzed by FACS using a flow cytometer (FACSCalibur; BD). Cells that stained positive for early apoptosis (annexin V–FITC-stained only) and for late apoptosis (annexin V–FITC- and PI-stained) were combined for analysis.
Mitochondrial membrane potential (ΔΨm) assay
After the cells were treated with different concentrations of magnolol for 48 h, they were collected and washed twice with cold PBS. Then the cells were incubated with rhodamine 123 (Sigma-Aldrich) for 30 min in the dark. Subsequently, the cells were washed twice with cold PBS and analysed by flow cytometry.
Detection of morphological apoptosis by Hoechst 33342 staining
The GBC cell lines were treated with different concentrations of magnolol for 48 h, then washed with PBS and fixed in methanol:acetic acid (3:1) for 15 min at room temperature. The fixed cells were washed with PBS and stained with 5 μg/mL Hoechst 33342 for 10 min. Then the morphological changes in the Hoechst 33342-stained nuclei of cells were observed using a fluorescence microscope (Leica, Wetzlar, Germany).
Western blot analysis
Total cell lysates were separated on 10% SDS-PAGE gels and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked and then probed with primary antibodies directed against corresponding proteins overnight at 4°C. After the membranes were washed, they were incubated with the appropriate HRP-linked secondary antibodies and visualized using enhanced chemiluminescent detection reagent.
In vivo efficacy of magnolol
BALB/c homozygous (nu/nu) nude mice (6–8 weeks old, 18–20 g body weight) that were bred in-house were maintained in a specific pathogen-free environment. Exponentially growing GBC-SD cells (2.5 × 106) were suspended in 100 μL PBS and injected s.c. into the left axilla of recipient mice. On day 5, tumor-bearing mice were randomly divided into four groups (control, 5, 10, and 20 mg/kg), with five animals in each group. Magnolol was administered to the mice in the latter three treatment groups by i.p. injection every day at 5, 10, or 20 mg/kg, respectively. Control animals received i.p. injections of DMEM. On day 28, all mice were killed, and tumors were dissected and weighed. Animal procedures were carried out in accordance with the institutional guidelines of Shanghai Jiaotong University (Shanghai, China).
Statistical analysis
The results of each experiment are presented as the mean ± SD where applicable. Statistically significant differences in each assay were determined using spss version 18.0 (SPSS, Chicago, IL, USA). Differences in each group were tested for significance using anova. P < 0.05 was considered statistically significant.
Results
Magnolol inhibits cell proliferation and colony formation in GBC-SD and SGC996 cells
Deregulated cell proliferation is a hallmark of cancer.18 To determine the effects of magnolol on GBC cell proliferation and independent growth, we investigated the proliferative activities and independent growth of GBC-SD and SGC-996 cell lines by CCK-8 and colony formation assays. The results indicated that magnolol significantly diminished the proliferative activities of both cancer cell lines in a dose- and time-dependent manner compared with the control group (Fig.1b,c). The IC50 values of magnolol against GBC-SD and SGC-996 cell viability were 20.5 ± 6.8 μmol/L and 14.9 ± 5.30 μmol/L, respectively, after 48 h. However, magnolol treatment did not markedly inhibit HUVEC viability (Fig.1d). In addition, the colony formation rates of GBC-SD and SGC-996 cells in magnolol-treated groups were markedly lower than that of the control group (Fig.1e,f; P < 0.001 each).
