Abstract
Background:
Primary axillary hyperhidrosis (PAH) is a common condition with a great impact on the patient's quality of life (QOL). It is associated with serious social, emotional, and occupational distress. The aim of this study was to investigate the QOL in patients with PAH before and after treatment with fractionated microneedle radiofrequency (FMR).
Materials and Methods:
We evaluated 25 patients with severe PAH. Each patient had three sessions of FMR treatment using a novel applicator at 3-week intervals. The study was based on Dermatology Life Quality Index (DLQI) Questionnaires. Patients were evaluated at baseline and 3 months after the last session.
Results:
Our patients included 32% males and 68% females. The mean ± standard deviation (SD) age of subjects was 30.2 ± 6.27 years. The mean ± SD of the DLQI before and after treatment was 12.96 ± 5.93, and 4.29 ± 2.21, respectively. There was a statistically significant difference between the before and after intervention (P < 0.001). No major, permanent adverse effects were shown.
Conclusion:
Treatment with FMR can improve the DLQI of patients with PAH.
Keywords: Fractionated microneedle radiofrequency, primary axillary hyperhidrosis, quality of life
INTRODUCTION
Hyperhidrosis is a common condition rarely due to significant underlying pathology that may have serious social, emotional, and professional consequences.[1] The prevalence estimates range from 1% to 3% of the population.[1,2]
Hyperhidrosis is always accompanied by the subjective reduction in quality of life (QOL) for the patient who feels uncomfortable in the world, where sweating is considered anti-esthetic and may hinder socialization.[3] Furthermore, hyperhidrosis represents a disease with a severe psychosocial impact, and it interferes with patients’ daily activities.[1,4] Hyperhidrosis is a common disease for which most patients do not seek medical advice because they do not appreciate the condition as a disease.[1]
Therapy for hyperhidrosis can be challenging for both the patient and the physician. There are several different treatment options for hyperhidrosis, including topical therapy, botulinum toxin A (BTX-A), oral medications (anti-cholinergic and a-adrenergic blockers), and surgical therapy.[5,6]
Recently, studies have used radiofrequency (RF) energy in the treatment of primary axillary hyperhidrosis (PAH), which suggests that newer bipolar RF devices are effective in reducing the amount of sweating.[7] RF energy is conducted electrically to tissue and provides tissue heating, depending on the specific tissue resistance.[8,9] The combination of RF and fractional microneedle technology creates an effective method with a better safety profile for treating various dermatological ailments.[10]
At present, QOL questionnaires have become an important tool for quantifying results in medicine, and this psychosocial factor has important implications for better management of patients.[3,11] Dermatology Life Quality Index (DLQI) is a dermatology-specific, validated questionnaire that applies to a variety of skin diseases for the determination of the impact on the patient's QOL and for the evaluation of change during therapy. The use of this index is more indicative of the extent to which treatment helps the patient.[11,12]
The aim of this study was to investigate the DLQI in patients with PAH, who had been treated with fractionated microneedle RF (FMR).
MATERIALS AND METHODS
This prospective study was conducted on patients with PAH, who had been referred to the dermatology clinic of Isfahan University of Medical Sciences, Iran in 2014. The Ethics Committee of Isfahan University of Medical Sciences approved the study. All patients signed informed consent forms. A total of 25 patients were included in our study using a simple sampling method.
The inclusion criteria were diagnoses of axillary PAH confirmed clinically by a dermatologist. Patients with pregnancy or intention to become pregnant, breastfeeding, history of having received botulinum toxin injections in the axillae within the previous 6 months, history of pacemaker implantation, active infection in the treatment area, and propensity for keloid formation were not included. The exclusion criteria were not able to continue the study.
Each patient had three sessions of FMR treatment using a novel applicator (INFINI TM; Lutronic, Goyang, Korea) at 3-weeks interval.
All subjects were pretreated with a topical anesthesia (EMLA; Astra-Zeneca, Sodertalje, Sweden) in axilla under occlusion 45 min prior to FMR treatment. The treatment included the following parameters:
A depth of 2-3 mm;
A time range of 120-180 ms,
An energy level of 6-10 j/cm2 with a small range (±10%), and time settings determining the optimal parameters for the population being treated.[6,7]
Patients were evaluated at baseline and 3 months after the last session.