Magnolol induces mitochondrial-related apoptosis in GBC-SD and SGC-996 cells
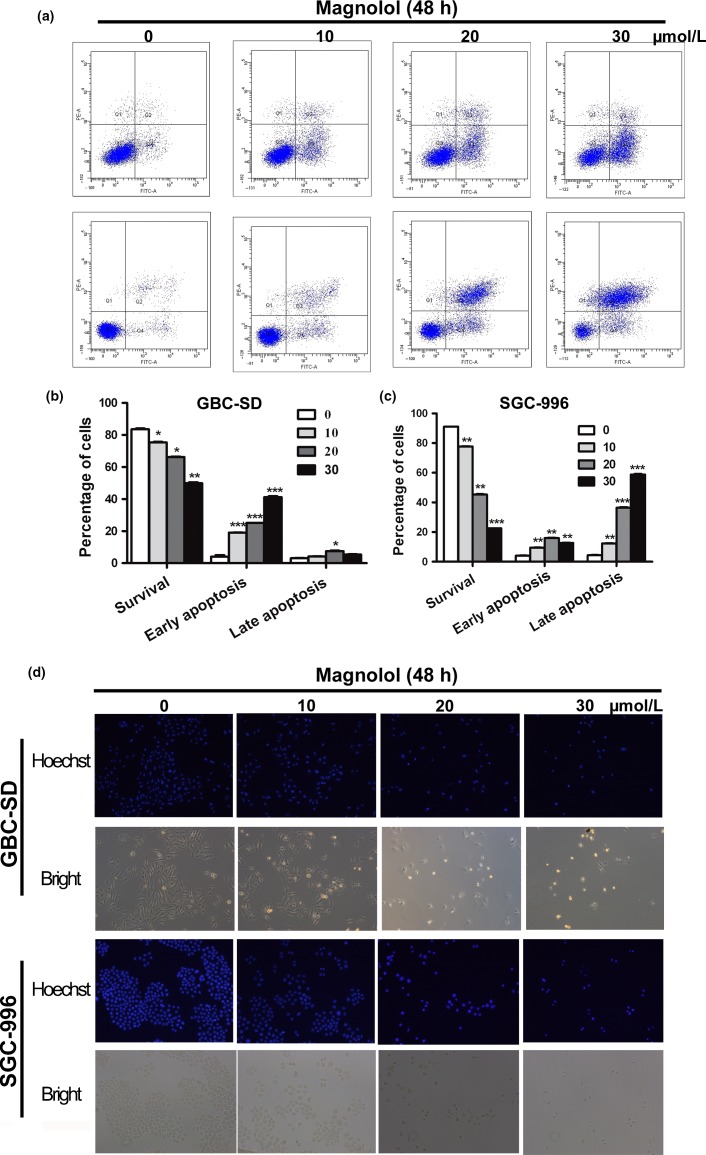
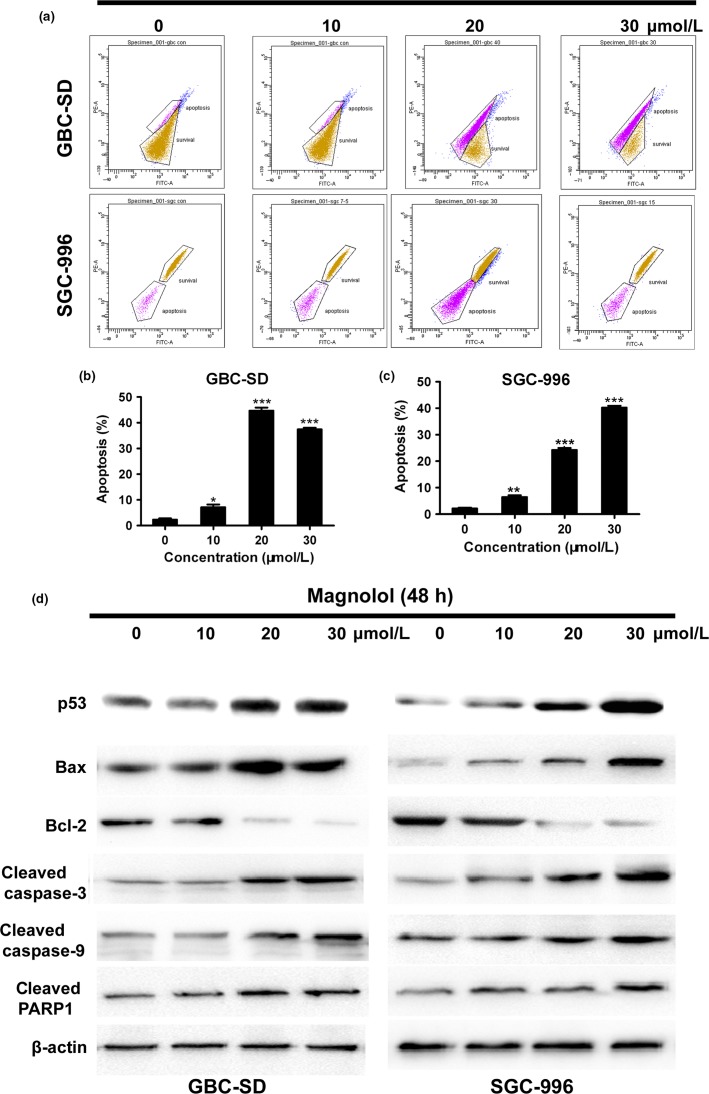
Apoptotic cells with nuclear condensation and fragmentation can be visualized using Hoechst 33258 and DAPI staining. To determine the effects of magnolol treatment on GBC cell apoptosis, Hoechst 33258 staining and flow cytometric analysis were carried out. After GBC cells were exposed to four concentrations of magnolol (0, 10, 20, and 30 μmol/L) for 48 h, the apoptosis index of GBC cells in magnolol-treated groups was markedly higher than in the control group) (Fig.2a–c; *P < 0.05, **P < 0.01, ***P < 0.001). Moreover, GBC cell apoptosis was confirmed using Hoechst 33258 staining, which revealed increased cell membrane permeability and nuclear condensation (Fig.2d). In addition, a decline in the ΔΨm is a characteristic of apoptosis. In this study, the ΔΨm in magnolol-treated GBC cells was examined using rhodamine 123, which can be used to estimate membrane integrity and membrane potential changes. A dose-dependent dissipation of potential was observed in the mitochondrial membranes of GBC cells after 48 h of incubation with magnolol (Fig.3a–c). In addition, magnolol treatment resulted in a time-dependent increase in Bax/Bcl-2 levels, as well as the caspase-9-dependent activation of caspase-3 and of a cleaved form of poly(ADP-ribose) polymerase (Fig.3d). These results indicate that magnolol-induced GBC cell apoptosis occurs through the mitochondria caspase-dependent pathway.
Figure 2.
Magnolol induces apoptosis in gallbladder cancer (GBC) cells. (a–c) GBC-SD and SGC-996 cells were analyzed by flow cytometry with annexin V–FITC/propidium iodide (PI) staining after magnolol treatment. Annexin V versus PI plots from the gated cells show the populations corresponding to viable (annexin V−/PI−), necrotic (annexin V−/PI+), early apoptotic (annexin V+/PI−), and late apoptotic (annexin V+/PI+) cells. The data are presented as the mean ± SD of three independent experiments. (d) Changes in nuclear morphology during apoptosis were observed by Hoechst 33342 staining and visualized by fluorescence microscopy. *P < 0.05, **P < 0.01 versus control.
Figure 3.
Magnolol disrupts mitochondrial integrity in gallbladder cancer (GBC) cells. (a–c) Flow cytometric analysis of the mitochondrial membrane potential (ΔΨm). GBC-SD and SGC-996 cells were treated with magnolol and stained with rhodamine 123. Cells with high ΔΨm are marked “survival”, and those with low ΔΨm are marked “apoptosis”. The percentages of cells with low ΔΨm (apoptosis) are shown. (d) Western blot analysis of apoptosis-related proteins in both cell lines. β-Actin was used as a loading control. The data are presented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus control. PARP1, poly(ADP-ribose) polymerase 1.