Data on QOL was obtained from a questionnaire using the DLQI.[13] It is a 10-item questionnaire that covers six aspects of daily life experienced during the past week. Each question has four alternative responses: “Not at all,” “a little,” “a lot,” and “very much,” with corresponding scores of 0, 1, 2, and 3, respectively. The answer “not relevant” is scored as 0. The total score of every individual's QOL would be the sum of total scores of all the questions, that is, between 0 and 30.[12] Patients completed the questionnaire to evaluate their subjective perception of the improvement in hyperhidrosis symptoms.
The total scores refer to: DLQI scores 0-1 = no effect on patient's life, DLQI scores 2-5 = small effect on patient's life, DLQI scores 6-10 = moderate effect on patient's life, DLQI scores 11-20 = very large effect on patient's life and DLQI scores 21-30 = extremely large effect on patient's life.[13]
The reliability and validity of the Persian (the Iranian official language) version of the DLQI questionnaire had been proved through a study in patients with vitiligo in Shiraz.[14]
The first evaluation was based on a review of the medical records before the start of the treatment and completion of demographic data. Body mass index (BMI: kg/m2) was calculated as weight (in kilograms) divided by height (in meters) squared.
To evaluate side effects, in addition to close dermatological examinations, patients were instructed to report experiencing any side effects including pruritus, scaling, crusting, compensatory hyperhidrosis, postinflammatory pigmentation, pain in the area of the FMR, erythema, swelling, reduction in muscular force, dysesthesia, and paresthesia in the arm and forearm, etc. Side effects were categorized from Grade 1 (minor) to Grade 3 (severe).
Data were analyzed using statistical package for social science (SPSS) version 18 for windows (IBM Corporation, New York, USA). Mean and standard deviation (SD) were used to describe continuous data, number, and percentage for categorical data. The results were analyzed using two-sided, paired nonparametric tests including the Wilcoxon test. Statistical significance was defined as P < 0.05.
RESULTS
The study involved 25 patients with PAH, aged 18-47 years with an average age (±SD) of 30.2 ± 6.27 years; 68% of patients were female and BMI ranged from 18 to 28 kg/m2 with an average (±SD) of 21.82 ± 2.80 kg/m2. Twenty-four of the 25 enrolled subjects completed the study. One patient was excluded from the study after the first session of treatment due to transient dysesthesia development on the arm.
The mean (±SD) DLQI score before intervention was 12.96 ± 5.93 and less after intervention at 4.29 ± 2.21 [P < 0.001; Table 1].
Table 1.
DLQI before and after treatment (Wilcoxon test)

Based on the obtained scores, the disease had very large and extremely large effects on each patient's life in 15 (62.5%), and 3 (12.5%), respectively, while after treatment with FMR none of the patients showed these statuses [Figure 1].
Figure 1.
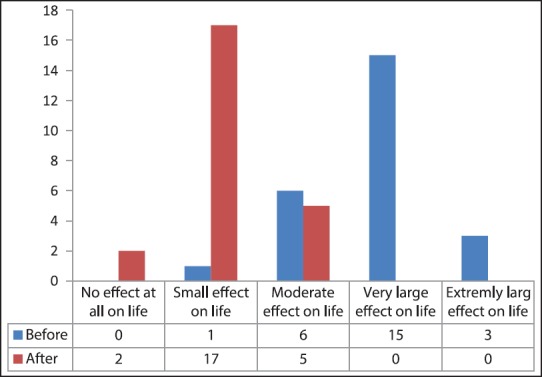
Dermatology Life Quality Index scores translation before and after intervention
Our results showed that treatment of PAH with FMR improved the score of DLQI, regardless of gender, marital status, educational level, family history of hyperhidrosis, and duration of disease [P < 0.05; Table 2].
Table 2.