Magnolol induces G0/G1 arrest in GBC-SD and SGC-996 cells
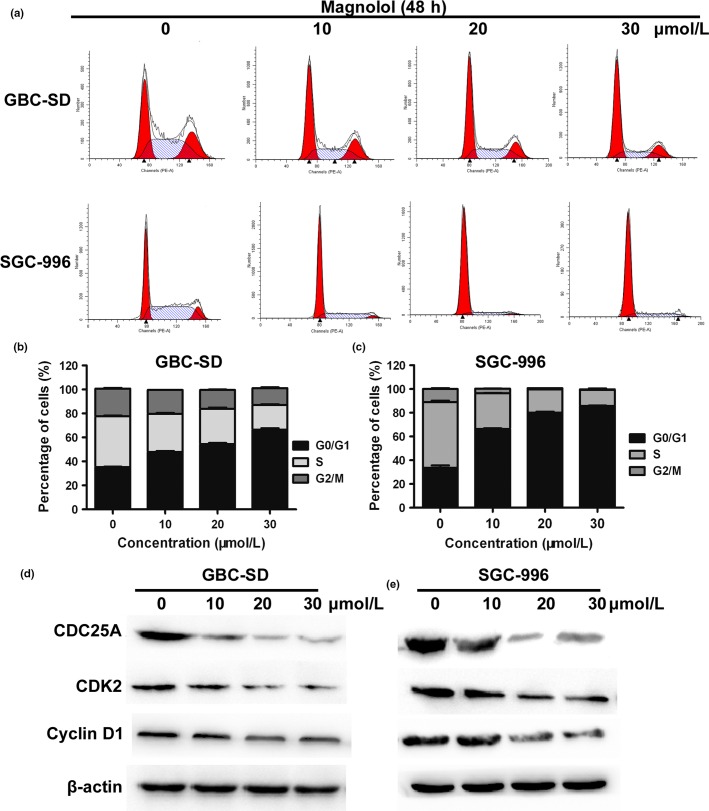
To determine whether magnolol inhibited cell cycle progression, cell cycle distribution proteins and related regulators were studied. Magnolol induced cell cycle arrest in G0/G1 phase in a concentration-dependent manner in both GBC cell lines (Fig.4a–c). Moreover, magnolol treatment resulted in a time-dependent decrease in the expression of CDK2, CDC25A, and cyclin D1 proteins (Fig.4d). These results showed that cell cycle arrest in G0/G1 phase also accounts for the antiproliferative effect of magnolol in both cell lines possibly by disturbing the expression of cell cycle regulators.
Figure 4.
Magnolol induces cell cycle arrest at G0/G1 phase and regulates expression of cell cycle-related proteins in gallbladder (GBC) cells. (a–c) GBC-SD and SGC-996 cells were treated with magnolol (0, 10, 20, and 30 μmol/L) for 48 h. The cell cycle phases of the treated cells were evaluated by flow cytometry. The data are expressed as the mean ± SD (n = 3). (d,e) Expression levels of CDC25A, cyclin D1, and CDK2 were measured by Western blot analysis, and β-actin was used as a loading control. The results are representative of three independent experiments.
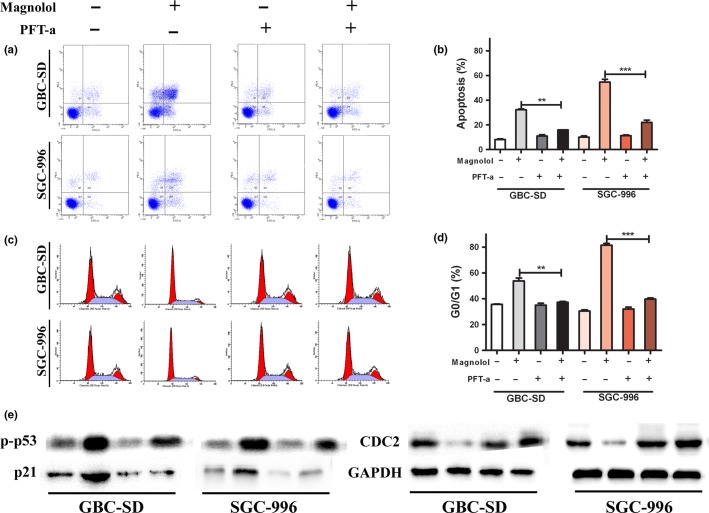
Magnolol-induced apoptosis and G0/G1 arrest regulated by p53
As a transcription factor, p53 plays a central role in apoptosis and cell cycle arrest.6 Therefore, the role of p53 in magnolol-induced apoptosis and G0/G1 arrest was studied. Cells were pretreated with 20 mmol/L PFT-a, a p53 inhibitor, for 1 h before magnolol treatment for an additional 48 h. As shown in Figure5a–d, pretreatment with PFT-a prevented the magnolol-induced apoptosis and reduced G0/G1 phase cell accumulation. To investigate the possible mechanism of magnolol’s effect, we detected the protein levels of p53 and its downstream regulator p21 using Western blot analysis. The results indicated that pretreatment with PFT-a reduced the phosphorylation of p53 and the level of p21 protein (Fig.5e), suggesting that p53 and p21 activation regulate magnolol-induced apoptosis and G0/G1 phase arrest. Taken together, these data showed that magnolol initiated apoptosis and G0/G1 phase arrest through the p53 pathway.
Figure 5.
Magnolol-induced apoptosis and G0/G1 arrest regulated by p53. Cells were incubated for 1 h in the presence or absence of pifithrin-a (PFT-a; 20 mmol/L), and then 20 μmol/L magnolol was added for an additional 48 h. (a,b) Distribution of cells undergoing apoptosis was determined by flow cytometry. (c,d) Cell cycle distribution was determined by flow cytometry. (e) Apoptosis and G0/G1 checkpoint-related proteins were detected by Western blot. The values presented are means ± SD from three independent experiments. **P < 0.01, ***P < 0.001 versus control. CDC2, Cell division cycle 2.
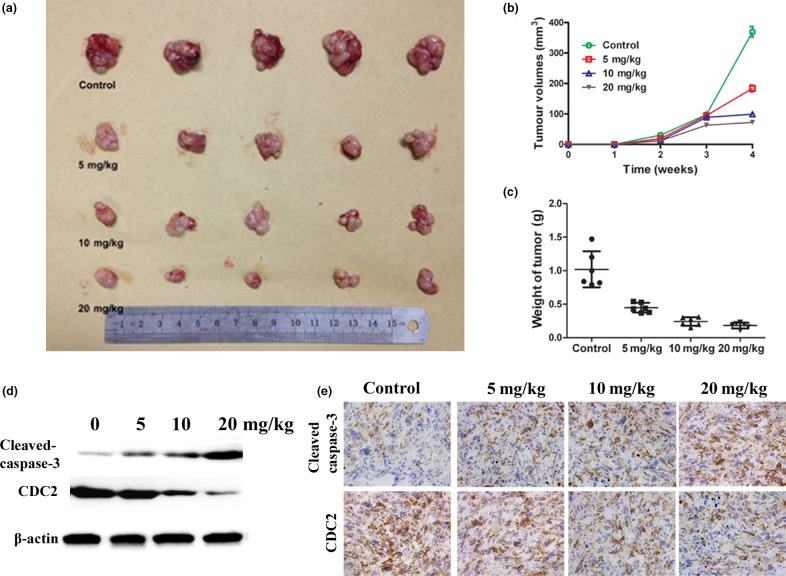
Magnolol inhibits tumor xenograft growth
To further investigate the effect of magnolol on tumor growth in vivo, we i.p. injected DMEM or three increasing doses of magnolol into nude mice with s.c. GBC-SD tumor xenografts. Our results showed that tumor growth in the three treatment groups was significantly inhibited in a dose–response manner compared with that in the control group (Fig.6a–c). Furthermore, Western blot and IHC analyses showed a significant increase in activated caspase-3 and a decrease in CDC2 expression in the tumors of magnolol-treated mice, which was consistent with the in vitro results (Fig.6d,e).
Figure 6.