DLQI before and after treatment regarding variables of study
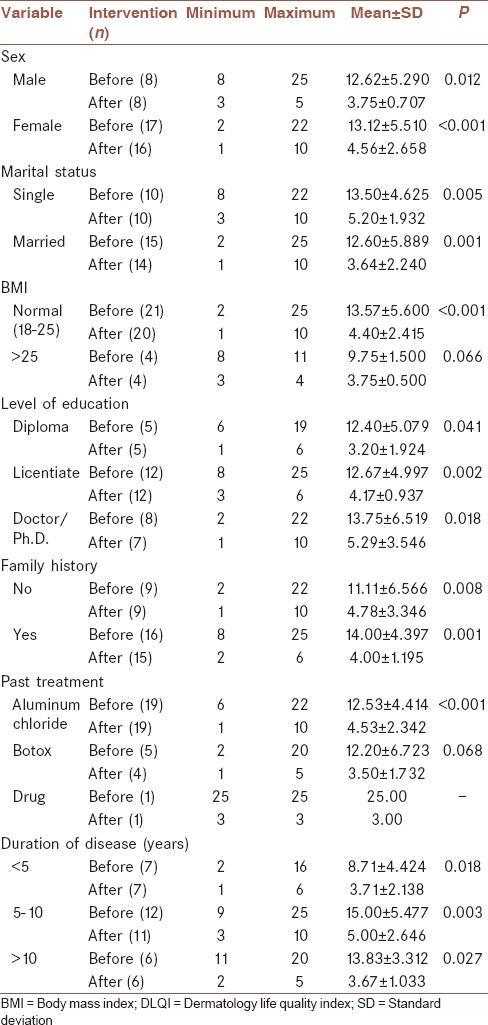
The mean DLQI score (±SD) of patients before and after treatment who had a history of treatment with topical aluminum chloride was 4.41 ± 12.53, and 2.34 ± 4.53, respectively, which improved the QOL with FMR (P < 0.001). In patients who had a history of treatment with BTX-A in the past, we did not find differences in the QOL before and after treatment with FMR [P = 0.068; Table 2].
The mean DLQI score (±SD) in patients before and after treatment who had a BMI of over 25 were 1.50 ± 9.75, and 0.50 ± 3.75, respectively, despite the improvement of QOL in these patients; the difference was not significant (P = 0.066). In those with normal BMI, the improvement of QOL was significant [P < 0.001; Table 2].
Reduction of DLQI ranged from 1 to 22, with an average score (± SD) of 8.54 ± 4.40.
About 80% of patients had more than a 5 score reduction, and 6 patients (24%) had more than a 10 score reduction in the DLQI.
No major permanent adverse effects such as scarring or ulceration were experienced with the FMR treatment. In most patients, temporary side effects such as swelling, pain, redness, transient hyperpigmentation, and mild compensatory hyperhidrosis were observed in the treated areas. Only one subject experienced transient tingling and numbness of the left arm after the procedure that completely resolved without any sequels after 2 months of discontinuing the treatment.
DISCUSSION
To the best of our knowledge, no studies have been performed on the QOL in patients with PAH after treatment with FMR. Our study shows that treatment with FMR can improve the DLQI of patients with PAH.
The correlation between axillary hyperhidrosis and impaired QOL and consequential psychosocial disorders is mutual; the anxiety and stress condition can be considered as a trigger factor for initiation or exacerbation of the disease.[1,3,4] The disease, through its negative impacts on the patient's life, causes psychosocial problems.[15] Although PAH is not a life threatening disease, its psychosocial problem can severely affect the social and psychological functioning of the affected individuals, causing anxiety, depression, decreased body satisfaction, and low QOL.[15] The high incidence of primary hyperhidrosis and its negative effect on QOL have led to demand effective treatments.[16]
The combination of RF and fractional microneedle technology creates an effective method with a better safety profile, which selectively destroys intradermal targets such as sweat glands.[10,17]
The effectiveness of FMR devices in the treatment of PAH has been shown in a number of studies. Kim et al. showed that FMR treatment was effective for the treatment of PAH without significant adverse reactions due to direct volumetric heating of the lower dermis.[10] In our recent study, we reported that FMR can be used as an effective, harmless, and nonaggressive method for treatment of PAH.[18,19] Also, we reported histopathological evidence of efficacy of FMR for treatment of axillary hyperhidrosis.[20]
The importance of our study lies in observing the improvement of QOL with FMR treatment in PAH, which has not been studied before.