Magnolol suppresses growth of xenograft gallbladder cancer (GBC) tumors in nude mice by causing apoptotic cell death. Tumor xenografts were established by s.c. implantation of GBC-SD cells into the left flank of nude mice. (a,b) Mice were treated with 0.1 mL vehicle (PBS) or magnolol (5, 10, and 20 mg/kg) i.p. every day for up to 4 weeks. Tumor volumes were measured. (c) Tumors were excised from the animals and weighed. (d) Western blot analysis revealed an increase in active caspase-3 and a decrease in CDC2 expression in magnolol-treated tumors compared with vehicle-treated tumors. (e) Immunohistochemical analysis (×200) illustrates an increase in active caspase-3 and a decrease in CDC2 (Cell division cycle 2) expression in the magnolol-treated tumors compared with the vehicle-treated tumors. The data represent the mean ± SD of three independent experiments. ***P < 0.001 versus control.
Discussion
Gallbladder cancer is the sixth most common form of digestive tract malignancy worldwide.1 At present, no effective therapy for advanced GBC is available. Therefore, it is necessary to identify new therapeutic agents to treat this disease. Chinese herbal medicines exhibit effective antitumor activities with minimal toxicity and are a valuable source of novel chemotherapeutic agents. Magnolol, which is isolated from M. officinalis, is a novel, natural hydroxylated biphenyl compound.13,19 Magnolol is commonly used to treat various ailments due to its muscle relaxant, anti-oxidative, anti-inflammatory, and steroidogenesis-stimulating activities. In addition, magnolol has dose- and time-dependent antitumor activity in several cancers, including colon, bladder, lung, and gastric carcinoma.19–23 In lung cancer, magnolol induces apoptosis of non-small-cell lung cancer cell lines through a caspase-independent pathway.20 Magnolol led to the downregulation of β-catenin/TCF (T cell factor)-targeted downstream genes such as c-myc, MMP-7, and urokinase-type plasminogen activator in human colon cancer cells.23 In human epidermal growth factor receptor 2 (HER2)-overexpressing ovarian cancers, magnolol acts against HER2 and its downstream PI3K/Akt/mTOR signaling network to result in suppression of HER2-mediated transformation and metastatic potential.17 However, magnolol’s cytotoxicity towards GBC cells and its underlying mechanisms of action have not yet been elucidated.
In this study, our results showed that magnolol significantly suppressed tumor cell growth in vivo and in vitro. In addition, because apoptosis and cell cycle arrest in cancer cells are major indicators of anticancer effects, we investigated the effects of magnolol on these two phenomena. The results indicated that magnolol induced G0/G1 phase cell cycle arrest and mitochondria-related apoptosis through the p53 signaling pathway. Moreover, we observed that magnolol had no toxic effect on HUVECs. Therefore, magnolol is most likely a safe molecule for potential clinical use in cancer therapy.
Apoptosis plays a crucial role in development and health maintenance by eliminating unhealthy cells.24 One of the major apoptotic pathways in cancer cells involves mitochondria.25 In our study, we found that the mitochondrial apoptosis pathway played an important role in magnolol-mediated apoptosis. A decrease in the ΔΨm was detected after treating two GBC cell lines with magnolol for 48 h, indicating that magnolol-induced apoptosis in GBC cells might occur by way of the mitochondrial pathway. Cell cycle arrest is closely linked to apoptosis. Deregulation of cell cycle progression is a hallmark of tumor growth.8,26 In the present study, we observed that magnolol inhibited GBC cell proliferation by arresting the cell cycle in G0/G1 phase in a dose-dependent manner. Furthermore, we explored the molecular mechanisms underlying magnolol-induced apoptosis and cell cycle arrest in GBC cells.
Tumor suppressor p53 is a cell cycle regulator with a short half-life.6 The function of p53 is achieved by increasing p53 transcription and post-translational stabilization to escape ubiquitin-dependent degradation.27 Apoptosis mediated by p53 is associated with Bcl2 and Bax, a pro-apoptotic member of the Bcl2 family.28 To examine the involvement of the mitochondrial pathway in magnolol-induced apoptosis, we examined the levels of Bcl2, Bax, and p53. Our data indicated an increase in p53 and Bax expression and a decrease in Bcl2 expression, which resulted in a reduction in the ΔΨm and increased cytochrome c release into the cytosol. Caspases are another important family of proteins involved in the downstream events of p53-mediated apoptosis.9 Our results showed that magnolol could induce p53-mediated apoptosis by activating the caspase-3 and caspase-9 apoptotic cascade. These results indicated that magnolol-induced apoptosis occurred through the p53 pathway in GBC cells.
Cell cycle progression is negatively regulated by the tumor suppressor protein p53 and Cdk inhibitors, including p21.18,29 A member of the cyclin-dependent kinase inhibitor family, p21, is widely recognized as a critical regulator that functions during the transition from G1 phase into S phase. Activation of p21 can induce G1 phase arrest through a p53-dependent pathway.30 Magnolol treatment significantly increased the expression of p53 and p21 proteins in a dose-dependent manner. Indeed, pretreatment with a p53 inhibitor (PFT-a) prevented magnolol-induced apoptosis and reduced the accumulation of p21 and G0/G1 phase cells, suggesting that magnolol initiated apoptosis and G0/G1 phase arrest through the p53 pathway.
In summary, the current study presents the first evidence regarding the roles of magnolol in inducing cell cycle arrest and promoting apoptosis in GBC cells by regulating the p53 signaling pathway. These anticancer properties indicate that magnolol might provide new hope in the search for an effective therapeutic approach for GBC.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81172029, 81272402, and 81402403), the National High Technology Research and Development Program (863 Program) (Grant No. 2012AA022606), the Foundation for Interdisciplinary Research of Shanghai Jiao Tong University (Grant No. YG2011ZD07), the Shanghai Science and Technology Commission Medical-guiding Project (Grant No. 12401905800), the Program for Changjiang Scholars, the Natural Science Foundation of Shanxi Province (Grant No. 20110313013-3 and 2014021037-3), and Shanghai Rising-Star Program (Grant No. 15QA1403100).
Disclosure Statement
The authors have no conflict of interest.
References
- Li M, Zhang Z, Li X, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46:872–6. doi: 10.1038/ng.3030. [DOI] [PubMed] [Google Scholar]
- Li M, Lu J, Zhang F, et al. Yes-associated protein 1 (YAP1) promotes human gallbladder tumor growth via activation of the AXL/MAPK pathway. Cancer Lett. 2014;355:201–9. doi: 10.1016/j.canlet.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- Boutros C, Gary M, Baldwin K, et al. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21:e183–91. doi: 10.1016/j.suronc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Wu XS, Shi LB, Li ML, et al. Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Oncol. 2014;21:449–57. doi: 10.1245/s10434-013-3292-z. [DOI] [PubMed] [Google Scholar]
- Hasty P, Christy BA. p53 as an intervention target for cancer and aging. Pathobiol Aging Age Relat Dis. 2013;3:1–11. doi: 10.3402/pba.v3i0.22702. doi: 10.3402/pba.v3i0.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhang M, Qian Y, et al. Rbm24, an RNA-binding protein and a target of p53, regulates p21 expression via mRNA stability. J Biol Chem. 2014;289:3164–75. doi: 10.1074/jbc.M113.524413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnvajanawong C, Ketnimit S, Pattanapanyasat K, et al. Involvement of p53 and nuclear factor-kappaB signaling pathway for the induction of G1-phase cell cycle arrest of cholangiocarcinoma cell lines by isomorellin. Biol Pharm Bull. 2012;35:1914–25. doi: 10.1248/bpb.b12-00118. [DOI] [PubMed] [Google Scholar]
- Lin Y, Xu J, Liao H, et al. Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumour Biol. 2014;35:3305–10. doi: 10.1007/s13277-013-1433-4. [DOI] [PubMed] [Google Scholar]
- Tazawa H, Kagawa S, Fujiwara T. Advances in adenovirus-mediated p53 cancer gene therapy. Expert Opin Biol Ther. 2013;13:1569–83. doi: 10.1517/14712598.2013.845662. [DOI] [PubMed] [Google Scholar]
- Li X, Yang X, Liu Y, et al. Japonicone A suppresses growth of Burkitt lymphoma cells through its effect on NF-kappaB. Clin Cancer Res. 2013;19:2917–28. doi: 10.1158/1078-0432.CCR-12-3258. [DOI] [PubMed] [Google Scholar]