In this study, the mean reduction of the DLQI score was about 8.5, also as in the study by Hong et al., 3 months after medical intervention.[7]
At least a 5-point reduction in DLQI is the change in the DLQI that represents a clinically significant improvement after therapy for PAH.[21]
In this study, about 80% of subjects had at least a 5-point reduction in DLQI after a 3-month visit, similar to the study by Hong et al.[7] These results are significantly higher than improvements that represent a clinically significant change in DLQI after therapy for PAH.[21]
Our results show that improvement of DLQI can occur after treatment of FMR, regardless of the marital status and educational level. As previous studies had not concerned with the evaluation of social backgrounds regarding these items, no supportive or contrary results were found.
In this study, the patients who had a past history of treatment with botulinum toxin did not have differences in QOL before and after treatment with FMR. This may perhaps be due to the treatment with BTX-A, which can improve QOL significantly, but the duration of the effect is transient.[22] Several studies have shown BTX-A to be particularly effective in the improvement of QOL in PAH. Although Tan and Solish showed that the DLQI score was reduced significantly after the treatment of axillary and hyperhidrosis with BTX-A, but the duration of the effect was on average 4-7 months.[22] In addition, Naumann reported 320 patients with axillary hyperhidrosis treated with BTX-A which showed significant improvement in emotional status in their daily lives, in their social activities, and in their productivity at work.[6] The limitations of these modalities, such as botulinum toxin therapy are high cost, and transient results that must be considered.[22,23,24] Because FMR therapy directly damages sweat glands; FMR was expected to have a lasting effect on sweat reduction compared with botulinum toxin.[10]
In our patients, none of the subject's experience worsened their QOL, which is unlike the study of Wolosker et al., in which the difficult control of abundant compensatory sweating following endoscopic thoracic sympathectomy worsened the QOL.[3] Among this modality of treatment, only surgical sympathectomy and axillary curettage have been capable of conferring a permanent solution. In addition, potential risks of these methods include burning, scarring, symptom recurrence, Horner's syndrome, compensatory hyperhidrosis in surgical sympathectomy,[3,25] and scar formation in axillary curettage.[23]
CONCLUSION
For patients with PAH and poor QOL, FMR appears to be a safe and effective treatment option to improve QOL. It can be a valid alternative to current surgical treatment. Further studies are required to determine the long-term outcomes of FMR on QOL of patients with PAH.
Financial support and sponsorship
This study was sponsored by the Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
BAN contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. FFN contributed to the conception and design of the work, conducting the study, approval of the final version of the manuscript and agreed for all aspects of the work. NA contributed to the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MP contributed in the design of the work, contributed in data analysis, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This study was supported by Skin Disease and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241–8. doi: 10.1016/j.jaad.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Leung AK, Chan PY, Choi MC. Hyperhidrosis. Int J Dermatol. 1999;38:561–7. doi: 10.1046/j.1365-4362.1999.00609.x. [DOI] [PubMed] [Google Scholar]
- 3.Wolosker N, de Campos JR, Kauffman P, de Oliveira LA, Munia MA, Jatene FB. Evaluation of quality of life over time among 453 patients with hyperhidrosis submitted to endoscopic thoracic sympathectomy. J Vasc Surg. 2012;55:154–6. doi: 10.1016/j.jvs.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 4.Tupker RA, Harmsze AM, Deneer VH. Oxybutynin therapy for generalized hyperhidrosis. Arch Dermatol. 2006;142:1065–6. doi: 10.1001/archderm.142.8.1065. [DOI] [PubMed] [Google Scholar]
- 5.Wolosker N, Teivelis MP, Krutman M, de Paula RP, Kauffman P, de Campos JR, et al. Long-term results of the use of oxybutynin for the treatment of axillary hyperhidrosis. Ann Vasc Surg. 2014;28:1106–12. doi: 10.1016/j.avsg.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Naumann M. Evidence-based medicine: Botulinum toxin in focal hyperhidrosis. J Neurol. 2001;248(Suppl 1):31–3. doi: 10.1007/pl00007817. [DOI] [PubMed] [Google Scholar]