- Ba Q, Zhou N, Duan J, et al. Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PLoS ONE. 2012;7:e42703. doi: 10.1371/journal.pone.0042703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee YM, Lee CK, et al. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–76. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Chen MC, Lee CF, Huang WH, et al. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1alpha/VEGF signaling pathway in human bladder cancer cells. Biochem Pharmacol. 2013;85:1278–87. doi: 10.1016/j.bcp.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Park JB, Lee MS, Cha EY, et al. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol Pharm Bull. 2012;35:1614–20. doi: 10.1248/bpb.b12-00352. [DOI] [PubMed] [Google Scholar]
- Lin SY, Liu JD, Chang HC, et al. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem. 2002;84:532–44. [PubMed] [Google Scholar]
- Chuang TC, Hsu SC, Cheng YT, et al. Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett. 2011;311:11–9. doi: 10.1016/j.canlet.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Lv C, Hong Y, Miao L, et al. Wentilactone A as a novel potential antitumor agent induces apoptosis and G2/M arrest of human lung carcinoma cells, and is mediated by HRas-GTP accumulation to excessively activate the Ras/Raf/ERK/p53-p21 pathway. Cell Death Dis. 2013;4:e952. doi: 10.1038/cddis.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Cho YH, Park K, et al. Magnolol elicits activation of the extracellular signal-regulated kinase pathway by inducing p27KIP1-mediated G2/M-phase cell cycle arrest in human urinary bladder cancer 5637 cells. Biochem Pharmacol. 2008;75:2289–300. doi: 10.1016/j.bcp.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Tsai JR, Chong IW, Chen YH, et al. Magnolol induces apoptosis via caspase-independent pathways in non-small cell lung cancer cells. Arch Pharm Res. 2014;37:548–57. doi: 10.1007/s12272-013-0232-1. [DOI] [PubMed] [Google Scholar]
- Rasul A, Yu B, Khan M, et al. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int J Oncol. 2012;40:1153–61. doi: 10.3892/ijo.2011.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilampalli C, Guillermo R, Zhang X, et al. Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action. BMC Cancer. 2011;11:456. doi: 10.1186/1471-2407-11-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Park HJ, Chung HJ, et al. Wnt/beta-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol Pharmacol. 2012;82:168–77. doi: 10.1124/mol.112.078535. [DOI] [PubMed] [Google Scholar]
- Ma GF, Chen SY, Sun ZR, et al. FoxP3 inhibits proliferation and induces apoptosis of gastric cancer cells by activating the apoptotic signaling pathway. Biochem Biophys Res Commun. 2013;430:804–9. doi: 10.1016/j.bbrc.2012.11.065. [DOI] [PubMed] [Google Scholar]
- Basanez G, Soane L, Hardwick JM. A new view of the lethal apoptotic pore. PLoS Biol. 2012;10:e1001399. doi: 10.1371/journal.pbio.1001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KS, Cho SH, Shin JS, et al. Ginsenoside Rh2 induces cell cycle arrest and differentiation in human leukemia cells by upregulating TGF-beta expression. Carcinogenesis. 2013;34:331–40. doi: 10.1093/carcin/bgs341. [DOI] [PubMed] [Google Scholar]
- Bansal N, Kadamb R, Mittal S, et al. Tumor suppressor protein p53 recruits human Sin3B/HDAC1 complex for down-regulation of its target promoters in response to genotoxic stress. PLoS ONE. 2011;6:e26156. doi: 10.1371/journal.pone.0026156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R, Kausar H, Munjal C, et al. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- Noori S, Hassan ZM. Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic Biol Med. 2012;52:1987–99. doi: 10.1016/j.freeradbiomed.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang K, Zhang K, et al. Ziyuglycoside II inhibits the growth of human breast carcinoma MDA-MB-435 cells via cell cycle arrest and induction of apoptosis through the mitochondria dependent pathway. Int J Mol Sci. 2013;14:18041–55. doi: 10.3390/ijms140918041. [DOI] [PMC free article] [PubMed] [Google Scholar]