- 7.Hong HC, Lupin M, O'shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2012;38:728–35. doi: 10.1111/j.1524-4725.2012.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lolis MS, Goldberg DJ. Radiofrequency in cosmetic dermatology: A review. Dermatol Surg. 2012;38:1765–76. doi: 10.1111/j.1524-4725.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick R, Geronemus R, Goldberg D, Kaminer M, Kilmer S, Ruiz-Esparza J. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232–42. doi: 10.1002/lsm.10225. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Shin JY, Lee J, Kim JY, Oh SH. Efficacy of fractional microneedle radiofrequency device in the treatment of primary axillary hyperhidrosis: A pilot study. Dermatology. 2013;227:243–9. doi: 10.1159/000354602. [DOI] [PubMed] [Google Scholar]
- 11.Campanati A, Penna L, Guzzo T, Menotta L, Silvestri B, Lagalla G, et al. Quality-of-life assessment in patients with hyperhidrosis before and after treatment with botulinum toxin: Results of an open-label study. Clin Ther. 2003;25:298–308. doi: 10.1016/s0149-2918(03)90041-5. [DOI] [PubMed] [Google Scholar]
- 12.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The dermatology life quality index 1994-2007: A comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x. [DOI] [PubMed] [Google Scholar]
- 13.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol. 2005;125:659–64. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 14.Aghaei S, Sodaifi M, Jafari P, Mazharinia N, Finlay AY. DLQI scores in vitiligo: Reliability and validity of the Persian version. BMC Dermatol. 2004;4:8. doi: 10.1186/1471-5945-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouris A, Armyra K, Christodoulou C, Karimali P, Karypidis D, Kontochristopoulos G. Quality of Life in Patients with Focal Hyperhidrosis before and after treatment with botulinum toxin A. ISRN Dermatol 2014. 2014:308650. doi: 10.1155/2014/308650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerfolio RJ, De Campos JR, Bryant AS, Connery CP, Miller DL, DeCamp MM, et al. The society of thoracic surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011;91:1642–8. doi: 10.1016/j.athoracsur.2011.01.105. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T. Electrosurgery using insulated needles: Treatment of axillary bromhidrosis and hyperhidrosis. J Dermatol Surg Oncol. 1988;14:749–52. doi: 10.1111/j.1524-4725.1988.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 18.Fatemi Naeini F, Abtahi-Naeini B, Pourazizi M, Nilforoushzadeh MA, Mirmohammadkhani M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: A sham control study. Australas J Dermatol. 2014 Dec 13; doi: 10.1111/ajd.12260. doi: 10.1111/ajd.12260 [Epub ahead of print] Link of web is: http://www.ncbi.nlm.nih.gov/pubmed/25496000 . [DOI] [PubMed] [Google Scholar]
- 19.Fatemi Naeini F, Pourazizi M, Abtahi-Naeini B, Nilforoushzadeh MA, Najafian J. A novel option for treatment of primary axillary hyperhidrosis: fractionated microneedle radiofrequency. J Postgrad Med. 2015;61:141–3. doi: 10.4103/0022-3859.153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naeini FF, Saffaei A, Pourazizi M, Abtahi-Naeini B. Histopathological evidence of efficacy of microneedle radiofrequency for treatment of axillary hyperhidrosis. Indian J Dermatol Venereol Leprol. 2015;81:288–90. doi: 10.4103/0378-6323.154789. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski JW, Eadie N, Dagget S, Lai PY. Validity and reliability of the hyperhidrosis disease severity scale (HDSS) J Am Acad Dermatol. 2004;50:P51. [Google Scholar]
- 22.Tan SR, Solish N. Long-term efficacy and quality of life in the treatment of focal hyperhidrosis with botulinum toxin A. Dermatol Surg. 2002;28:495–9. doi: 10.1046/j.1524-4725.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol. 2012;26:1–8. doi: 10.1111/j.1468-3083.2011.04173.x. [DOI] [PubMed] [Google Scholar]
- 24.Doft MA, Hardy KL, Ascherman JA. Treatment of hyperhidrosis with botulinum toxin. Aesthet Surg J. 2012;32:238–44. doi: 10.1177/1090820X11434506. [DOI] [PubMed] [Google Scholar]
- 25.de Campos JR, Kauffman P, Werebe Ede C, Andrade Filho LO, Kusniek S, Wolosker N, et al. Quality of life, before and after thoracic sympathectomy: Report on 378 operated patients. Ann Thorac Surg. 2003;76:886–91. doi: 10.1016/s0003-4975(03)00895-6. [DOI] [PubMed] [Google Scholar]


